Ultrasound-Mediated Atom Transfer Radical Polymerization (ATRP)
Abstract
1. Introduction
2. Mechanically Controlled ATRP
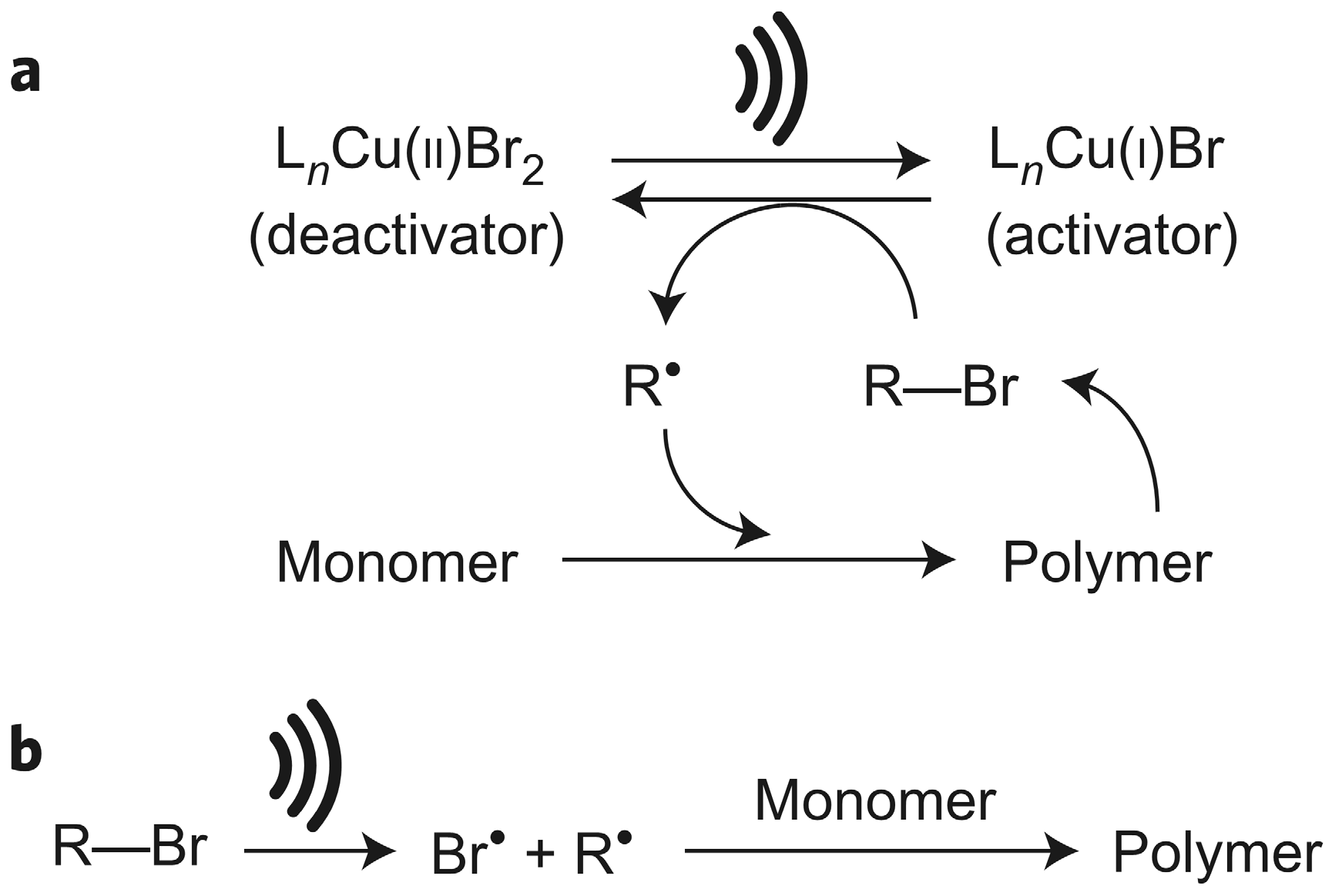
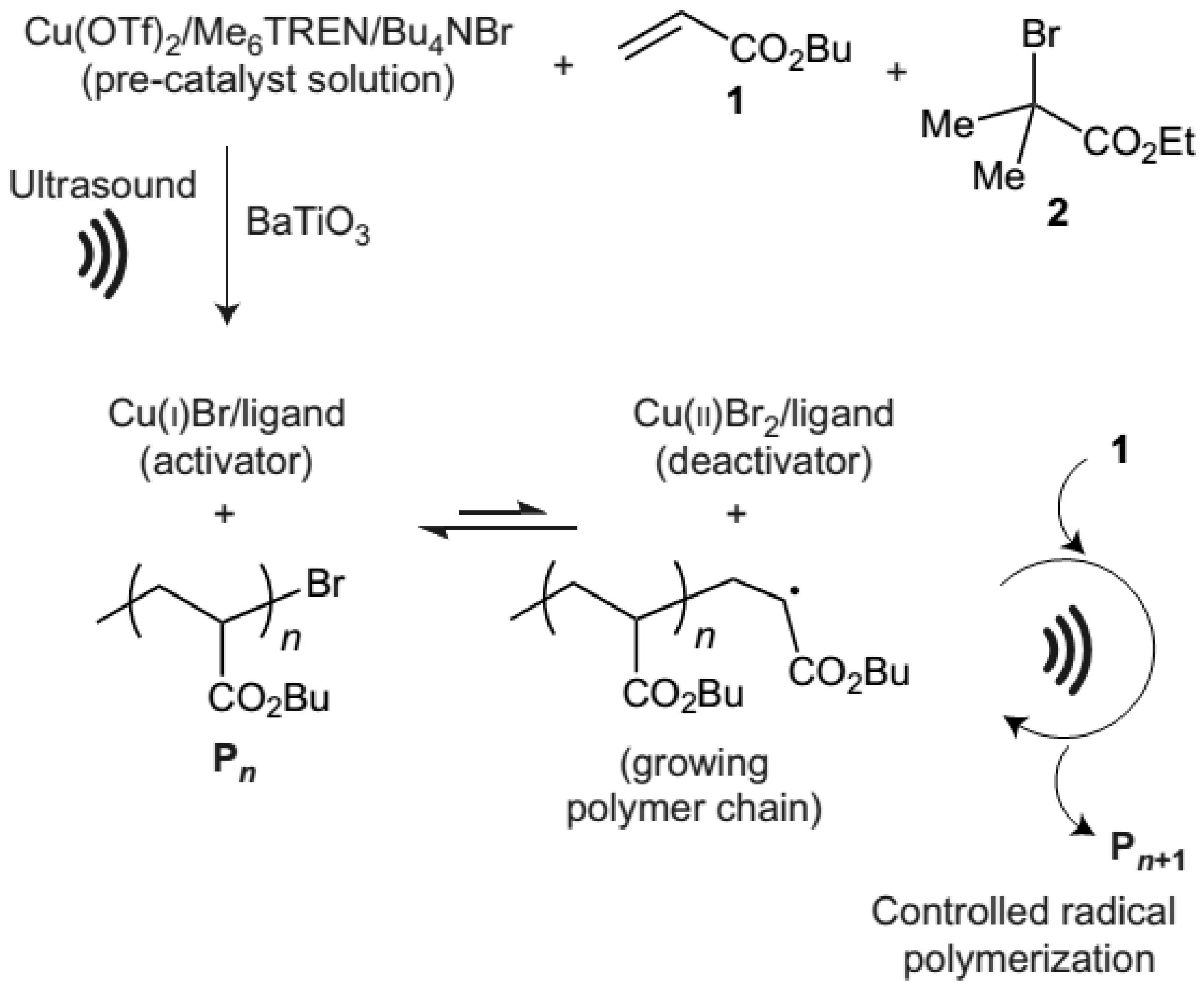
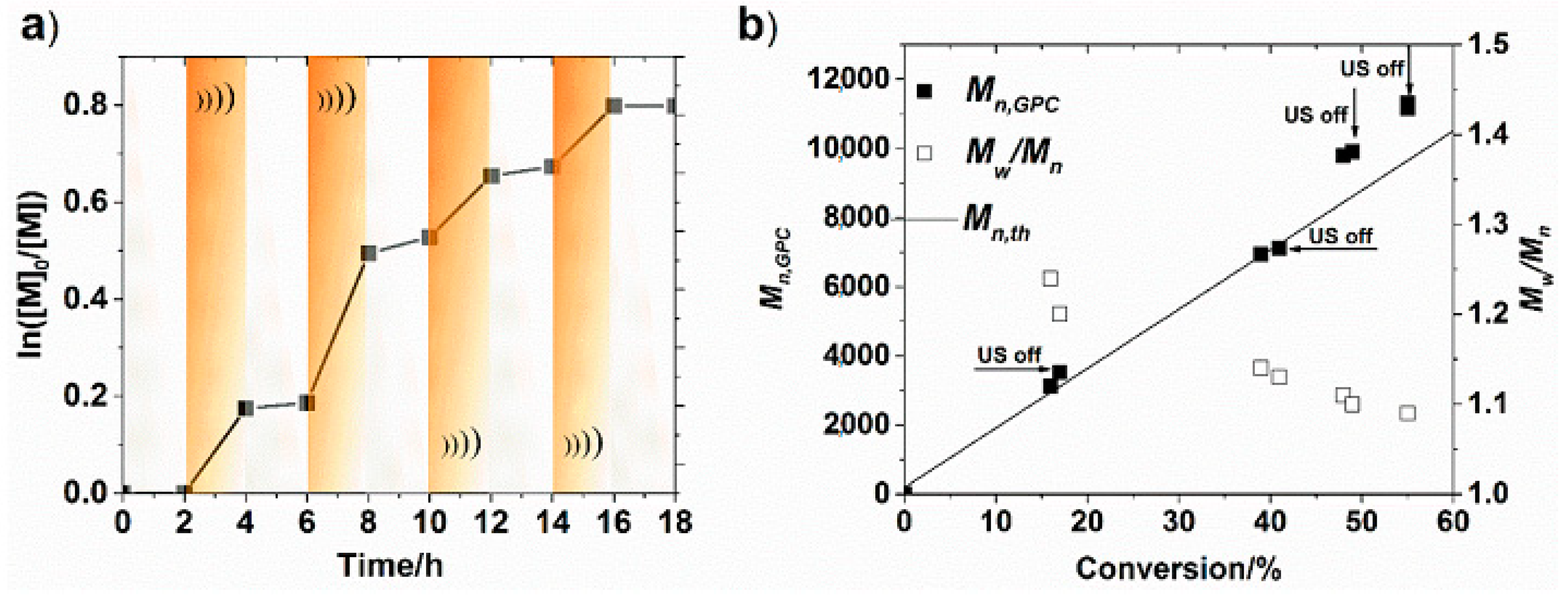
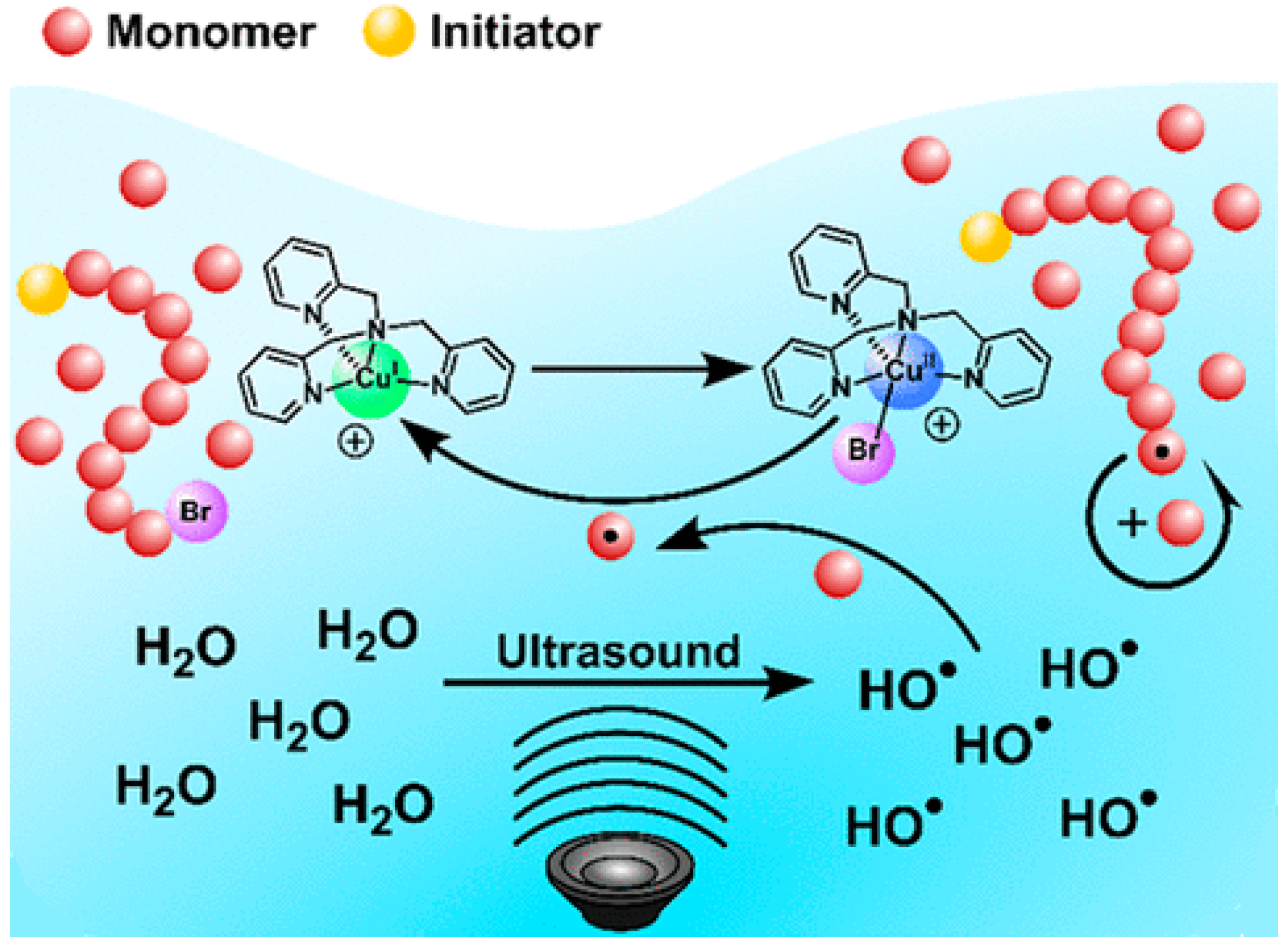
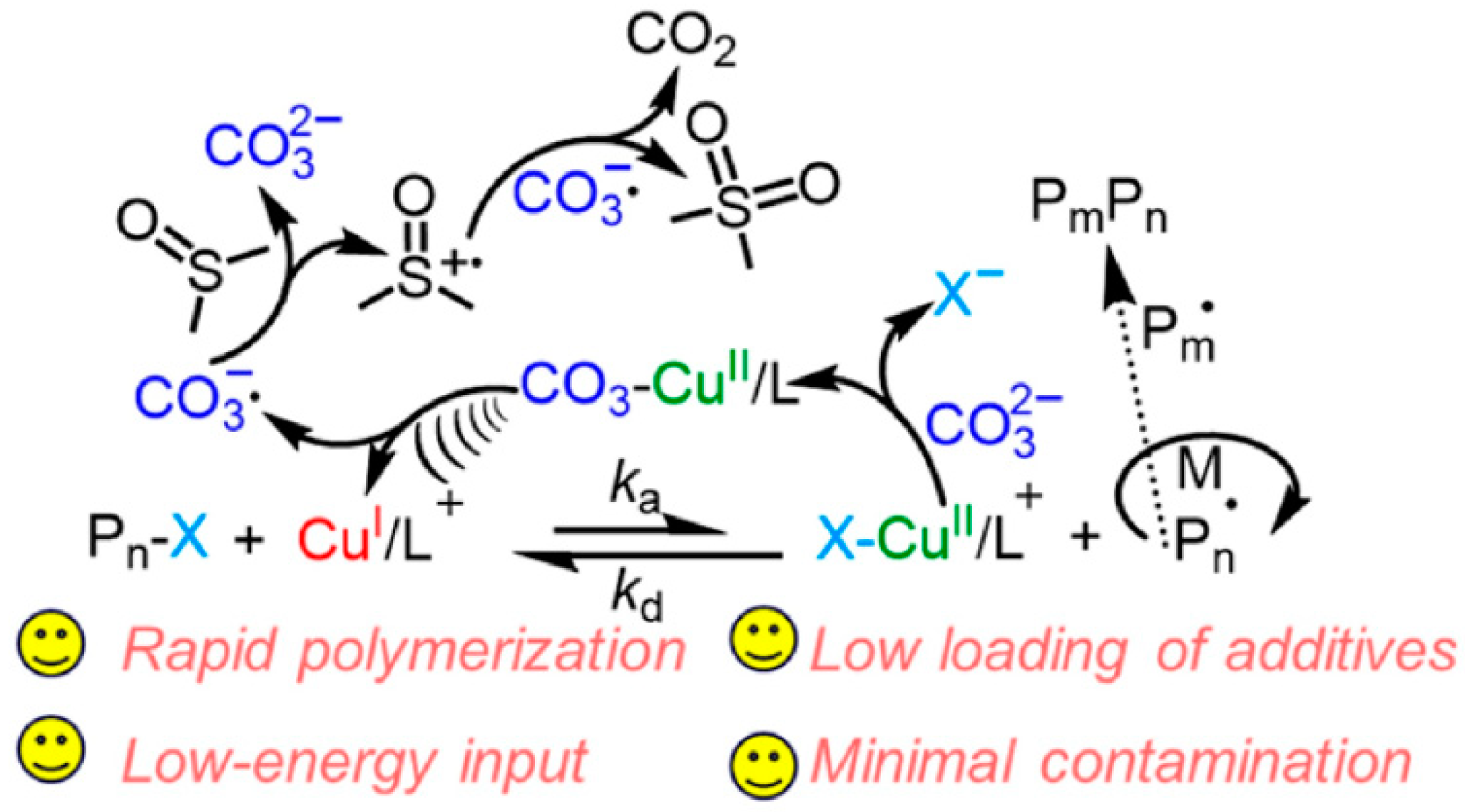
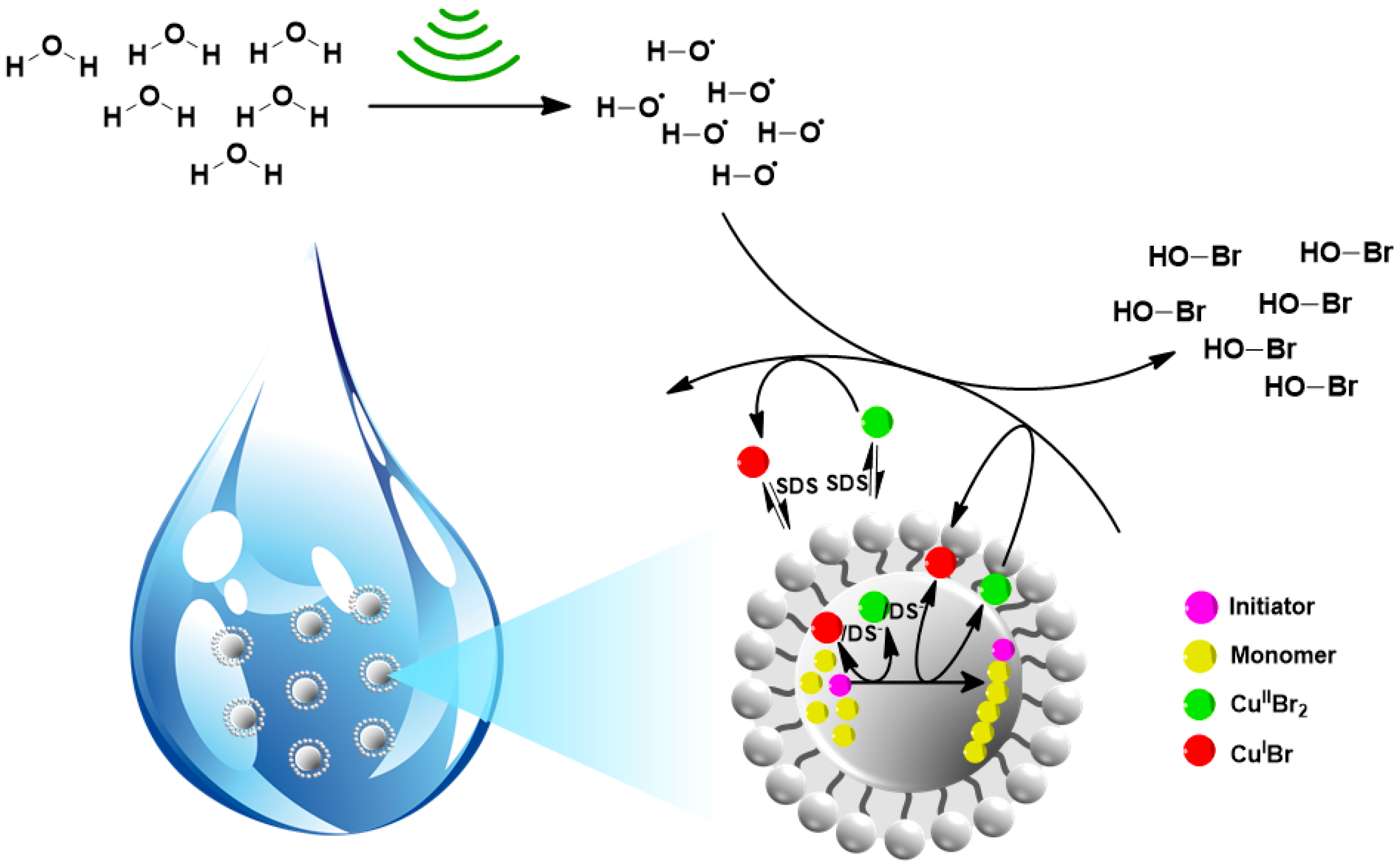
3. Ultrasonication-Induced ATRP in Homogenous and Dispersed Media
4. Future Prospective
Funding
Conflicts of Interest
References
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Satoh, K. Controlled/living polymerization of renewable vinyl monomers into bio-based polymers. Polym. J. 2015, 47, 527–536. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.D.; Ajayaghosh, A. Living supramolecular polymerization. Science 2015, 349, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, A.; Nikolaou, V.; Nurumbetov, G.; Wilson, P.; Kempe, K.; Quinn, J.F.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. Cu(0)-mediated living radical polymerization: A versatile tool for materials synthesis. Chem. Rev. 2016, 116, 835–877. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Pan, X.; Fantin, M.; Yuan, F.; Matyjaszewski, K. Externally controlled atom transfer radical polymerization. Chem. Soc. Rev. 2018, 47, 5457–5490. [Google Scholar] [CrossRef]
- Park, S.; Chmielarz, P.; Gennaro, A.; Matyjaszewski, K. Simplified electrochemically mediated atom transfer radical polymerization using a sacrificial anode. Angew. Chem. Int. Ed. 2015, 54, 2388–2392. [Google Scholar] [CrossRef]
- Williams, V.A.; Ribelli, T.G.; Chmielarz, P.; Park, S.; Matyjaszewski, K. A silver bullet: Elemental silver as an efficient reducing agent for atom transfer radical polymerization of acrylates. J. Am. Chem. Soc. 2015, 137, 1428–1431. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Matyjaszewski, K. Iron-catalyzed atom transfer radical polymerization of semifluorinated methacrylates. ACS Macro Lett. 2019, 8, 1110–1114. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of α-d-glucose-based star polymers through simplified electrochemically mediated ATRP. Polymer 2016, 102, 192–198. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Temporally controlled ultrasonication-mediated atom transfer radical polymerization in miniemulsion. Macromol. Chem. Phys. 2019, 220, 1900285. [Google Scholar] [CrossRef]
- Lu, C.; Wang, C.; Yu, J.; Wang, J.; Chu, F. Metal-free ATRP “grafting from” technique for renewable cellulose graft copolymers. Green Chem. 2019, 21, 2759–2770. [Google Scholar] [CrossRef]
- Mao, B.; Gan, L.; Gan, Y. Ultra high molar mass poly[2-(dimethylamino)ethyl methacrylate] via atom transfer radical polymerization. Polymer 2006, 47, 3017–3020. [Google Scholar] [CrossRef]
- Mueller, L.; Jakubowski, W.; Matyjaszewski, K.; Pietrasik, J.; Kwiatkowski, P.; Chaladaj, W.; Jurczak, J. Synthesis of high molecular weight polystyrene using AGET ATRP under high pressure. Eur. Polym. J. 2011, 47, 730–734. [Google Scholar] [CrossRef]
- Xie, G.; Martinez, M.R.; Daniel, W.F.M.; Keith, A.N.; Ribelli, T.G.; Fantin, M.; Sheiko, S.S.; Matyjaszewski, K. Benefits of catalyzed radical termination: High-yield synthesis of polyacrylate molecular bottlebrushes without gelation. Macromolecules 2018, 51, 6218–6225. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Wolski, K.; Grześ, G.; Isse, A.A.; Gennaro, A.; Zapotoczny, S.; Sobkowiak, A. Tannic acid-inspired star-like macromolecules via temporally controlled multi-step potential electrolysis. Macromol. Chem. Phys. 2019, 220, 1900073. [Google Scholar] [CrossRef]
- Chmielarz, P.; Park, S.; Sobkowiak, A.; Matyjaszewski, K. Synthesis of β-cyclodextrin-based star polymers via a simplified electrochemically mediated ATRP. Polymer 2016, 88, 36–42. [Google Scholar] [CrossRef]
- Xiu, K.; Yang, J.; Zhao, N.; Li, J.; Xu, F. Multiarm cationic star polymers by atom transfer radical polymerization from β-cyclodextrin cores: Influence of arm number and length on gene delivery. Acta Biomater. 2013, 9, 4726–4733. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P. Surface-initiated atom transfer radical polymerization for the preparation of well-defined organic-inorganic hybrid nanomaterials. Materials 2019, 12, 3030. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Chmielarz, P.; Yan, J.; Krys, P.; Wang, Y.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis of nanoparticle copolymer brushes via surface-initiated seATRP. Macromolecules 2017, 50, 4151–4159. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Liu, T.; Wei, Q.; Li, S.; Olszewski, M.; Wu, J.; Sobieski, J.; Fantin, M.; Bockstaller, M.R.; et al. Control of dispersity and grafting density of particle brushes by variation of ATRP catalyst concentration. ACS Macro Lett. 2019, 8, 859–864. [Google Scholar] [CrossRef]
- Krys, P.; Matyjaszewski, K. Kinetics of atom transfer radical polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef]
- Boyer, C.; Corrigan, N.A.; Jung, K.; Nguyen, D.; Nguyen, T.-K.; Adnan, N.N.M.; Oliver, S.; Shanmugam, S.; Yeow, J. Copper-mediated living radical polymerization (atom transfer radical polymerization and copper(0) mediated polymerization): From fundamentals to bioapplications. Chem. Rev. 2016, 116, 1803–1949. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom transfer radical polymerization: Billion times more active catalysts and new initiation systems. Macromol. Rapid Comm. 2019, 40, 1800616. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Wang, Y.; Zhong, M.J.; Krys, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Reversible-deactivation radical polymerization in the presence of metallic copper. A critical assessment of the SARA ATRP and SET-LRP mechanisms. Macromolecules 2013, 46, 8749–8772. [Google Scholar] [CrossRef]
- Chmielarz, P.; Król, P. PSt-b-PU-b-PSt copolymers using tetraphenylethane-urethane macroinitiator through SARA ATRP. Express Polym. Lett. 2016, 10, 302–310. [Google Scholar] [CrossRef]
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via interfacial and ion-pair catalysis: From ppm to ppb of residual copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of inositol-based star polymers through low ppm ATRP methods. Polym. Adv. Technol. 2017, 28, 1804–1812. [Google Scholar] [CrossRef]
- Lei, Q.; Peng, B.; Ma, K.K.Y.; Zhang, Z.; Wang, X.; Luo, J.; Tam, K.C. ARGET ATRP of triblock copolymers (PMMA-b-PEO-b-PMMA) and their microstructure in aqueous solution. ACS Omega 2018, 3, 15996–16004. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Malhotra, N.; Simakova, A.; Wang, Z.; Konkolewicz, D.; Matyjaszewski, K. Photoinduced atom transfer radical polymerization with ppm-level Cu catalyst by visible light in aqueous media. J. Am. Chem. Soc. 2015, 137, 15430–15433. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- Chmielarz, P.; Pacześniak, T.; Rydel-Ciszek, K.; Zaborniak, I.; Biedka, P.; Sobkowiak, A. Synthesis of naturally-derived macromolecules through simplified electrochemically mediated ATRP. Beilstein J. Org. Chem. 2017, 13, 2466–2472. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yağci, Y. Photoinduced metal-free atom transfer radical polymerizations: State-of-the-art, mechanistic aspects and applications. Polym. Chem. 2018, 9, 1757–1762. [Google Scholar] [CrossRef]
- Cohen-Karni, D.; Kovaliov, M.; Ramelot, T.; Konkolewicz, D.; Graner, S.; Averick, S. Grafting challenging monomers from proteins using aqueous ICAR ATRP under bio-relevant conditions. Polym. Chem. 2017, 8, 3992–3998. [Google Scholar] [CrossRef]
- Jones, G.R.; Whitfield, R.; Anastasaki, A.; Haddleton, D.M. Aqueous copper(II) photoinduced polymerization of acrylates: Low copper concentration and the importance of sodium halide salts. J. Am. Chem. Soc. 2016, 138, 7346–7352. [Google Scholar] [CrossRef]
- Zhang, Y.; Bradley, M.; Geng, J. Photo-controlled one-pot strategy for the synthesis of asymmetric three-arm star polymers. Polym. Chem. 2019, 10, 4769–4773. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, X.; Li, L.; Fantin, M.; Yan, J.; Wang, Z.; Wang, Z.; Xia, H.; Matyjaszewski, K. Enhancing mechanically induced ATRP by promoting interfacial electron transfer from piezoelectric nanoparticles to Cu catalysts. Macromolecules 2017, 50, 7940–7948. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, X.; Yan, J.; Dadashi-Silab, S.; Xie, G.; Zhang, J.; Wang, Z.; Xia, H.; Matyjaszewski, K. Temporal control in mechanically controlled atom transfer radical polymerization using low ppm of Cu catalyst. ACS Macro Lett. 2017, 6, 546–549. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Li, J.-J.; Ljubic, D.; Luo, Z.-H.; Zhu, S. Mechanically mediated atom transfer radical polymerization: Exploring its potential at high conversions. Macromolecules 2018, 51, 6911–6921. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Pan, X.; Fu, L.; Lathwal, S.; Olszewski, M.; Yan, J.; Enciso, A.E.; Wang, Z.; Xia, H.; et al. Ultrasonication-induced aqueous atom transfer radical polymerization. ACS Macro Lett. 2018, 7, 275–280. [Google Scholar] [CrossRef]
- McKenzie, T.G.; Karimi, F.; AshokKumar, M.; Qiao, G.G. Ultrasound and sonochemistry for radical polymerization: Sound synthesis. Chem. A Eur. J. 2019, 25, 5372–5388. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Mechanochemistry and sonochemistry: Concluding remarks. Faraday Discuss. 2014, 170, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Tang, J.; Jiang, J.; Gu, Z.; Dai, L.; Zhu, X. Controlled grafting modification of silica gel via RAFT polymerization under ultrasonic irradiation. Mater. Chem. Phys. 2009, 114, 402–406. [Google Scholar] [CrossRef]
- McKenzie, T.G.; Colombo, E.; Fu, Q.; Ashokkumar, M.; Qiao, G.G. Sono-RAFT polymerization in aqueous medium. Angew. Chem. Int. Ed. 2017, 56, 12302–12306. [Google Scholar] [CrossRef]
- Mohapatra, H.; Kleiman, M.; Esser-Kahn, A.P. Mechanically controlled radical polymerization initiated by ultrasound. Nat. Chem. 2016, 9, 135–139. [Google Scholar] [CrossRef]
- Mohapatra, H.; Ayarza, J.; Sanders, E.C.; Scheuermann, A.M.; Griffin, P.J.; Esser-Kahn, A.P. Ultrasound promoted step-growth polymerization and polymer crosslinking via copper catalyzed azide-alkyne “click” reaction. Angew. Chem. 2018, 130, 11378–11382. [Google Scholar] [CrossRef]
- Wang, Z.; Ayarza, J.; Esser-Kahn, A.P. Mechanically initiated bulk-scale free-radical polymerization. Angew. Chem. Int. Ed. 2019, 58, 12023–12026. [Google Scholar] [CrossRef]
- Collins, J.; McKenzie, T.G.; Nothling, M.D.; AshokKumar, M.; AshokKumar, M.; Qiao, G.G. High frequency sonoATRP of 2-hydroxyethyl acrylate in an aqueous medium. Polym. Chem. 2018, 9, 2562–2568. [Google Scholar] [CrossRef]
- Wang, Z.; Lorandi, F.; Fantin, M.; Wang, Z.; Yan, J.; Wang, Z.; Xia, H.; Matyjaszewski, K. Atom transfer radical polymerization enabled by sonochemically labile Cu-carbonate species. ACS Macro Lett. 2019, 8, 161–165. [Google Scholar] [CrossRef]
- Bian, C.; Zhou, Y.; Luo, Z. Mechanistic and kinetic investigation of Cu(II)-catalyzed controlled radical polymerization enabled by ultrasound irradiation. AIChE J. 2019, 16746. [Google Scholar] [CrossRef]
- Cintas, P.; Cravotto, G.; Barge, A.; Martina, K. Interplay between mechanochemistry and sonochemistry. In Polymer Mechanochemistry; Boulatov, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 239–284. [Google Scholar] [CrossRef]
- Suslick, K.S.; Flannigan, D.J. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef]
- Kang, J.-E.; Yoon, W.-H.; Ahn, C.-W.; Jeong, S.; Ryu, J.; Zhou, Y.; Choi, S.-Y.; Park, D.-S.; Hahn, B.-D.; Kim, J.-W.; et al. Ubiquitous magneto-mechano-electric generator. Energy Environ. Sci. 2015, 8, 2402–2408. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A force of synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Gautschi, G. Piezoelectric Sensors. In Piezoelectric Sensorics: Force Strain Pressure Acceleration and Acoustic Emission Sensors Materials and Amplifiers; Springer-Verlag Berlin Heidelberg: Berlin/Heidelberg, Germany, 2002; pp. 73–91. [Google Scholar] [CrossRef]
- Khan, A.; Abas, Z.; Kim, H.S.; Oh, I.-K. Piezoelectric thin films: An integrated review of transducers and energy harvesting. Smart Mater. Struct. 2016, 25, 53002. [Google Scholar] [CrossRef]
- Hong, K.-S.; Xu, H.; Konishi, H.; Li, X. Direct water splitting through vibrating piezoelectric microfibers in water. J. Phys. Chem. Lett. 2010, 1, 997–1002. [Google Scholar] [CrossRef]
- Novak, N.; Pirc, R.; Kutnjak, Z. Impact of critical point on piezoelectric and electrocaloric response in barium titanate. Phys. Rev. B 2013, 87, 104102. [Google Scholar] [CrossRef]
- Thanki, A.; Goyal, R. Study on effect of cubic- and tetragonal phased BaTiO3 on the electrical and thermal properties of polymeric nanocomposites. Mater. Chem. Phys. 2016, 183, 447–456. [Google Scholar] [CrossRef]
- Petrovsky, V.; Petrovsky, T.; Kamlapurkar, S.; Dogan, F. Dielectric constant of barium titanate powders near curie temperature. J. Am. Ceram. Soc. 2008, 91, 3590–3592. [Google Scholar] [CrossRef]
- Mohod, A.V.; Gogate, P.R. Ultrasonic degradation of polymers: Effect of operating parameters and intensification using additives for carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA). Ultrason. Sonochem. 2011, 18, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, G.; Xin, J.; Deng, Y.; Miller, J.T.; Kropf, A.J.; Bunel, E.E.; Qi, X.; Lan, Y.; Lee, J.-F.; et al. Homolytic cleavage of the O–Cu(II) bond: XAFS and EPR spectroscopy evidence for one electron reduction of Cu(II) to Cu(I). Chem. Commun. 2016, 52, 6914–6917. [Google Scholar] [CrossRef] [PubMed]
- Fantin, M.; Chmielarz, P.; Wang, Y.; Lorandi, F.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Harnessing the interaction between surfactant and hydrophilic catalyst to control eATRP in miniemulsion. Macromolecules 2017, 50, 3726–3732. [Google Scholar] [CrossRef] [PubMed]


| Initiator | Monomer | Catalyst Complex | Piezoelectrics | Solvent | Temp. (°C) | Ultrasound Frequencies (kHz) | Catalyst Concentration | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| ppm (Catalyst/Monomer) | ppm (by Weight) | ||||||||
| EBiB | BA | CuII(OTf)2/Me6TREN a | BaTiO3 | DMF | 15–25 °C | 20 | 10,042 | 14,475 | [48] |
| EBiB | MA | CuIIBr2/TPMA | BaTiO3 | DMSO | R.T. | 40 | 150 | 172 | [41] |
| EBiB | MA | CuIIBr2/TPMA | ZnO | DMSO | R.T. | 40 | 75–150 | 174 b | [40] |
| EBiB | EA | CuIIBr2/TPMA | ZnO | DMSO | R.T. | 40 | 150 | – | [40] |
| EBiB | tBA | CuIIBr2/TPMA | ZnO | DMSO | R.T. | 40 | 150 | – | [40] |
| EBiB | BA | CuIIBr2/TPMA | ZnO | DMSO | R.T. | 40 | 150 | – | [40] |
| EBiB | MMA | CuIIBr2/TPMA | ZnO | DMSO | R.T. | 40 | 150 | – | [40] |
| PMA-Br | MA | CuIIBr2/TPMA | ZnO | DMSO | R.T. | 40 | 97 | 95 | [40] |
| EBiB | MA | CuIIBr2/Me6TREN | BaTiO3 | DMSO | 50 °C | 40 | 100–200 | 172 | [42] |
| EBiB | MSEA | CuIIBr2/Me6TREN | BaTiO3 | DMSO | 50 °C | 40 | 150–1500 | – | [42] |
| EBiB/MBiB/MBP/MBAc/mPEG bromoisobutyrate 5000 | BA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 10,000 | 9730 c | [50] |
| EBiB | tBA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 10,000 | 9730 | [50] |
| EBiB | MA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 20,000 | 14,609 | [50] |
| EBiB | MMA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 20,000 | 14,029 | [50] |
| EBiB | EA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 10,000 | 10,933 | [50] |
| EBiB | EHMA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 10,000 | 7630 | [50] |
| PMMA-Br | BA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 150 | – | [50] |
| EBiB | MA, EGDMA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 20,000 | 14,609 | [50] |
| EBiB | HEA | FeIIICl3·6H2O/TDA-1 | ZnO | DMF/DMSO/NMP | 20–30 °C | 40 | 10,000 | 10,212 | [50] |
| Initiator | Monomer | Catalyst Complex | Solvent | Temp. (°C) | Ultrasound Frequencies (kHz) | Catalyst Concentration | Ref. | |
|---|---|---|---|---|---|---|---|---|
| ppm (Catalyst/Monomer) | ppm (by Weight) | |||||||
| PEG2k-BPA | OEOMA500 | CuIIBr2/TPMA a | H2O | R.T. | 40 | 40–4000 | 39 b | [43] |
| PEG2k-BPA | OEOMA500 | CuIIBr2/TPMA a | H2O/EtOH | R.T. | 40 | 120–2000 | 40 b | [43] |
| PEG2k-BPA | HEA | CuIIBr2/TPMA a | H2O | R.T. | 40 | 200–500 | 312 c | [43] |
| P(OEOMA500)-Br | OEOMA500 | CuIIBr2/TPMA a | H2O | R.T. | 40 | 3800 | 58 | [43] |
| DNA-iBBr | OEOMA500 | CuIIBr2/TPMA d | H2O | R.T. | 40 | 1000 | – | [43] |
| HEBriB | HEA | CuIIBr2/Me6TREN | H2O | R.T. | 490 | 50–1000 | 8–153 | [51] |
| EBiB | MA | CuIIBr2/TPMA e | DMSO | R.T. | 40 | 150 | 178 f | [52] |
| EBiB | MA | CuIIBr2/Me6TREN | DMSO | 50 °C | 40 | 150 | 177 | [53] |
| EBiB | MA | CuIIBr2/TPMA | DMSO | 50 °C | 40 | 150 | – | [53] |
| EBiB | MA | CuIIBr2/PMDETA | DMSO | 50 °C | 40 | 150 | – | [53] |
| EBiB | MMA | CuIIBr2/Me6TREN | DMSO | 50 °C | 40 | 150 | – | [53] |
| EBiB | St | CuIIBr2/Me6TREN | DMSO | 50 °C | 40 | 150 | – | [53] |
| EBIB | BA | CuIIBr2/TPMA g | H2O h | 65 °C | 40 | 717 | 192–218 | [12] |
| EBiB | MMA | CuIIBr2/TPMA | H2O | 65 °C | 40 | 717 | 290 | [12] |
| EBPA | MMA | CuIIBr2/TPMA | H2O | 65 °C | 40 | 717 | 154 | [12] |
| PBA-Br | BA | CuIIBr2/TPMA | H2O | 65 °C | 40 | 717 | 150 | [12] |
| EBiB | tBA | CuIIBr2/TPMA | H2O | 65 °C | 40 | 717 | 151 | [12] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborniak, I.; Chmielarz, P. Ultrasound-Mediated Atom Transfer Radical Polymerization (ATRP). Materials 2019, 12, 3600. https://doi.org/10.3390/ma12213600
Zaborniak I, Chmielarz P. Ultrasound-Mediated Atom Transfer Radical Polymerization (ATRP). Materials. 2019; 12(21):3600. https://doi.org/10.3390/ma12213600
Chicago/Turabian StyleZaborniak, Izabela, and Paweł Chmielarz. 2019. "Ultrasound-Mediated Atom Transfer Radical Polymerization (ATRP)" Materials 12, no. 21: 3600. https://doi.org/10.3390/ma12213600
APA StyleZaborniak, I., & Chmielarz, P. (2019). Ultrasound-Mediated Atom Transfer Radical Polymerization (ATRP). Materials, 12(21), 3600. https://doi.org/10.3390/ma12213600






