Abstract
Single phase β-Si3N4 with microcrystals was synthesized via carbothermal reduction-nitridation (CRN) of quartz and carbon coke powder as starting materials. The effects of reaction parameters, i.e., heating temperature, holding time, C/SiO2 ratio, Fe2O3 additive and β-Si3N4 seeds on the phase transformation and morphology of products were investigated and discussed. Rather than receiving a mixture of both α- and β- phases of Si3N4 in the products, we synthesized powders of β-Si3N4 single polymorph in this work. The mechanism for the CRN synthesis of β-Si3N4 from quartz and the formation mechanism of Fe3Si droplets were discussed. We also firstly reported the formation of Fe3Si Archimedean solids from a CRN process where Fe2O3 was introduced as additive. Comparing to the gear-like short columnar morphology observed in samples without β-Si3N4 seeding, the addition of β-Si3N4 seeds led to an elongated morphology of final products and much finer widths. In addition, the β-Si3N4 microcrystals exhibited a violet‒blue spectral emission range, which could be highly valuable for their future potential optoelectronic applications.
1. Introduction
Silicon nitride (Si3N4) is an important high temperature structural material because of its excellent properties, including high strength, high decomposition temperature (1900 °C), good resistance to oxidation, thermal shock, corrosive environments, which have been investigated extensively over the past three decades [1,2,3,4].
The most prevalent methods for preparing Si3N4 powders include direct nitridation method, carbothermal reduction-nitridation (CRN) method and thermal decomposition method [5,6,7]. By CRN method, Si3N4 powders or columnar grains with excellent size distribution and physical properties could be synthesized and used as thermal conductive fillers or commercial applications for manufacturing engineering devices [8,9]. For example, Karakus et al. [9]. synthesized α-Si3N4 powders by CRN of synthetic silica and activated charcoal at 1470 °C, and then used the obtained α-Si3N4 powders as the raw materials to prepare the Si3N4 ceramic by a pressureless sintering method. Comparing the results with commercial Si3N4 powders, the resultant Si3N4 powders by CRN method indicated a similar or ever better density and β-phase conversion by the pressureless sintering. Yin et al. prepared ZrN–Si3N4 composite powders from natural zircon and quartz via CRN reaction at temperatures below 1600 °C [10]. Similarly, Arik prepared Si3N4 powders by CRN from diatomite with C/SiO2 molar ratio 4 at 1400 °C for 16 h [11]. Thus, it is feasible to prepare Si3N4 with quartz and carbon black. On the other hand, CRN method takes advantage from low-cost starting materials [8,9]. The high cost of raw materials is a primary limitation for large scale production of Si3N4 powders. Through the CRN method, it is possible to synthesize Si3N4 powders from low-cost quartz with abundant reserves in the world. It was reported that the same problem for the massive production of SiC powders was overcome by this way, and the product was much finer for achieving excellent flexural strength [12].
Grain size and shape of Si3N4 powders can influence some properties of Si3N4-based products such as varying electrical and optical properties and mechanical properties [13,14]. Apart from Si3N4 seeds, Fe and its oxides have been used as additives to change the morphology of a product or to promote nitriding process [15,16,17,18]. However, to the best of our knowledge, the crystal microstructure/morphology of Fe-containing compounds formed in the nitridation process while Fe or iron oxide being used as additive was rarely reported.
In this study, quartz and carbon coke powders were selected as raw materials to prepare Si3N4 powders via CRN method. The influence of temperature, holding times, C/SiO2 molar ratio, additive amount of Fe2O3 and β-Si3N4 seeds were studied on the phase transformation and morphology of products. Faceted Fe3Si Archimedean solids, a novel morphology of the iron silicide, were observed firstly which have the potential application in spintronics devices [19]. The formation mechanism of products from the nitridation reaction was discussed and photoluminescence (PL) properties of samples were also detected.
2. Experimental
2.1. Materials
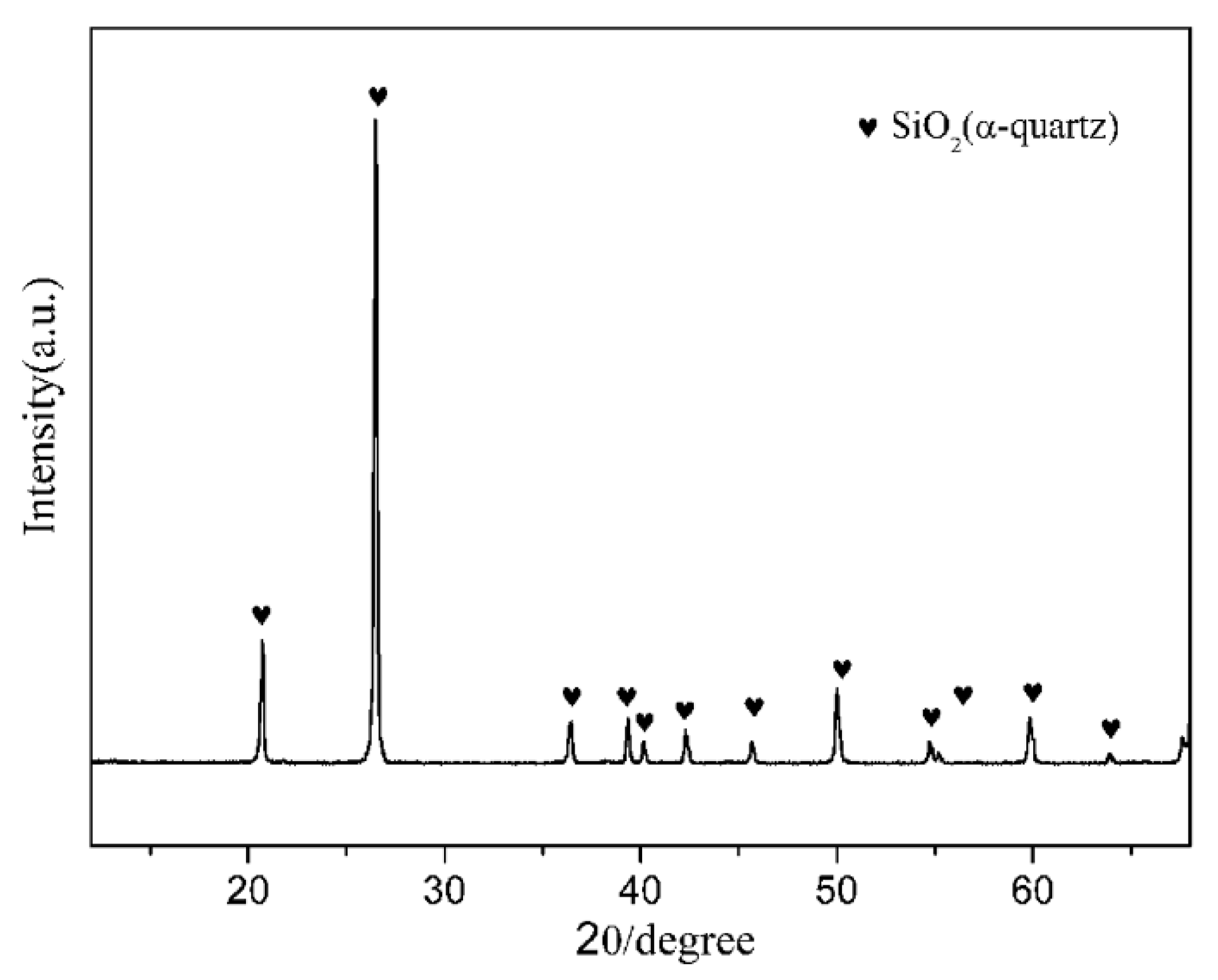
Natural quartz powders (granularity ≤ 400 mesh, chemical composition (wt.%): SiO2: 97.8, Al2O3: 0.63, Fe2O3: 0.13, CaO: 0.08, K2O: 0.05, others: 1.31), and coke powders (granularity ≤ 200 mesh, carbon content = 88%) were used as the main starting raw materials. The crystalline phase of the natural quartz powders was hexagonal α-quartz (Figure 1). Fe2O3 (A.R. grade, Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China) and Si3N4 (A.R. grade, ~800 nm, Shanghai Pantian powder material Co., Ltd., Shanghai, China) were used as additives. The starting compositions of all the samples are listed in Table 1.
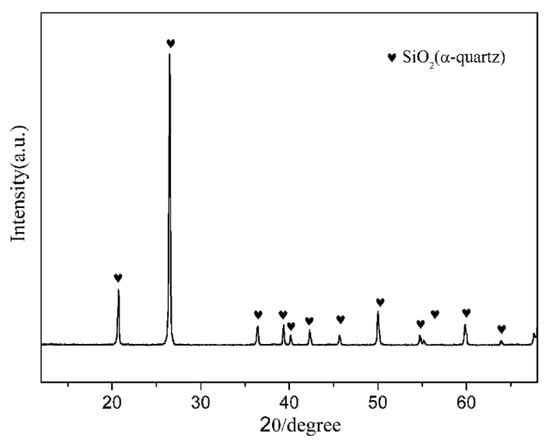
Figure 1.
X-ray diffraction (XRD) pattern of natural quartz.

Table 1.
Compositional design of the samples (wt.%).
2.2. Methods
The starting materials were ball-milled together for 2 h. Then, 2 g of the mixed powders were die-pressed under 20 MPa into a specimen of 10 mm in diameter. The specimens were placed in a graphite crucible and heated in flow nitrogen (purity 99.999%) in the reaction chamber of a tube furnace at temperatures in the range of 1450–1600 °C. A two stage heating schedule was used, i.e., heating up from ambient temperature to 1000 °C at 10 °C·min−1, then raising to the final temperature at 5 °C·min−1 and held for 3 h. The final temperatures were set as 1470 °C, 1500 °C, 1530 °C, 1550 °C, 1570 °C, and 1600 °C, respectively. In addition, in order to study the effect of holding time to the CRNed product, S1 sample was synthesized at 1600 °C and held for 1 h, 2 h, and 3 h, respectively. The fired samples were furnace-cooled to room temperature, and then reheated in air at 700 °C for 2 h to remove the residual carbon.
2.3. Characterization
The crystalline phases of the synthesized products were identified by X-ray diffraction (XRD; D8 Advance diffractometer, Bruker, Rheinstetten, Germany), using Cu Kα1 radiation (λ = 1.5406 Å) with a step of 0.02° (2θ) and a scanning rate of 4° min−1. The microstructures and morphologies of the products were observed by scanning electron microscopy (SEM; JEM-6460LV microscope, JEOL, Tokyo, Japan) and high-resolution transmission electron microscopy (HRTEM, JEM2010, JEOL, Tokyo, Japan). The energy dispersive X-ray spectroscopies (EDS) linked with the SEM and the HRTEM were employed to assist the micro-area chemical analysis of the products. FT-IR spectra were collected at room temperature using a FT-IR Spectrometer (FT/IR-4000 JASCO, Tokyo, Japan) equipped with a Michelson 28° interferometer with corner-cube mirrors, covering a range between 250,000 and 5 cm−1. Photoluminescence emission (PL) spectra were measured by F-7000 fluorescence spectrophotometer (Eppendorf, Shanghai, China) with a photomultiplier tube functioning at 700 V, and a 150 W Xe lamp as the excitation source.
3. Results and Discussion
3.1. Influence of Synthetic Schedule on the Phase Composition and Morphology of Products
3.1.1. Influence of Temperature on the Phase Composition and Morphology of Products
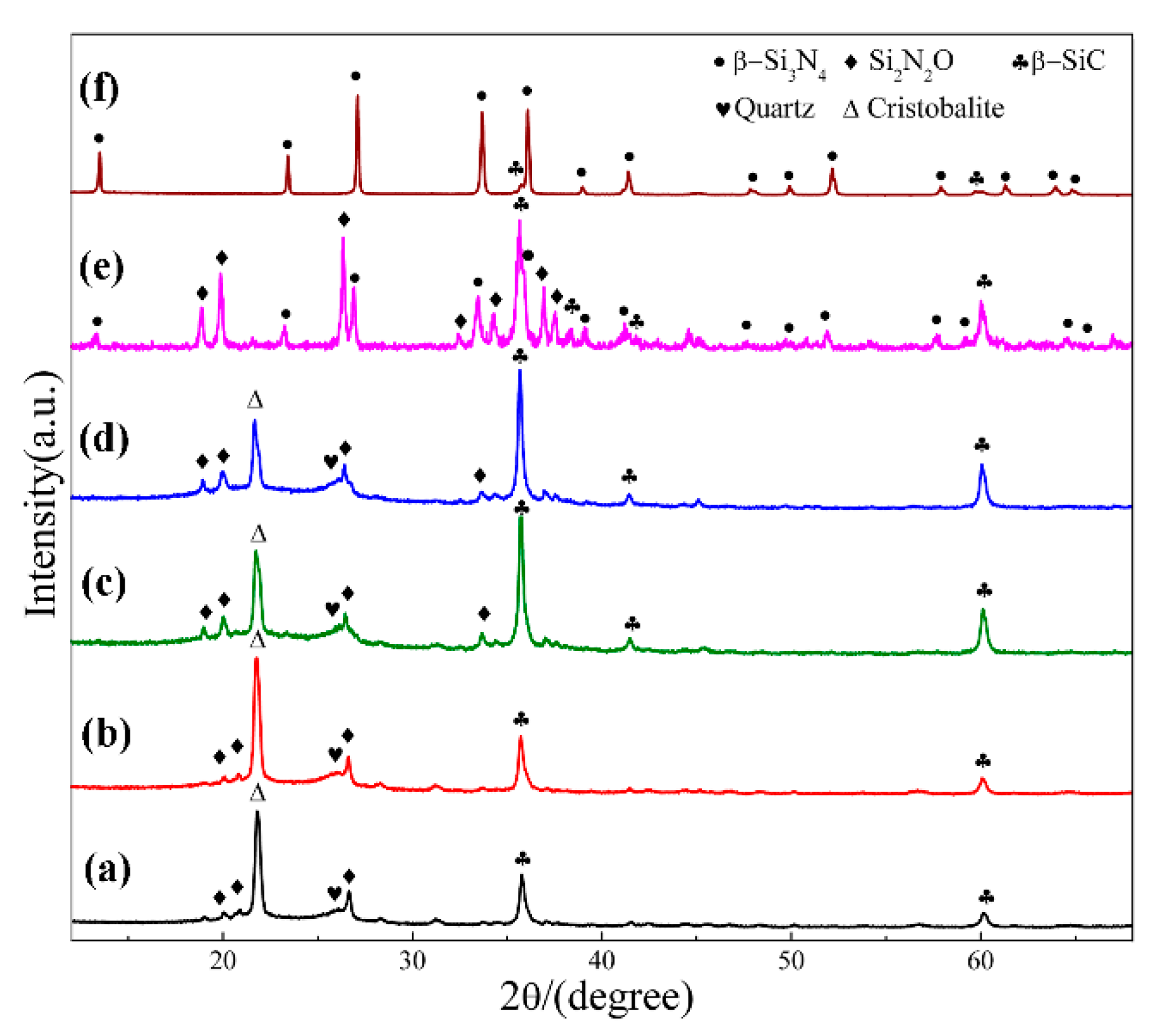
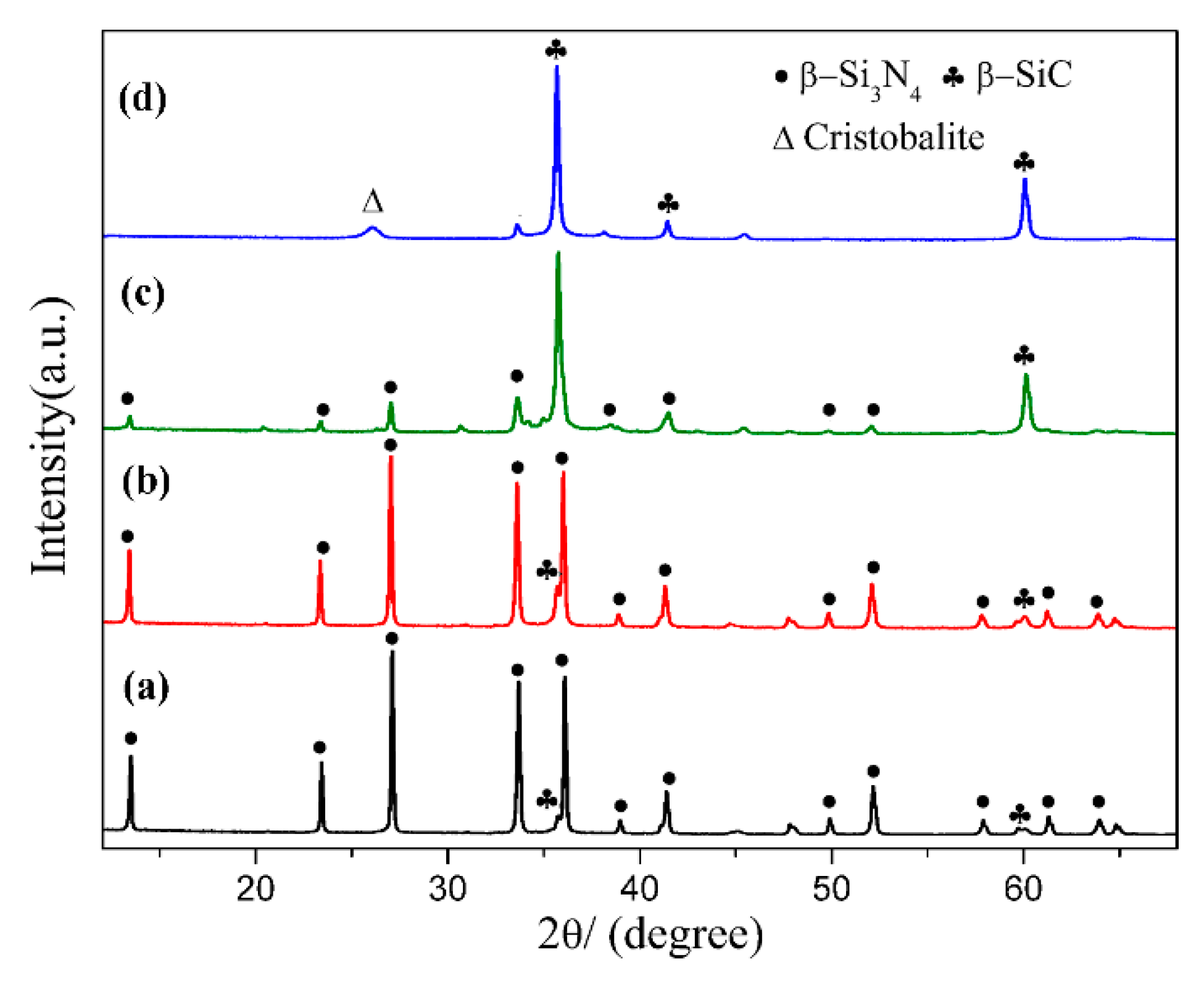
Figure 2 shows XRD patterns of sample S1 nitrided at different temperatures for 3 h. As can be seen, cristobalite was the dominant phase at 1470 °C, which was phase transition from quartz. In additional, some SiC, Si2N2O and residual quartz were present in the products (Figure 2a). With nitriding temperature being gradually elevated from 1470 °C to 1550 °C, intensity of β-SiC and Si2N2O diffraction peaks increased and that of cristobalite decreased (Figure 2a–d). After further increasing the temperature to 1570 °C, β-Si3N4 had just emerged while its diffraction intensity was much lower than that of β-SiC and Si2N2O; in the meantime, the peaks of cristobalite disappeared, and no other silica phases were detected (Figure 2e). By applying a higher temperature of 1600 °C, β-Si3N4 was formed in a relatively high purity (Figure 2f). The intensity of the secondary phase β-SiC was very weak. The β-Si3N4 synthesized at 1570 & 1600 °C (Figure 2e,f) in S1 crystallized as a hexagonal structure and their lattice parameters and cell volumes are listed in Table 2. They were increased from 1570 to 1600 °C. The weight fraction of each phase in S1 at 1570 & 1600 °C is shown Table 3, the weight fraction was remarkable increased from 32.30 to 91.52 with the increased of temperature to 1600 °C. Upon the above observation, it is clear that in this work, the effect of temperature on the phase formation from quartz by CRN is very significant. For the holding period of 3 h, the temperature 1570 °C was critical for the transformation of cristobalite into Si3N4, whereas it cannot be formed at a lightly lower temperature of 1550 °C. Moreover, a temperature of 1600 °C, 30 °C higher than 1570 °C contributed to the formation of high-purity Si3N4 powders.
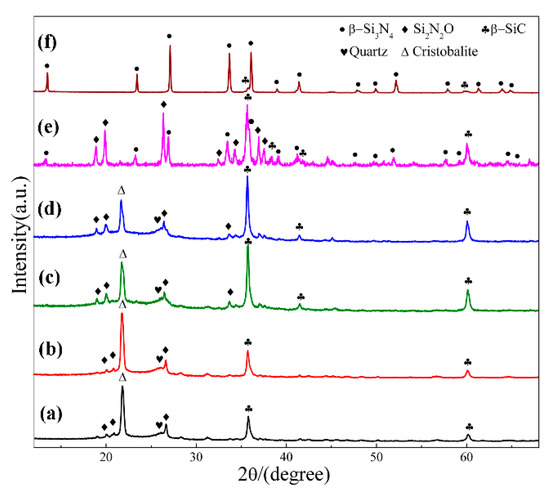
Figure 2.
XRD patterns of sample S1 nitrided at different temperatures for 3 h: (a) 1470 °C; (b) 1500 °C; (c) 1530 °C; (d) 1550 °C; (e) 1570 °C; (f) 1600 °C.

Table 2.
Lattice parameters and cell volume of β-Si3N4 in S1 & S5.

Table 3.
Weight fraction of each phase in S1 and S5 at 1570 and 1600 °C (wt. %).
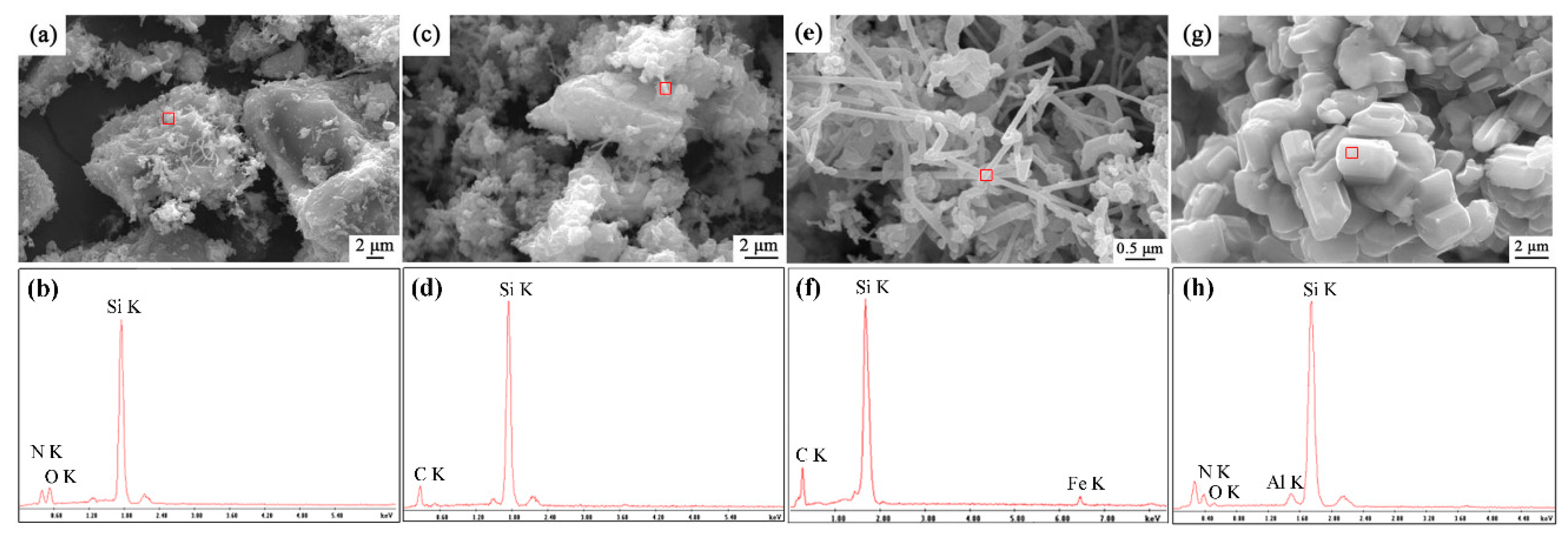
Morphological variation of sample S1 after being nitrided at different nitriding temperatures was investigated by SEM observation, and the elemental composition was performed by EDS analysis (Figure 3). There were many independent SiO2 particles with size of 20 μm, covered by some Si2N2O and SiC fibers at temperatures of 1500–1550 °C, confirmed by EDS (Figure 3a–d). Some Fe element in the fibers was detected in the EDS, which should be from original quartz raw material (Figure 3e,f). In the sample synthesized at 1600 °C, short columnar grains with diameters of about 2 μm were dominant throughout the sample, which is the typical morphology of β-phase Si3N4. The trace amounts of Al and O was detected in the grains; this might come from impurity of original quartz or ball milling.
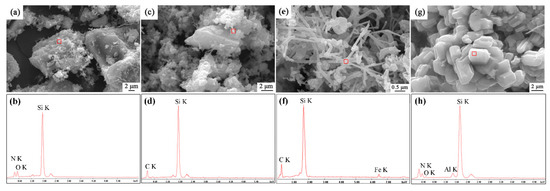
Figure 3.
SEM images and X-ray spectroscopies (EDS) patterns of sample S1 nitrided at different temperatures for 3 h: (a,b)1500 °C, (c,d) 1550 °C, (e,f) 1570 °C (g,h) 1600 °C.
3.1.2. Influence of Holding Time on the Phase Composition of Products
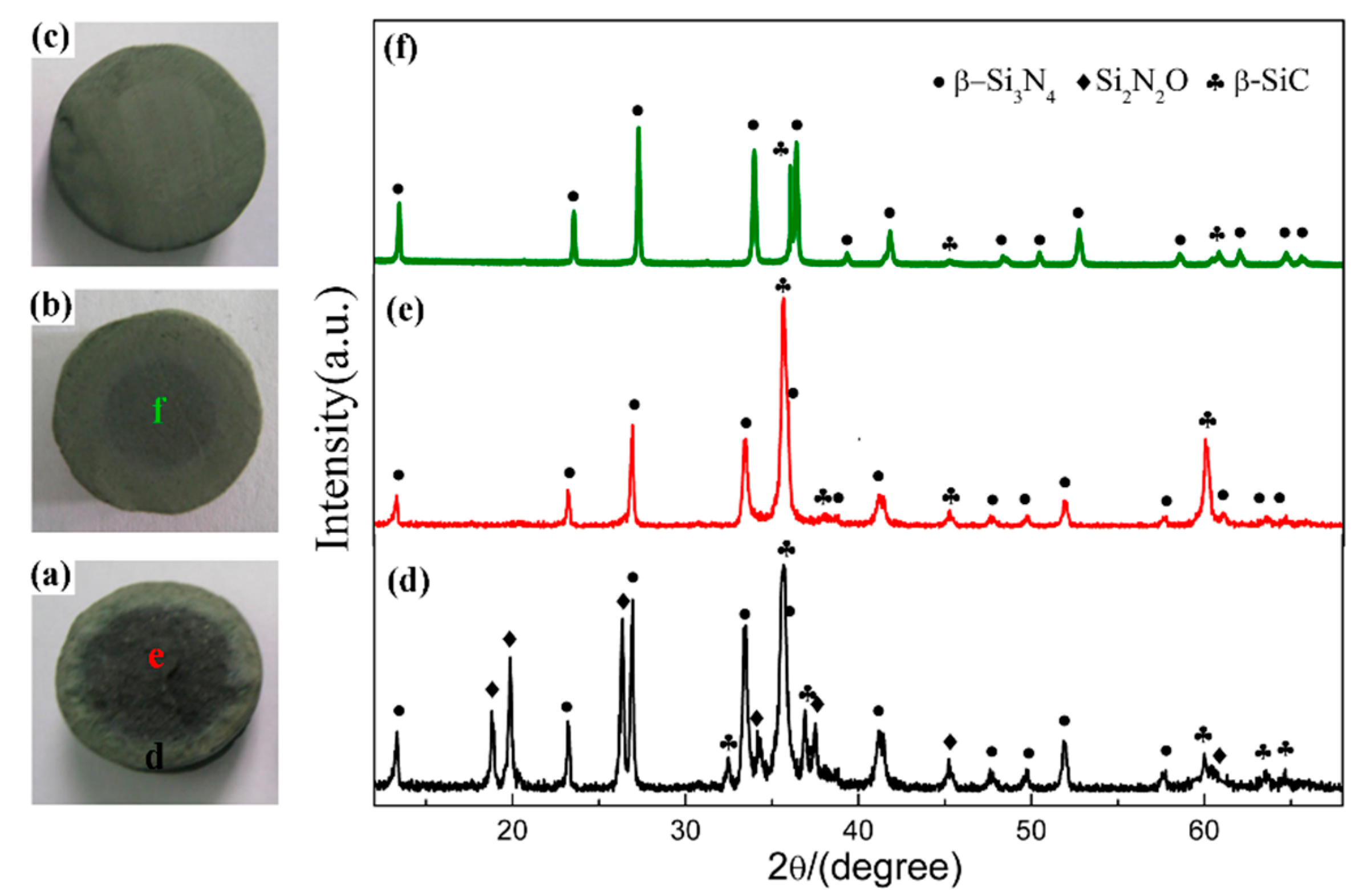
In order to elucidate the effect of holding time, the samples synthesized at 1600 °C for different holding times were compared. Figure 4a–c present the digital photos of three samples, which clearly demonstrated the degree of nitridation from the change in the nitridized area. As seen from the digital photos, 2 h was insufficient for CRN of quartz at 1600 °C. There were two different zones on the cross section of products (Figure 4a,b). The interior portion of pellets had a darker color while the exterior part was greenish gray. Interior regions of product narrowed down when holding time was 2 h and disappeared when 3 h. A longer holding time of 3 h enabled the complete nitridation of the entire pellet with diameter of <10 mm. The XRD patterns of them are depicted in Figure 4d,f. It is revealed that phase compositions were Si3N4, SiC, and Si2N2O for exterior region as well as SiC and Si3N4 for interior region with holding time of 1 h (Figure 4d,e). The diffraction peaks of SiC in interior region were evidently weakened with increasing the holding time to 2 h (Figure 4f) even almost disappeared after 3 h (Figure 2f). Since the permeation of N2 in samples was from outside to inside, the external N2 of samples was more abundant than that of internal. Increasing holding time allowed complete penetration of N2 into the inner pellets, leading to the fully nitridation of the entire pellets.
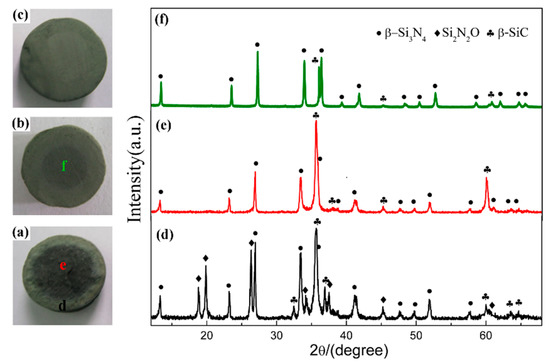
Figure 4.
Digital photos of sample S1 nitrided at 1600 °C with different holding time: (a) 1 h, (b) 2 h, (c) 3 h; and XRD patterns of sample S1 in different regions: (d) exterior region and (e) interior region of pellet reacted for 1 h, (f) interior region of the pellet reacted for 2 h.
3.1.3. The CRN Mechanism of Quartz
Based on the results described above and literatures reported previously [20,21], it is generally accepted that the CRN reaction of quartz would happen through several steps. The reduction of quartz into SiO, via the pathways as shown by Equation (1) (∆G is the Gibbs free energy of reaction, and T is the temperature), is the first but a critical step, which enables the further reduction process.
SiO2(s) + C(s) → SiO(g) + CO(g), ΔG = 665.578–0.33T kJ/mol
The as-reduced SiO (g) is then nitrided into silicon nitride phases by carbon through the Equation (2) [22,23].
3SiO(g) + 3C(s) + 2N2(g) → Si3N4(s) + 3CO(g), ΔG = −830.44 + 0.35T kJ/mol
At a temperature of 1550 °C or lower, intermediate phases Si2N2O and SiC were preferably formed through Equations (3) and (4), respectively [22,24]. It suggests that under the subcritical temperatures (1470 to 1550 °C), the reaction process was dominated by the reductive atmosphere/conditions while the nitridation processes were less thermodynamically preferred.
2SiO2(s) + 3C(s) + N2(g) → Si2N2O(s) + 3CO(g), ΔG = 572.54–0.32T kJ/mol
SiO(g) + 2C(s) → SiC(s) + CO(g), ΔG = −78.60 + 0.01 T kJ/mol
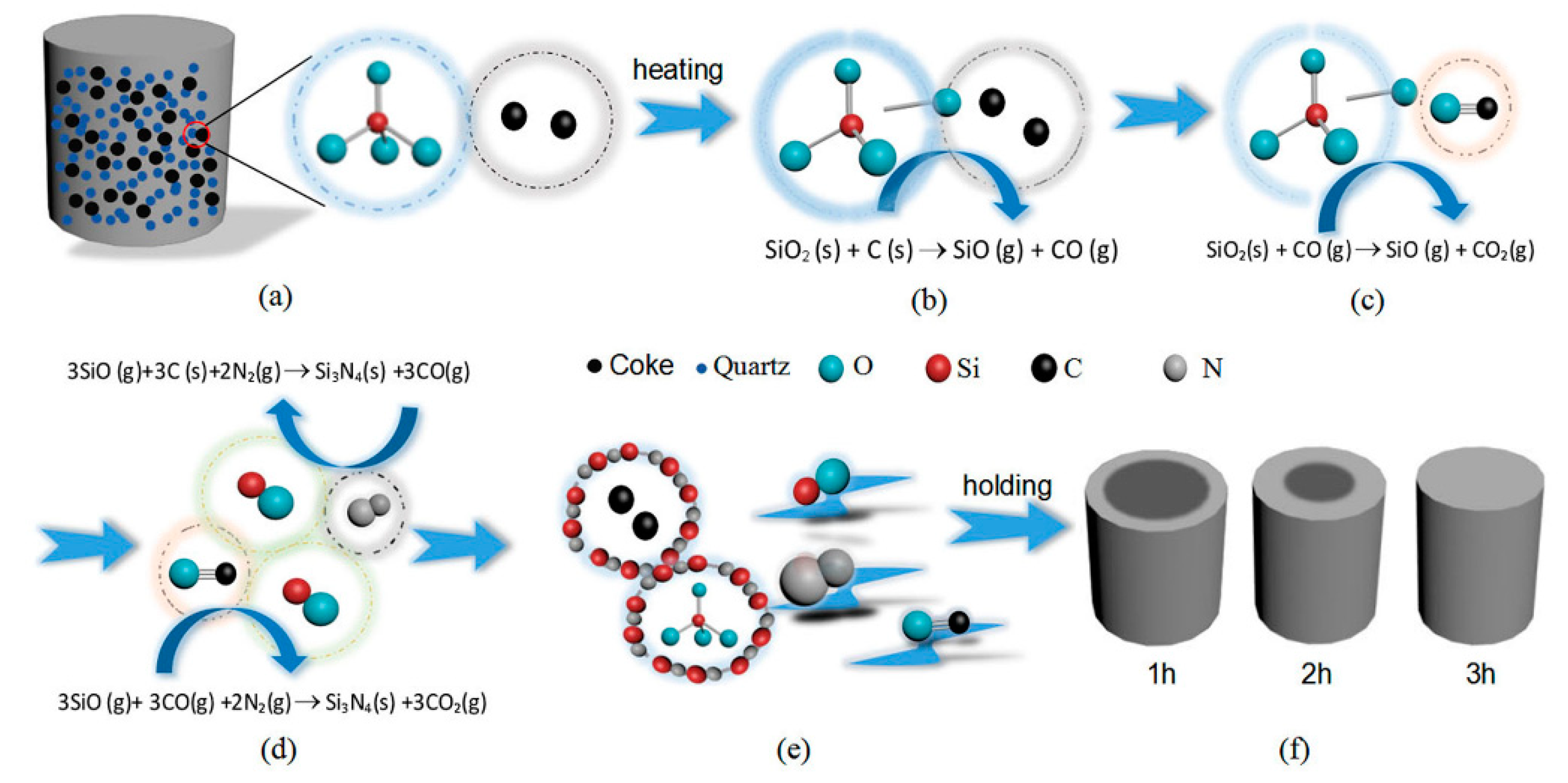
Figure 5 is a schematic diagram demonstrating the synthesis of β-Si3N4 by CRN of quartz in two major steps. As the first step, illustrated by Figure 5a–c, SiO(g) forms via Equation (1) beyond certain temperature. Equation (1) requires direct contact of carbon and SiO2. Then in the next step, as shown in Figure 5d, Si3N4 nucleates from Equation (2), and grows up on the surface of carbon and SiO2, which is associated with the diffusion rate of SiO. The reaction process is progressive from exterior to interior of the sample pellets as shown in Figure 5f.
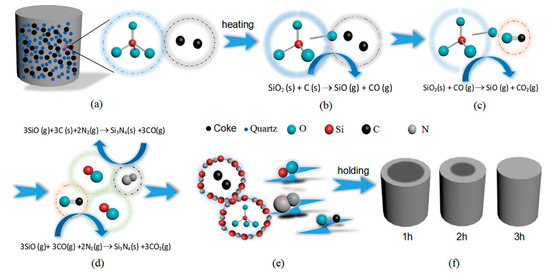
Figure 5.
Reaction schematic diagram for the formation of β-Si3N4 by carbothermal reduction-nitridation (CRN) of quartz: (a) the green body of specimen, (b–e) nitridation processes of raw materials, (f) specimens obtained under different holding times.
3.2. Influences of Starting Composition and Additive on Phase Composition and Morphology of Products
In order to elucidate the key factors for the synthesis of β-phase Si3N4 from quartz CRN synthesis, we investigated the effects of C/SiO2 molar ratio, Fe2O3 additive content, and the introduction of β-Si3N4 seeds on the products.
3.2.1. Influence of C/SiO2 Molar Ratio on Phase Composition
According to Equation (5), the theoretical C/SiO2 molar ratio of Si3N4 is 2 [25]. Thus, samples S1–S4 with different C/SiO2 molar ratios (2, 2.2, 3, and 4) were designed and subjected to CRN synthesis at 1600 °C for 3 h.
3SiO2(s) + 6C(s) + 2N2(g) → Si3N4(s) + 6CO(g), ΔG = 1166.30–0.64T kJ/mol
Figure 6 shows the XRD patterns of samples S1~S4 nitrided at 1600 °C for 3 h. As seen in the figure, β-Si3N4 was the main phase when C/SiO2 was 2 (sample S1) and 2.2 (Sample S2), with trace amount of β-SiC. Peaks of β-Si3N4 decreased significantly when a higher C/SiO2 molar ratio of 3 was adopted in sample S3, and almost disappeared when the C/SiO2 ratio further increased to 4 (sample S4). At the meantime, β-cristobalite was detected which was derived from the phase transformation of residual quartz at high temperature. The results indicate that the theoretical/stoichiometric carbon content as per Equation (5) was optimal for preparing single-phase β-Si3N4 from quartz by CRN. Increased amount of β-SiC and other by-products formed in the product of samples with a higher C/SiO2 molar ratio.
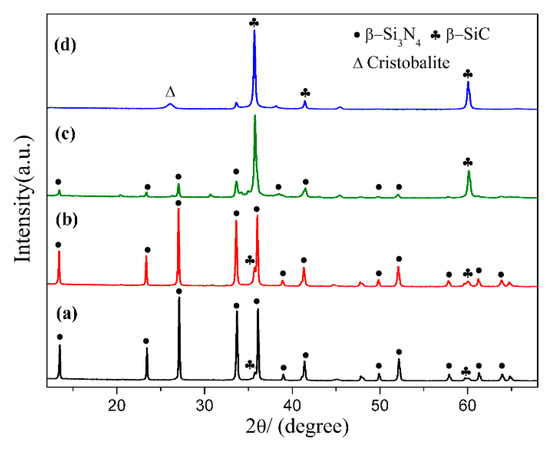
Figure 6.
XRD patterns of sample S1~S4 nitrided at 1600 °C for 3 h: (a) S1, C/SiO2 = 2; (b) S2, C/SiO2 = 2.2; (c) S3, C/SiO2 = 3; (d) S4, C/SiO2 = 4.
3.2.2. Influence of Fe2O3 Additive on Phase Composition and Morphology of Products
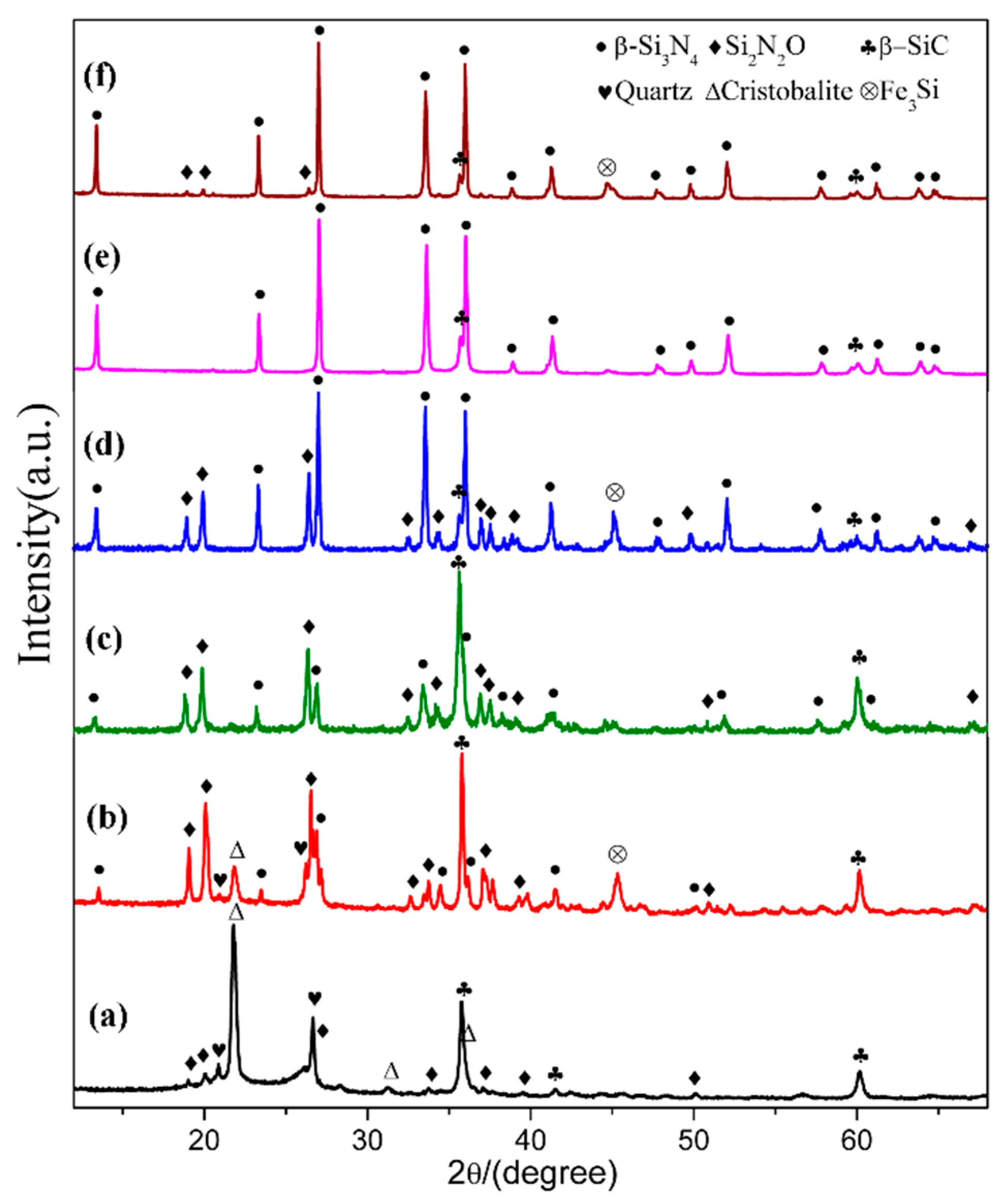
In order to see the effect of additive, we designed a composition (sample S5) which added extra 4 wt.% Fe2O3 into the starting mixture. The XRD patterns of sample S1 (Fe2O3-free) and S5 (with extra 4 wt.% Fe2O3) are plotted in Figure 7, where the phase assemblages in the samples at different temperatures are compared. At 1470 °C, β-cristobalite, quartz and β-SiC were the main phases in sample S1. In contrast, much weaker peaks of cristobalite showed in sample S5 whereas Si2N2O and β-Si3N4 formed with a considerable amount. At 1570 °C, β-Si3N4, β-SiC and Si2N2O were present in both sample S1 and sample S5, while there was significantly more β-Si3N4 and much less β-SiC formed in sample S5 (comparing to S1). At 1600 °C, both samples had β-Si3N4 as dominant phase and trace amount of β-SiC, and the trace amount of Si2N2O were found in S5. Additionally, peaks appeared at 45.5° in sample S5 at all temperatures can be assigned to Fe3Si. The parameters and cell volumes of β-Si3N4 synthesized in S1 and S5 at 1600 °C (Figure 7e,f) are listed in Table 2. Table 3 is the weight fraction of each phase in S1 and S5 at 1570 and 1600 °C. These results illustrate that Fe2O3 could enhance the carbothermal reduction process of quartz and nitridation transformation to β-Si3N4.
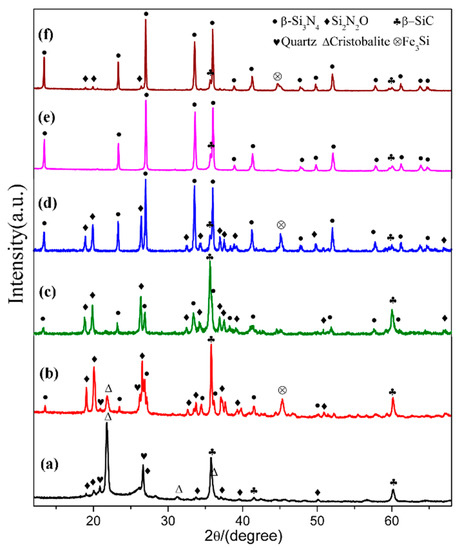
Figure 7.
XRD patterns of sample S1(a,c,e), and sample S5 (b,d,f) at 1470, 1570, and 1600 °C, respectively.
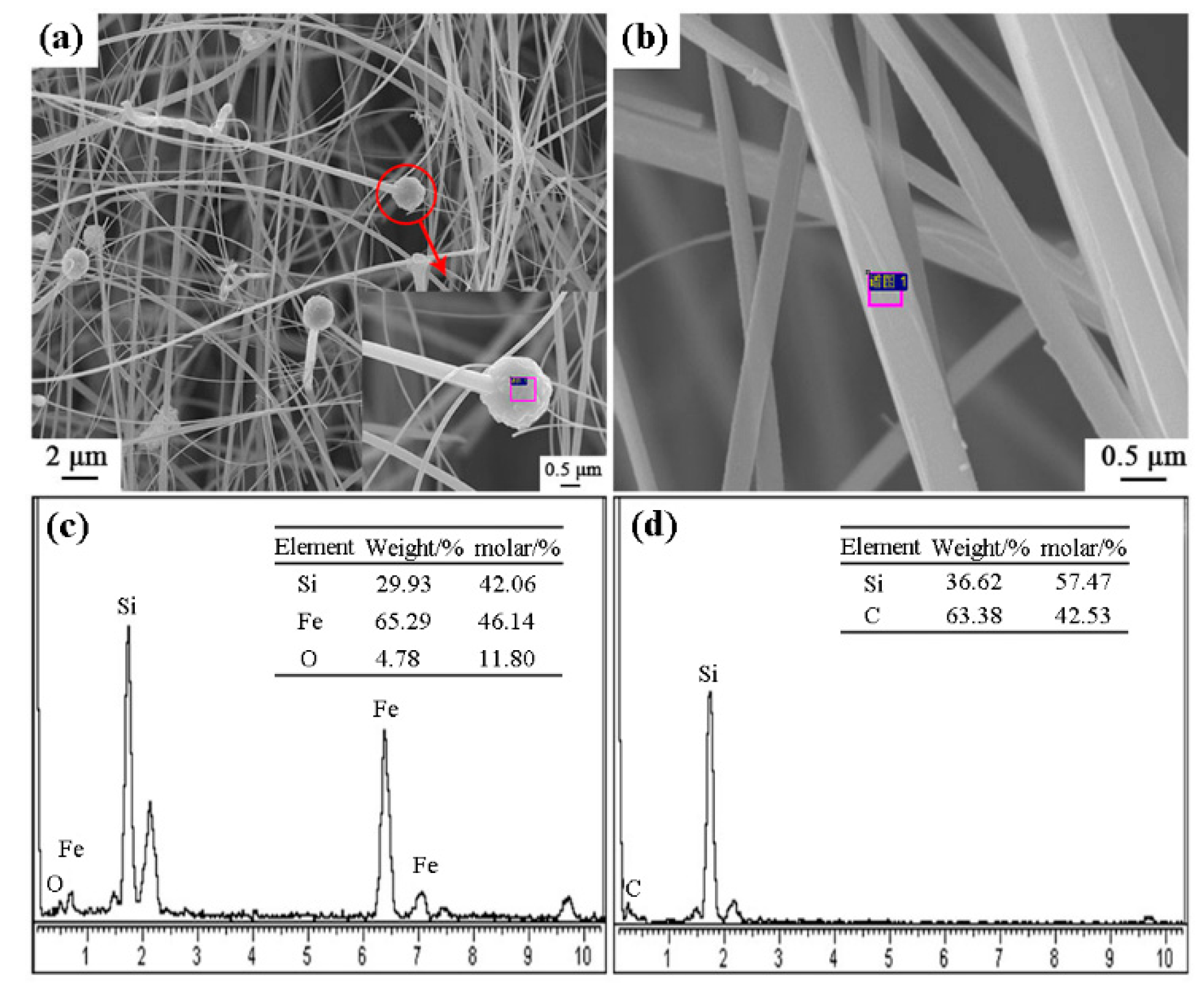
Figure 8 shows the SEM images and EDS results of sample S5 nitrided at 1600 °C. As seen in Figure 8a–d, the columnar grains had gear-like morphologies and dominate in the products, which could be assigned to β-Si3N4 upon XRD and EDS results. A trace amount of Fe element was detected (Figure 8f), which was originated from the additive added in this sample. Furthermore, a white-colored product layer was covered on the surface of sample S5 nitrided at 1600 °C for 3 h. SEM observation shows that it was composed of fibers with widths of about 0.3–0.5 μm (Figure 9a,b). EDS result reveals that the fibers were SiC phase (Figure 9d). At the tip of each fiber, there was a spherical particle containing Si-Fe-O elements (Figure 9a,c). It is therefore suggested that the SiC fibers formed through a Vapor - Liquid - Solid (VLS) mechanism [26].
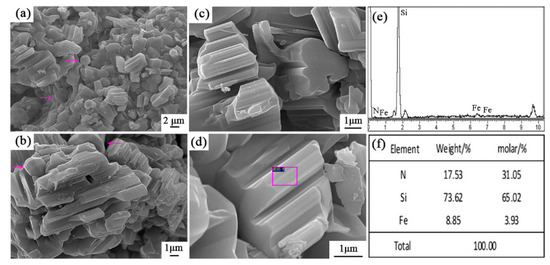
Figure 8.
SEM images and EDS patterns of β-Si3N4 in sample S5 nitrided at 1600 °C: (a–d) SEM images with different magnification, (e,f) EDS results of the selected area in (d).
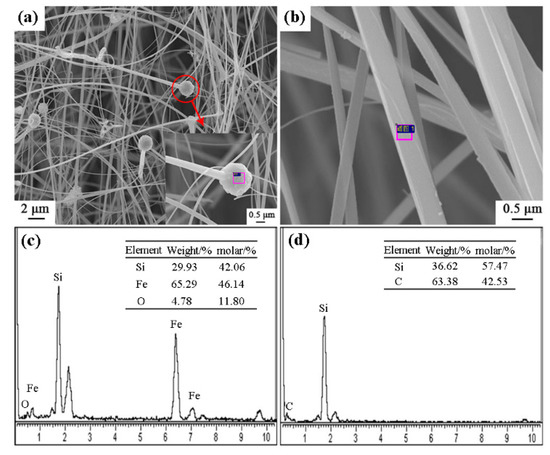
Figure 9.
SEM images and EDS patterns of SiC fibers on the surface of sample S5 nitrided at 1600 °C: (a,b) SEM images of white-colored product layer, (c,d) EDS results of the selected area in (a,b).
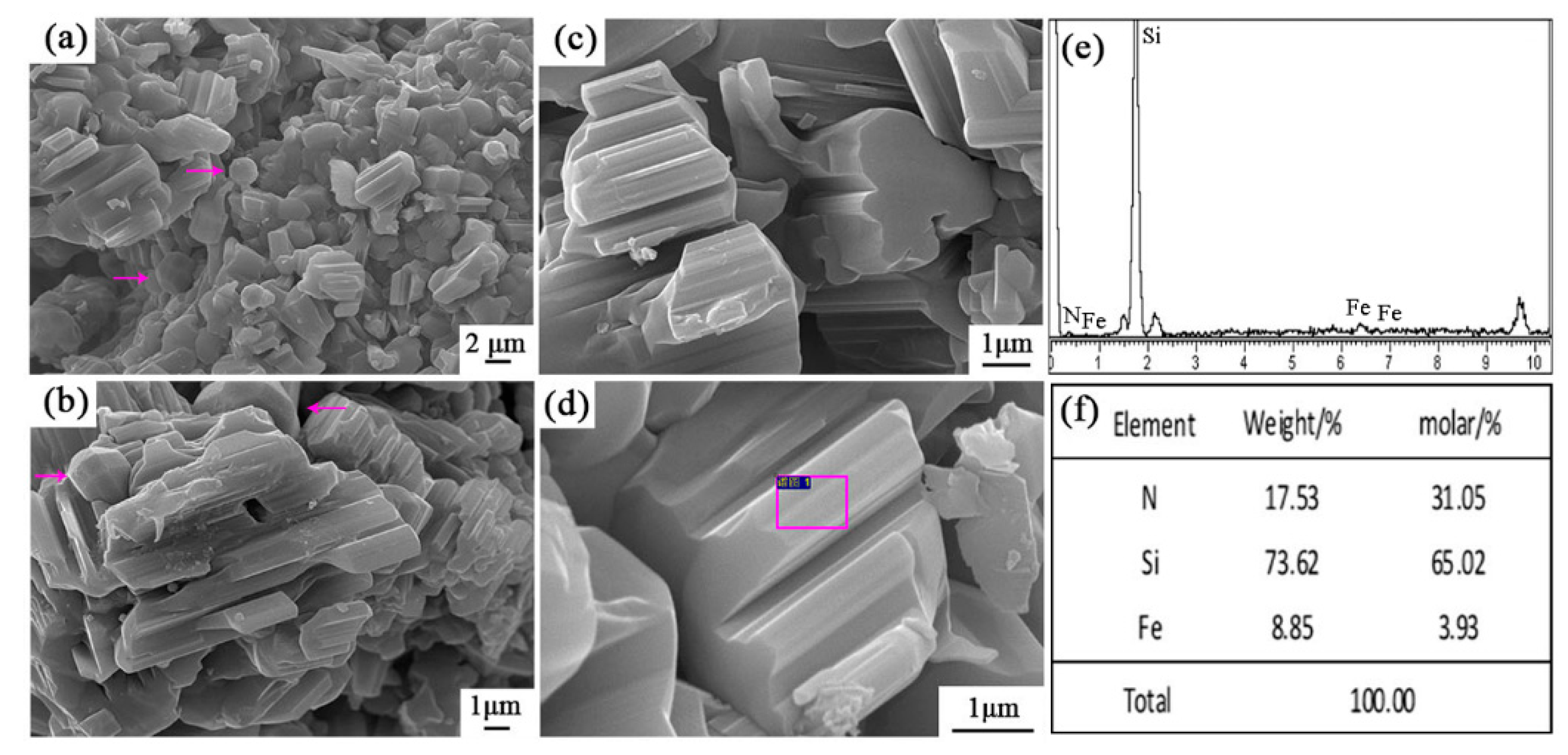
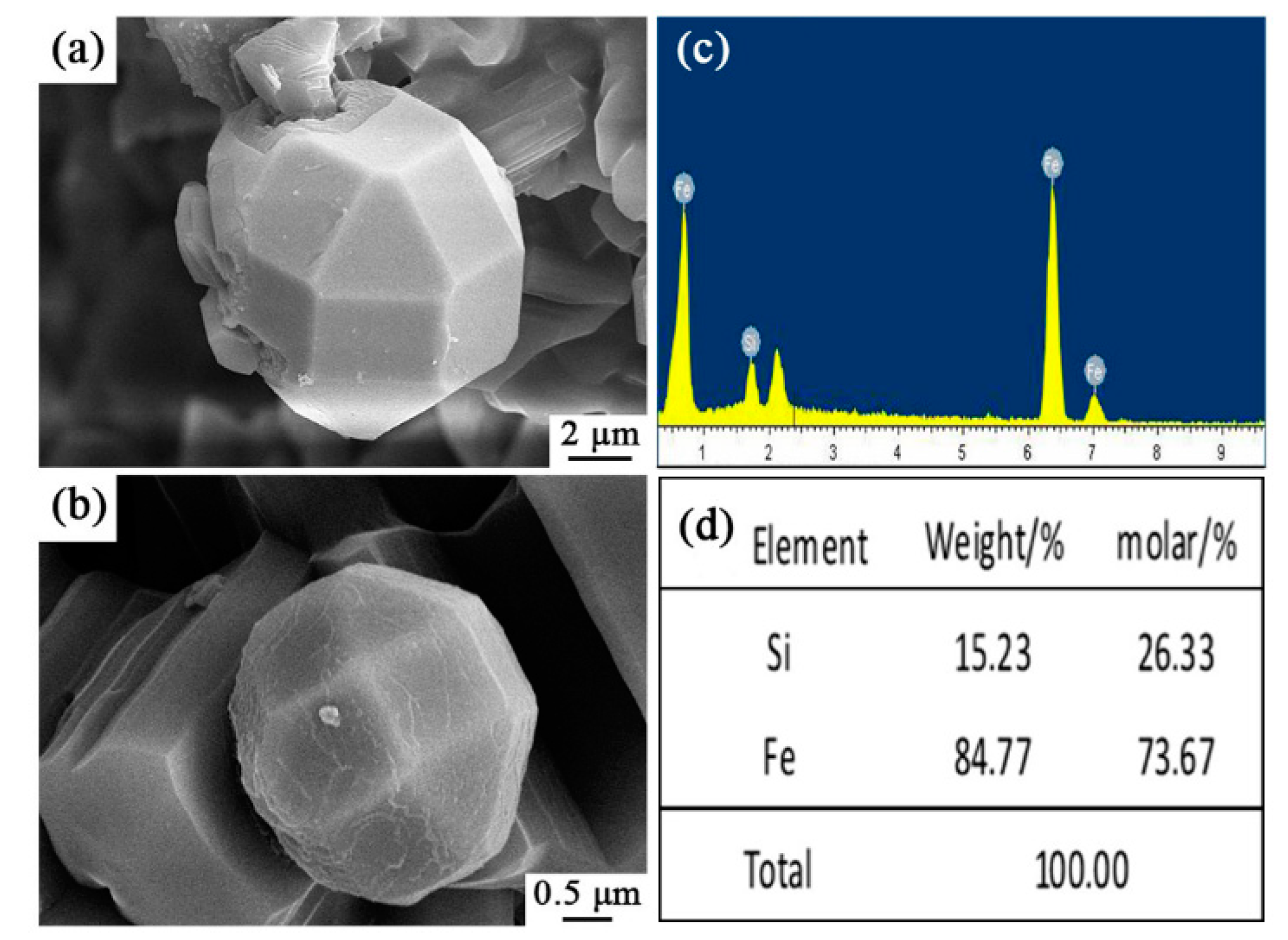
Very interestingly, some spherical particles were observed in sample S5 (arrowed in Figure 8a,b), which were further characterized by SEM and EDS (Figure 10). It is wonderful to see that they were Archimedean solids with sizes of several microns. EDS results illustrate that they contained only Fe and Si elements with the molar ratio of Fe/Si ~2.79. This result clearly reveals that the faceted crystal was Fe3Si as detected in XRD data. To the best of our knowledge, such a novel morphology of Fe3Si was never reported in the literature.
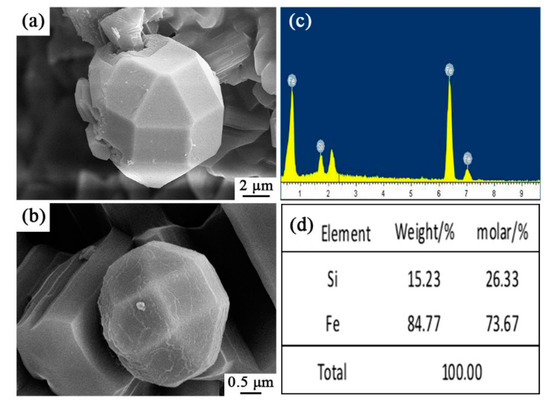
Figure 10.
SEM images and EDS patterns of Si-Fe alloy of sample S5 nitrided at 1600 °C: (a,b) SEM images of spherical particles in sample S5, (c,d) EDS results of the selected area in (a,b).
Based on the above results, it can be concluded that Fe2O3 had a remarkable catalytic effect on the CRN of quartz at a lower temperature than 1600 °C. Under the reductive environment at high temperatures, carbon or CO can reduce Fe2O3 to Fe by Equations (6) or (7). It is known that the melting point of pure iron is 1538 °C. Therefore, Fe generally attaches to a support material in the reaction system by forming a liquid phase. In the present study, we speculate that Fe-Si-O liquid formed first (Equation (8), Figure 9). The Fe-containing liquid could dissolve SiO2 and C, then enhance their reaction to form SiO(g) [27] (Equation (9)), consequently promoting the formation of Si2N2O, SiC and even β-Si3N4 at lower temperature (Equations (3) and (4), Figure 7). Fe-containing liquid could significantly enhance the nitridation reaction, and the precipitation of β-Si3N4 has been known to be favored by the presence of Fe-containing liquid [28] (Equation (10)). Fe-Si-O liquids were further reduced to be Fe-Si containing liquid (Equation (11)). The Fe-rich liquid then crystallized as Fe3Si crystals while cooling (Equation (12)). The Fe-containing liquid phases, as a catalytic phase, promoted the formation of Si3N4, which is clearly illustrated by the phase assemblages in the samples at the low temperatures (Figure 5). Nevertheless, it is yet unclear how the unique Archimedean solids formed, which is beyond the objectives of this work and needs further investigation in near future.
Fe2O3(s) + 3C(s) → 2Fe(l) + 3CO(g), ΔG = 474.35–0.51T kJ/mol
Fe2O3(s) + 3CO(g) → 2Fe(l)+3CO2(g), ΔG= −6.32–0.01T kJ/mol
Fe (l) + SiO2(s) + C(s) → Fe-Si-O(l) + CO(g)
Fe-Si-O(l)+ C(s)/CO(g) → Fe-Si(l) + CO2(g)
Fe-Si(l) → Fe3Si(s)
3.2.3. Influence of β-Si3N4 Seeds on Morphology of Products
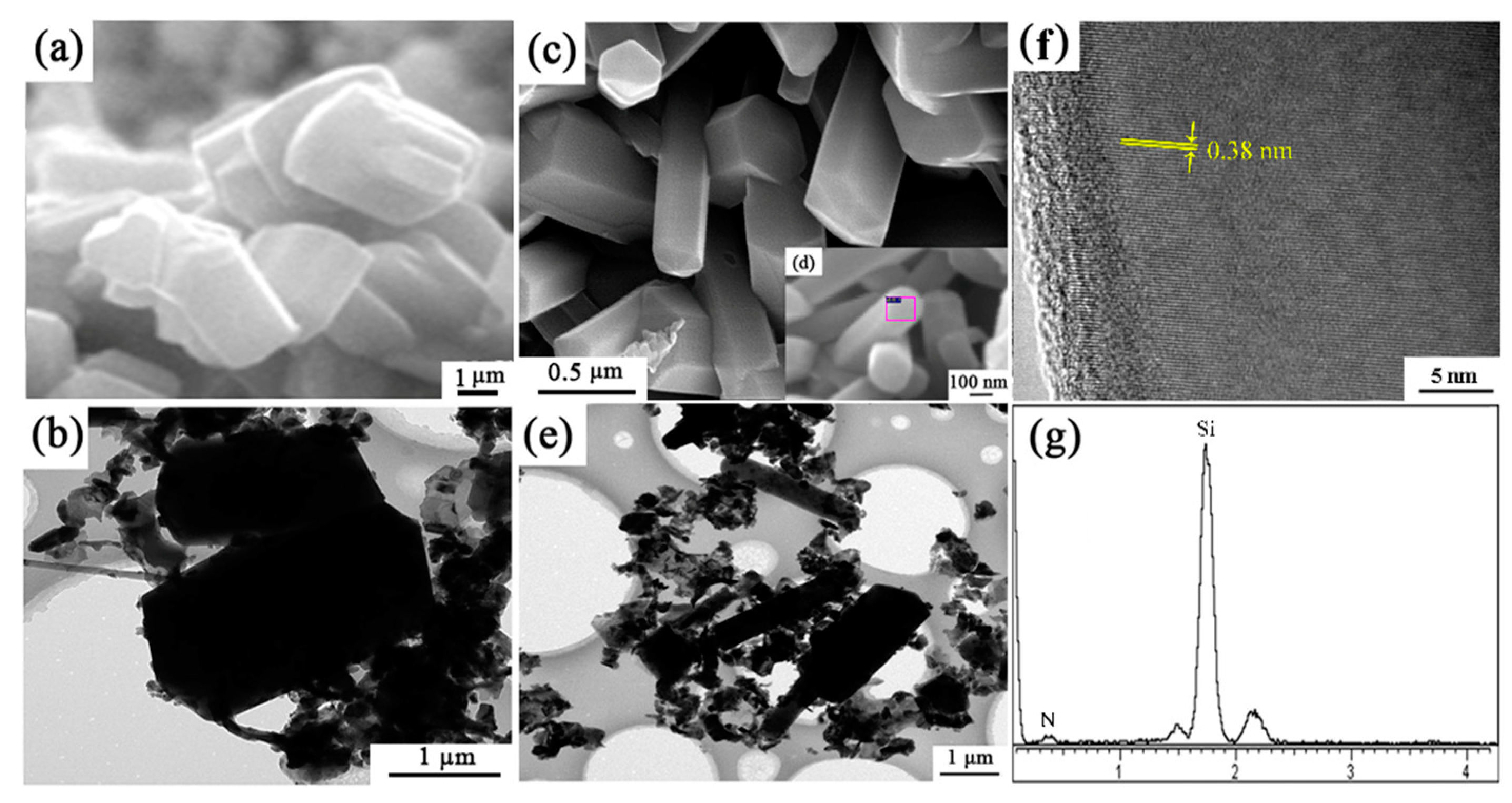
The microstructure of sample S1 (no seeds) and S6 (with extra 2 wt.% Si3N4 seeds) synthesized at 1600 °C for 3 h were shown in Figure 11. In sample S1, the morphology of β-Si3N4 grains are short columnar, having grain sizes of around 3 μm. While in sample S6, grains were elongated and the gear-like morphology was rarely observed. From Figure 11c, the widths of the elongated grains are much smaller (0.2–0.5 µm) than those of short columnar grains in S1 (2–3 µm). HRTEM lattice image of the Si3N4 elongated grain indicates that the lattice fringe had no obvious distortion (Figure 11f,g). The measured lattice fringe spacing of 0.38 nm matched well with the (110) plane of β-Si3N4 (Figure 11f).
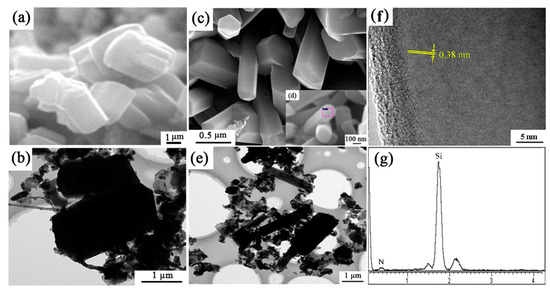
Figure 11.
SEM, TEM/HRTEM images and EDS patterns of (a,b) sample S1 and (c–g) S6 nitrided at 1600 °C for 3 h.
It is reported that introducing seeds into the starting materials could affect the microstructure of synthesized products and improve the physical properties of final ceramics [29,30]. For example, in the synthesis of Sialon powders, the addition of Sialon seeds would increase the growth competition among the grains, leading to an elongated columnar morphology, thus improving flexural strength and fracture toughness [24,31]. Herein, a significantly increased aspect ratio of Si3N4 grains was achieved by adding seeds into the starting mixtures. The addition of seeds could trigger heterogeneous nucleation [32]:
where θ is contact angle between new Si3N4 nucleus and Si3N4 seeds, f(θ) is the influence function of θ, and ∆G* is Gibbs free energy of homogeneous nucleation. Therefore, when the contact angle was 0–180°, nucleation power could be reduced. Moreover, while crystal structure of seeds was the same as that of Si3N4, θ would be very small, which would reduce ∆G and make more nucleus formed at the early stage of nitriding process. Thus, the growth competition between those nuclei would made the smaller and long columnization of Si3N4 grains.
3.3. The Possible Mechanisms of β-Si3N4 Single Polymorph Formation
Usually, α- and β- Si3N4 polymorphs are present in the products from carbothermal reduction nitridation of SiO2, and some related researches reported in the literature are summarized in Table 4. The phase composition ratio of α/β-Si3N4 in the CRN products was highly affected by additives (or impurities). For example, Sun et al. [8] reported that the yield of β-Si3N4 increased from 42.8% to 81.8% when improving CaF2 additive contents (Table 4 (1 and 2)). Wang et al. [22] revealed that the introduction of CaF2-Y2O3 additives significantly promoted the conversion of α-Si3N4 to β-Si3N4 (Tabele 4 (3 and 4)). Furthermore, the CRN temperature was another key factor. According to the literatures [33,34,35], the increasing CRN temperature was beneficial for the transformation of α-Si3N4 to β-Si3N4 (Table 4 (6 and 7), (8 and 9), (10 and 11)), and when it was higher than 1500 °C, β-Si3N4 emerged as the major phase, even single phase at 1700 °C (Table 4 (5)).

Table 4.
Comparison of reaction conditions and products by carbothermal reduction nitridation of SiO2 reported in the literature and the present study.
Herein, we successfully produced β-Si3N4 as the single polymorph (no α- phase co-exists), either with or without Fe2O3 as an additive. It is widely known that the α-Si3N4 derives from SiO vapor while β-Si3N4 from liquid phase during CNR process, and also there exist phase transformation between α-Si3N4 and β-Si3N4 [34,35]. As SiO vapor always forms during the reduction process, α-Si3N4 can form (temporarily at least) during the CRN process. In our study, the β-Si3N4 as the single polymorph formed regardless of adding Fe2O3 or not. Thus, we speculate the following three possible reasons that lead to the formation of single β-Si3N4 polymorph. Firstly, the silicon source used in this work was quartz mineral rather than commercial/pure amorphous or crystalline SiO2 powders in other literatures reported. The high temperature stability of quartz made nitriding formation process of Si3N4 took place at 1570 °C in catalyst-free sample. Secondly, according to the results and discussion above, α-Si3N4 was metastable at high temperature. α-phase might be generated in this work, but it was converted to β-phase at such high temperature. Last, the impurities in quartz raw material including 0.13 wt.% Fe2O3, or the formed eutectic liquid phases, contributed to the formation of pure β-phase in products, by either promoting the α-to-β phase transformation or direct formation of β-phase. The gear-like morphology of β-Si3N4 should be formed via coalescence in the iron-containing liquid phase.
3.4. PL Spectrum of Synthesized β-Si3N4 Samples
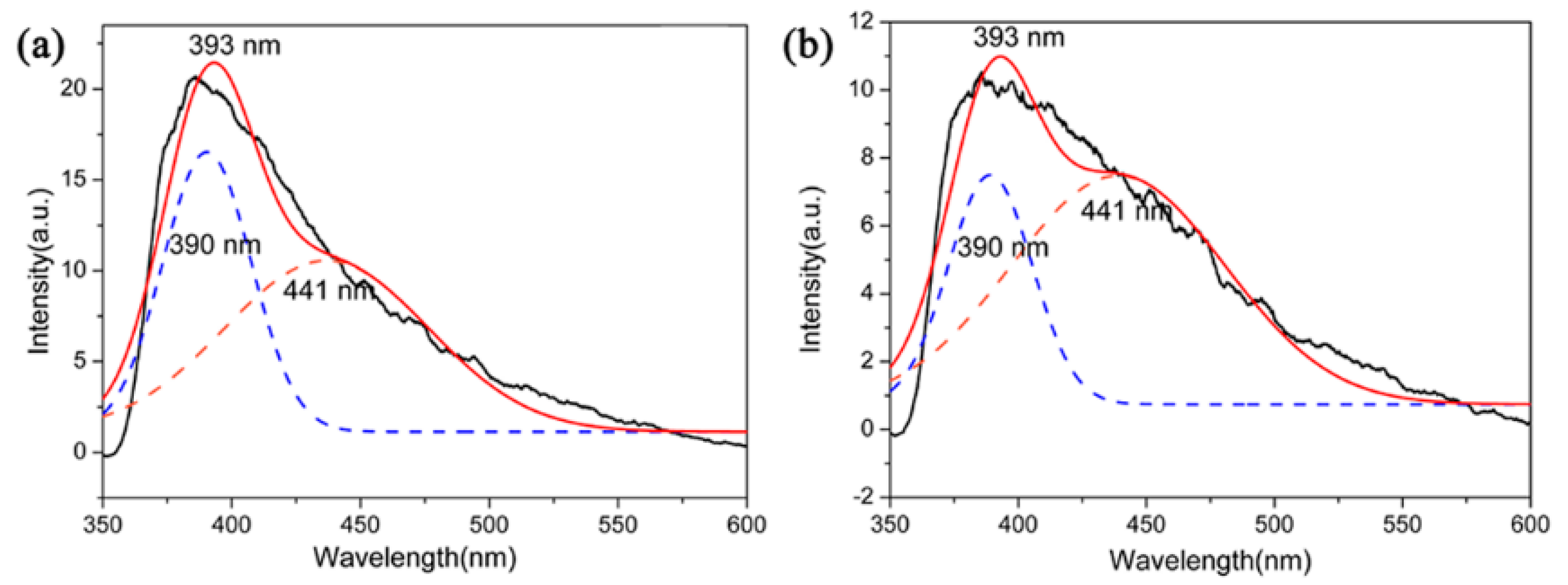
The functional applications of Si3N4 had attracted increasing interest, for instance, the optical properties of Si3N4 films or nanostructures have been studied [36,37]. However, Si3N4 microcrystals had not attracted much attention in terms of their optical properties. In this context, PL emission properties (excitation at 330 nm) of β-Si3N4 microcrystals with different morphologies were tested by using samples S5 and S6 nitrided at 1600 °C. The PL spectra are showed in Figure 12. The spectrum of these two samples showed a board purple emission band with the maximum at 393 nm and it could be further fitted into two Guassion peaks 390 nm (3.17 eV) of purple spectral region and 441 nm (2.81 eV) of blue spectral region. It has been reported that as an indirect gap semiconductor, the luminescence of Si3N4 is attributed to a defect luminescence mechanism. The PL process of Si3N4 could be caused by the existing defect of it which including ≡Si–Si≡, =N−, ≡Si0 and ≡Si−, and those were corresponding to four types of defects of Si–Si bond, N–N bond, and Si–N dangling bonds [38,39]. The experimental data may shed light on potential application of Si3N4 microcrystals in optical devices.
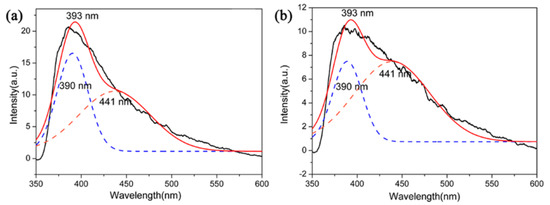
Figure 12.
PL spectrum of (a) S5 and (b) S6 nitrided at 1600 °C.
4. Conclusions
A carbothermal reduction-nitridation (CRN) method was used for the preparation of β-Si3N4 powders from natural quartz and coke powders. β-Si3N4 powders with relatively high purity were obtained at 1600 °C for 3 h, with trace amount of β-SiC formed as a by-product. The results indicated that β-Si3N4 powders were obtained in the products, accompanied by the appearance of β-SiC and Si2N2O during the CRN process. The temperature, holding time, C/SiO2 malor ratio, Fe2O3 addition, and β-Si3N4 seeds played important roles for the synthesis β-Si3N4 powders. The optimal nitriding temperature was 1600 °C and holding time was 3 h. A stoichiometric molar ratio of C/SiO2 of 2 was preferred for preparing β-Si3N4 with better phase purity. Fe2O3 under the reducing environment formed Fe-containing liquid phases, which promoted the reaction thermodynamics of the CRN process. The products were mainly gear-like β-Si3N4 grains and Fe3Si Archimedean solids. On the other hand, adding β-Si3N4 seeds into the starting mixture led to elongated β-Si3N4 grains with much finer widths and big specific ratio. The as-synthesized Si3N4 microcrystals exhibited an intense violet‒blue spectral range with two maximum peaks at 441 nm (3.17 eV) and 390 nm (2.81 eV), which may shed light on potential application of Si3N4 microcrystals in optical devices.
Author Contributions
Conceptualization, J.H. and Z.C.; methodology, M.Z.; software, S.H.; validation, Z.H., Z.F. and Q.X.; formal analysis, M.Z.; investigation, X.L.; resources, J.H.; data curation, Z.C.; writing—Original draft preparation, M.Z.; writing—Review and editing, J.H.; visualization, Z.C.; supervision, J.H.; project administration, Z.H.; funding acquisition, J.H. and X.L.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant No. 51772140, 51962023 and 51862024), Natural Science Foundation of Jiangxi Province (Grant No. 20171ACB21033), Shanghai Aerospace Science and Technology Innovation Fund (Grant No. SAST2017-116), Science and Technology Project of the Education Department of Jiangxi Province (GJJ170573), PHD Starting Foundation of Nanchang Hangkong University (EA201801233) and Graduate Innovation Special Fund of Nanchang Hangkong University (Grant No. YC2018-S358).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, T.F.; Chen, Y.J.; Li, W.; Li, J.B.; Luo, L.J.; Yang, T.; Liu, L.Y.; Wu, G.L. Fabrication and mechanical properties of boron nitride nanotube reinforced silicon nitride ceramics. Ceram. Int. 2018, 44, 6456–6460. [Google Scholar] [CrossRef]
- Huang, J.T.; Zhang, S.W.; Huang, Z.H.; Liu, Y.G.; Fang, M.H. Growth of α-Si3N4 nanobelts via Ni-catalyzed thermal chemical vapour deposition and their violet-blue luminescent properties. CrystEngComm 2013, 15, 785–790. [Google Scholar] [CrossRef]
- Hu, X.; Shao, C.W.; Wang, J.; Wang, H.; Cheng, J. Effects of residual radicals on compositional and structural stability of silicon nitride fibers. J. Eur. Ceram. Soc. 2017, 37, 4497–4503. [Google Scholar] [CrossRef]
- Wu, J.M.; Zhang, X.Y.; Xu, J.; Gan, K.; Li, J.L.; Li, C.H.; Yang, J.L.; Shi, Y.S. Preparation of porous Si3N4 ceramics via tailoring solid loading of Si3N4 slurry and Si3N4 poly-hollow microsphere content. J. Adv. Ceram. 2015, 4, 260–266. [Google Scholar] [CrossRef]
- Liu, X.Z.; Yi, X.M.; Guo, R.; Li, Q.D.; Nomura, T. Formation mechanisms of Si3N4 microstructures during silicon powder nitridation. Ceram. Int. 2017, 43, 16773–16779. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, M.J.; Kim, J.M.; Lee, J.W.; Ko, J.W.; Kim, H.D. Sintered reaction-bonded silicon nitrides with high thermal conductivity: The effect of the starting Si powder and Si3N4 diluents. J. Eur. Ceram. Soc. 2014, 34, 1105–1113. [Google Scholar] [CrossRef]
- Strong, K.T.; Arreguin, S.A.; Bordia, R.K. Controlled atmosphere pyrolysis of polyureasilazane for tailored volume fraction Si3N4/SiC nanocomposites powders. J. Eur. Ceram. Soc. 2016, 36, 3663–3669. [Google Scholar] [CrossRef]
- Sun, S.Y.; Wang, Q.; Ge, Y.Y.; Tian, Z.B.; Zhang, J.; Xie, Z.P. Synthesis of well-dispersed columnar Si3N4 using carbothermal reduction–nitridation method. Powder Technol. 2018, 331, 322–325. [Google Scholar] [CrossRef]
- Karakuş, N.; Kurt, A.O.; Duran, C.; Öztürk, C.; Toplan, H.Ö. Sintering behaviour of silicon nitride powders produced by carbothermal reduction and nitridation. Adv. Powder Technol. 2013, 24, 697–702. [Google Scholar] [CrossRef]
- Magnani, G.; Galvagno, S.; Sico, G.; Portofino, S.; Freda, C.; Burresi, E. Sintering and mechanical properties of β-SiC powder obtained from waste tires. J. Adv. Ceram. 2016, 5, 40–46. [Google Scholar] [CrossRef]
- Yin, L.; Xu, Y.G.; Huang, Z.H.; Liu, Y.G.; Fang, M.H.; Liu, B.L. Synthesis of ZrN–Si3N4 composite powders from zircon and quartz by carbothermal reduction and nitridation. Powder Technol. 2013, 246, 677–681. [Google Scholar] [CrossRef]
- Arik, H. Synthesis of Si3N4 by the carbo-thermal reduction and nitridation of diatomite. J. Eur. Ceram. Soc. 2003, 23, 2005–2014. [Google Scholar] [CrossRef]
- Anggraini, L.; Natsume, Y.; Ameyama, K. Effect of particle shape on dispersion formation of harmonic microstructure of Si3N4-ZrO2. Mater. Sci. Forum 2016, 864, 47–51. [Google Scholar] [CrossRef]
- Hu, Z.L.; Zhu, T.B.; Wu, W.W.; Peng, Z.J.; Hu, F.; Xie, Z.P. Growth mechanism of α-Si3N4 submicron rods prepared from amorphous Si3N4 powders. Ceram. Int. 2018, 44, 22003–22007. [Google Scholar] [CrossRef]
- Yu, J.J.; Guo, W.M.; Wei, W.X.; Lin, H.T.; Wang, C.Y. Fabrication and wear behaviors of graded Si3N4 ceramics by the combination of two-step sintering and β-Si3N4 seeds. J. Eur. Ceram. Soc. 2018, 38, 3457–3462. [Google Scholar] [CrossRef]
- Huang, J.T.; Huang, Z.H.; Yi, S.; Liu, Y.G.; Fang, M.H.; Zhang, S.W. Fe-catalyzed growth of one-dimensional α-Si3N4 nanostructures and their cathodoluminescence properties. Sci. Rep. 2013, 3, 3504. [Google Scholar] [CrossRef]
- Li, B.; Li, G.Q.; Chen, J.H.; Chen, H.Y.; Xing, X.M.; Hou, X.M.; Li, Y. Formation mechanism of elongated β-Si3N4 crystals in Fe-Si3N4 composite via flash combustion. Ceram. Int. 2018, 44, 9395–9400. [Google Scholar] [CrossRef]
- Chen, K.; Huang, Z.H.; Liu, Y.G.; Fang, M.H.; Huang, J.T.; Xu, Y.G. Synthesis of β-Si3N4 powder from quartz via carbothermal reduction nitridion. Powder Technol. 2013, 235, 728–734. [Google Scholar] [CrossRef]
- Dahal, N.; Chikan, V. Phase-controlled synthesis of iron silicide (Fe3Si and FeSi2) nanoparticles in solution. Chem. Mater. 2010, 22, 2892–2897. [Google Scholar] [CrossRef]
- Ortega, A.; Alcalá, M.D.; Real, C. Carbothermal synthesis of silicon nitride (Si3N4): Kinetics and diffusion mechanism. J. Mater. Process. Technol. 2008, 195, 224–231. [Google Scholar] [CrossRef]
- Li, J.; Shao, G.; Ma, Y.; Zhao, X.T.; Wang, H.L.; Zhang, R. Processing and properties of polycrystalline cubic boron nitride reinforced by SiC whiskers. Int. J. Appl. Ceram. Technol. 2019, 16, 32–38. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.Y.; Li, S.; Guo, Z.Y. Carbothermal synthesis of approximately spherical Si3N4 particles with homogeneous size distribution. Ceram. Int. 2018, 44, 22680–22685. [Google Scholar] [CrossRef]
- Ji, H.P.; Huang, Z.H.; Chen, K.; Li, W.J.; Gao, Y.F.; Fang, M.H.; Liu, Y.G.; Wu, X.W. Synthesis of Si3N4 powder with tunable α/β-Si3N4 content from waste silica fume using carbothermal reduction nitridation. Powder Technol. 2014, 252, 51–55. [Google Scholar] [CrossRef]
- Huang, J.T.; Miao, Y.P.; Zhang, M.; Feng, Z.J.; Hu, Z.H.; Li, X.B.; Luo, J.M. Hot-pressed sintered Ca-α-Sialon ceramics with grains from short prismatic to elongated morphology synthesized via carbothermal reduction and nitridation. J. Alloy. Compd. 2018, 767, 90–97. [Google Scholar] [CrossRef]
- Li, X.M.; Li, R.; Zhu, X.T.; Zhou, Y.L.; Ren, G.N.; Zhang, L. Properties of large-sized porous Si3N4 ceramic tubes fabricated by carbothermal reduction of diatomite preforms. Ceram. Int. 2017, 43, 10559–10565. [Google Scholar] [CrossRef]
- Charoo, M.S.; Wani, M.F. Friction and wear properties of nano-Si3N4/nano-SiC composite under nanolubricated conditions. J. Adv. Ceram. 2016, 5, 145–152. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, J.; Ji, H.M.; Xu, X.W. Catalytic Effect and Mechanism of Fe2O3 on Synthesis of Si2N2O by Carbothermal Reduction and Nitridation of SiO2. Aerosp. Mater. Technol. 2012, 2, 95–98. [Google Scholar]
- Boyer, S.M.; Moulson, A.J. A mechanism for the nitridation of Fe-contaminated silicon. J. Mater. Sci. 1978, 13, 1637–1646. [Google Scholar] [CrossRef]
- Guo, W.M.; Yu, J.J.; Xiong, M.; Wu, S.H.; Lin, H.T. High-toughness Lu2O3-doped Si3N4 ceramics by seeding. Ceram. Int. 2016, 42, 6495–6499. [Google Scholar] [CrossRef]
- Wang, B.; Yang, J.; Guo, R. Microstructure and property enhancement of silicon nitride-barium aluminum silicate composites with β-Si3N4 seed addition. J. Mater. Sci. 2009, 44, 1351–1356. [Google Scholar] [CrossRef]
- Marin, E.; Adachi, T.; Boschetto, F. Biological response of human osteosarcoma cells to Si3N4-doped Bioglasses. Mater. Des. 2018, 159, 79–89. [Google Scholar] [CrossRef]
- Jun, Y.S.; Kim, D.; Neil, C.W. Heterogeneous nucleation and growth of nanoparticles at environmental interfaces. Accounts Chem. Res. 2016, 49, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Topateş, G. Direct production of Si3N4 foams by carbothermal reduction and nitridation of SiO2. Ceram. Int. 2018, 44, 20545–20550. [Google Scholar] [CrossRef]
- Karakus, N.; Kurt, A.O.; Toplan, H.Ö. Synthesizing high α-phase Si3N4 powders containing sintering additives. Ceram. Int. 2009, 35, 2381–2385. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Mukerji, J. Reaction sequences in the synthesis of silicon nitride from quartz. Ceram. Int. 1991, 17, 171–179. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.F.; Yang, L.X. Synthesis and photoluminescence of Si3N4 nanowires from La/SiO2 composites and Si powders. Ceram. Int. 2015, 41, 1505–1510. [Google Scholar] [CrossRef]
- Huang, J.T.; Zhang, S.W.; Huang, Z.H. Catalyst-assisted synthesis and growth mechanism of ultra-long single crystal α-Si3N4 nanobelts with strong violet-blue luminescent properties. CrystEngComm 2012, 14, 7301–7305. [Google Scholar] [CrossRef]
- Robertson, J.; Powell, M.J. Gap states in silicon nitride. Appl. Phys. Lett. 1984, 44, 415–417. [Google Scholar] [CrossRef]
- Xiong, L.; Dai, J.H.; Song, Y. Effects of doping on photoelectrical properties of one-dimensional α-Si3N4 nanomaterials: A first-principles study. Phys. B Condens. Matter 2018, 550, 32–38. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).