Properties of Nanohydroxyapatite Coatings Doped with Nanocopper, Obtained by Electrophoretic Deposition on Ti13Zr13Nb Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Electrophoretic Deposition of Nanohydroxyapatite and Cu Nanoparticles
2.3. Thermal Treatment
2.4. Surface Analysis
2.5. Chemical and Phase Composition
2.6. Mechanical Studies
2.7. Contact Angle Studies
3. Results and Discussion
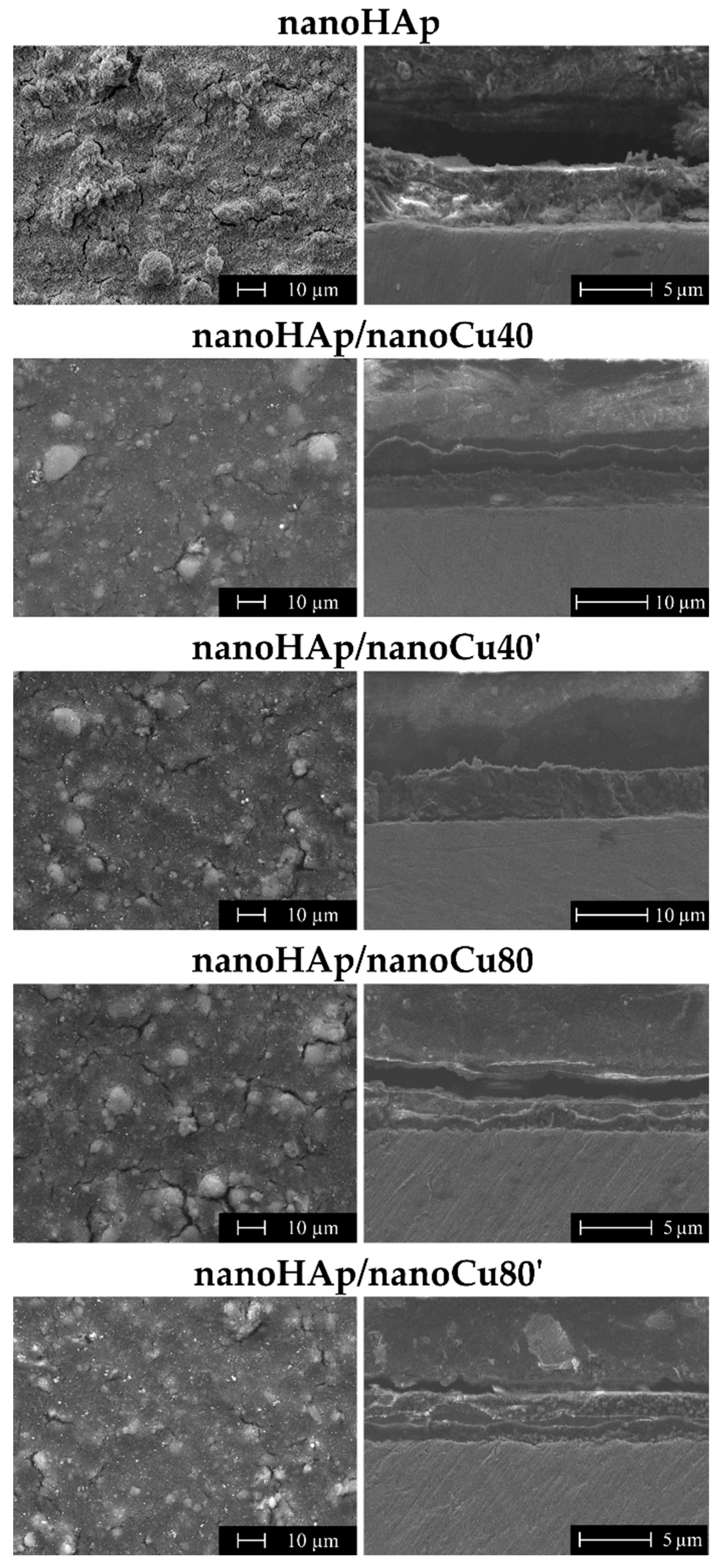
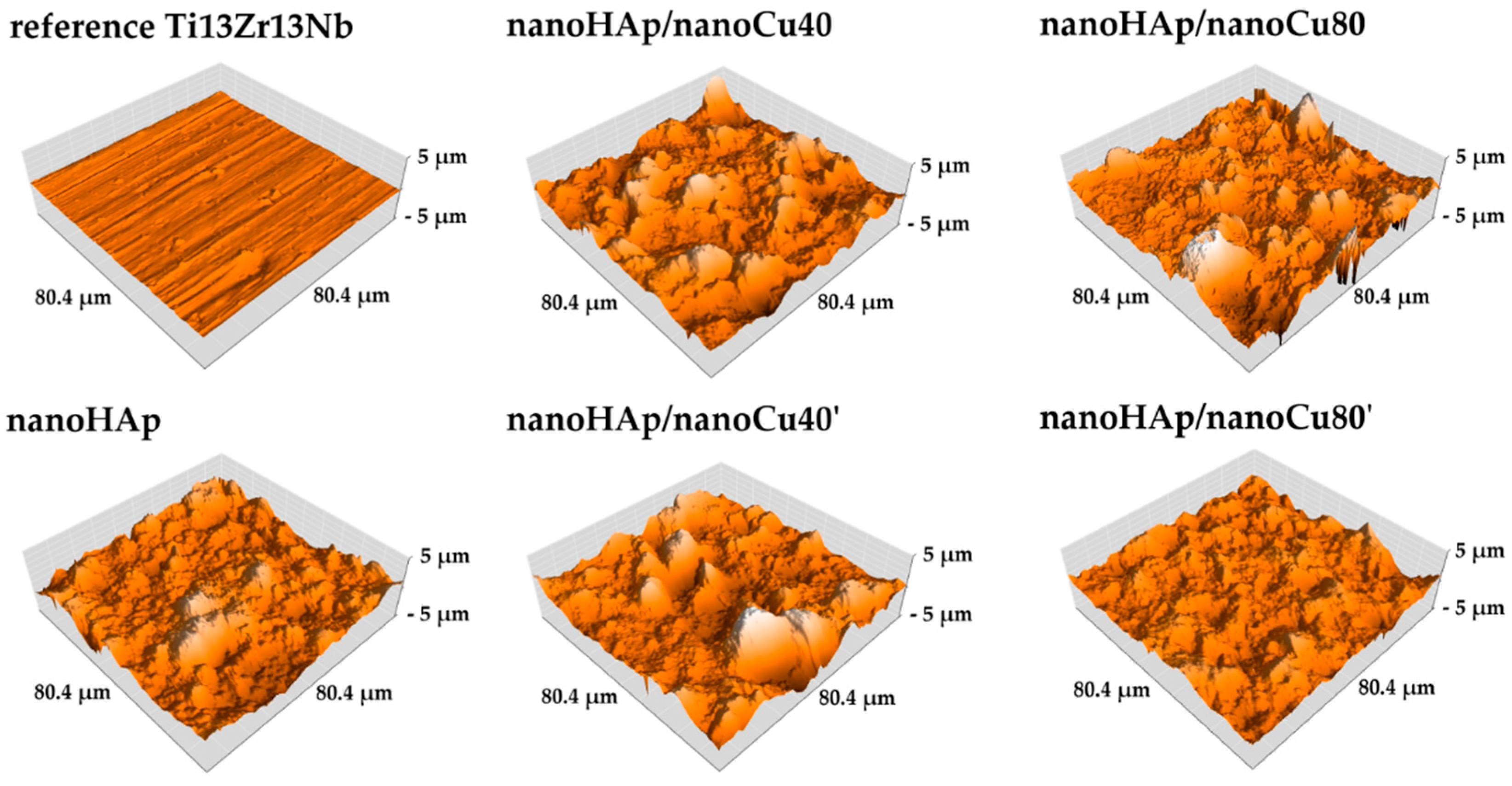
3.1. Morphology and Topography Studies
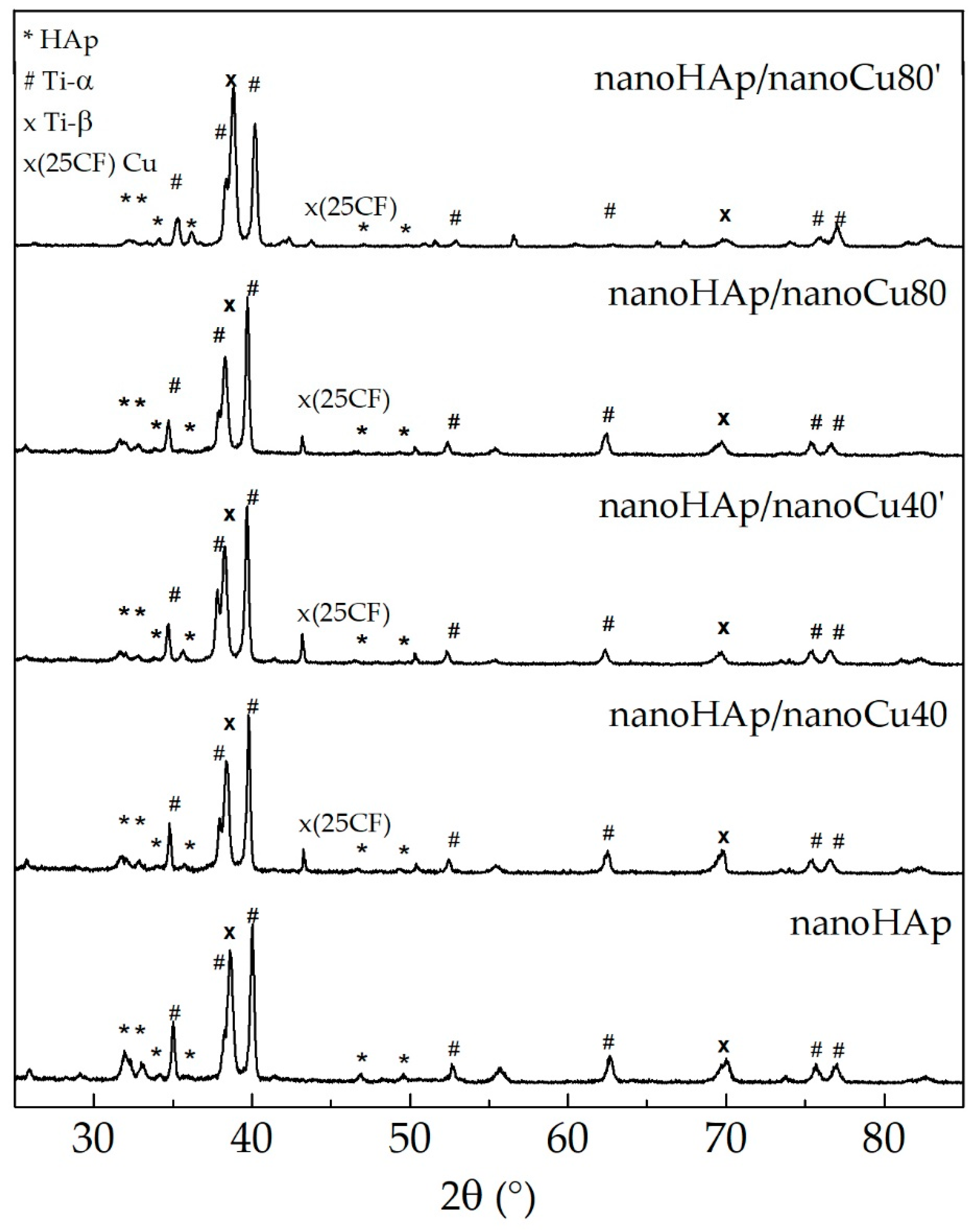
3.2. Chemical and Phase Analysis
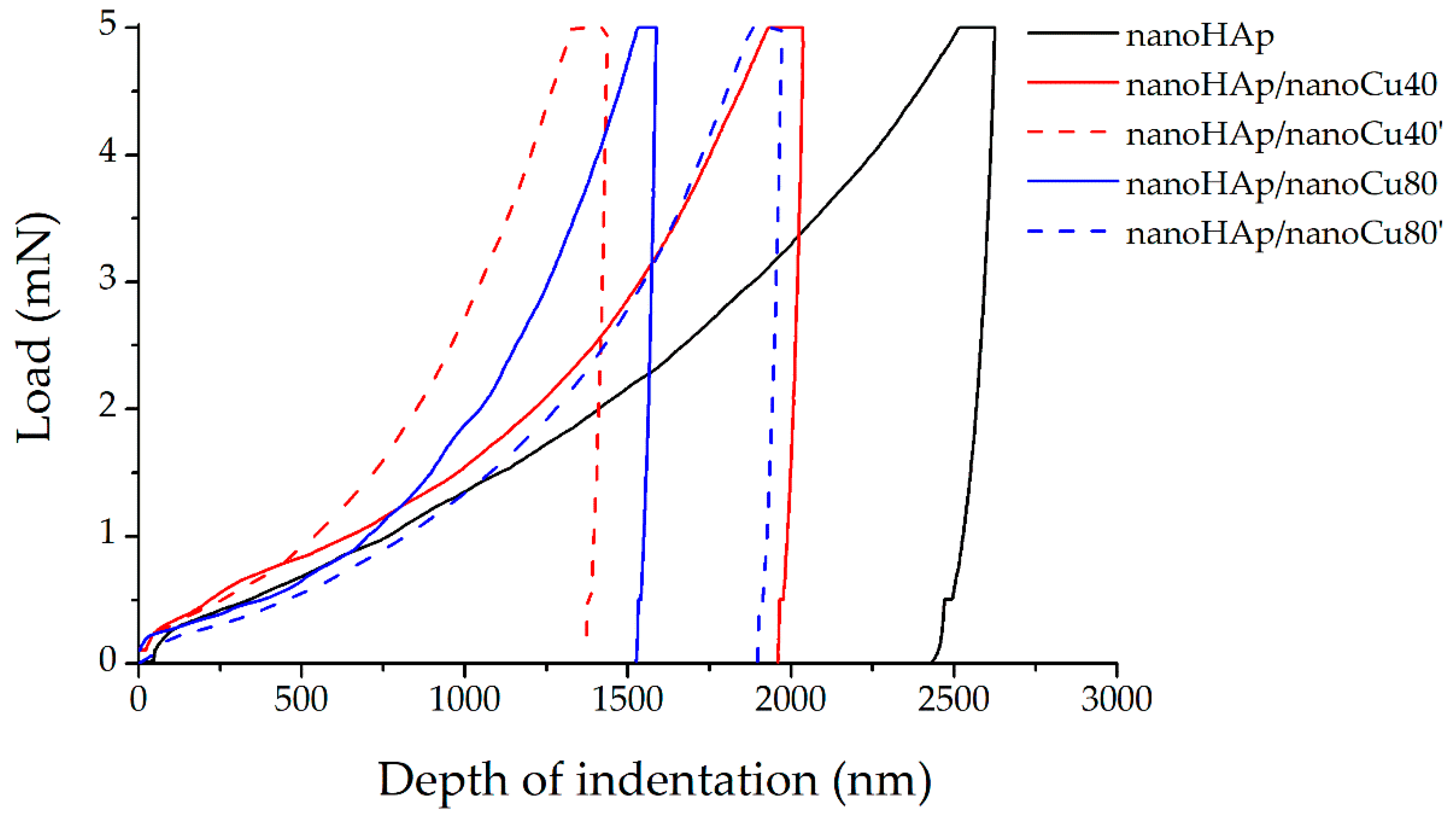
3.3. Nanomechanical Studies
3.4. Contact Angle Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Granato, R.; Bonfante, E.A.; Castellano, A.; Khan, R.; Jimbo, R.; Marin, C.; Morsi, S.; Witek, L.; Coelho, P.G. Osteointegrative and microgeometric comparison between micro-blasted and alumina blasting/acid etching on grade II and V titanium alloys (Ti-6Al-4V). J. Mech. Behav. Biomed. Mater. 2019, 97, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Vilardell, A.M.; Cinca, N.; Garcia-Giralt, N.; Müller, C.; Dosta, S.; Sarret, M.; Cano, I.G.; Nogués, X.; Guilemany, J.M. In-vitro study of hierarchical structures: Anodic oxidation and alkaline treatments onto highly rough titanium cold gas spray coatings for biomedical applications. Mater. Sci. Eng. C 2018, 91, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qian, Q.; Xu, L.; Zhu, H.; Xu, L.; Wang, Q. Effects of hydrothermal sterilization on properties of biological coating fabricated by alkaline-heat treatment on titanium. Surf. Coat. Technol. 2018, 342, 69–75. [Google Scholar] [CrossRef]
- Khodaei, M.; Hossein Kelishadi, S. The effect of different oxidizing ions on hydrogen peroxide treatment of titanium dental implant. Surf. Coat. Technol. 2018, 353, 158–162. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Jazdzewska, M.; Głodowska, J.; Kalka, P. Effects of electrophoretic deposition times and nanotubular oxide surfaces on properties of the nanohydroxyapatite/nanocopper coating on the Ti13Zr13Nb alloy. Ceram. Int. 2019, 45, 20002–20010. [Google Scholar] [CrossRef]
- Koshuro, V.; Fomin, A.; Rodionov, I. Composition, structure and mechanical properties of metal oxide coatings produced on titanium using plasma spraying and modified by micro-arc oxidation. Ceram. Int. 2018, 44, 12593–12599. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, H.; Lei, J.; Zhang, J.; Zhou, H.; Qi, M. Formation and in vitro/in vivo performance of “cortex-like” micro/nano-structured TiO 2 coatings on titanium by micro-arc oxidation. Mater. Sci. Eng. C 2018, 87, 90–103. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive coatings on Ti6Al4V alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- Acciari, H.A.; Palma, D.P.S.; Codaro, E.N.; Zhou, Q.; Wang, J.; Ling, Y.; Zhang, J.; Zhang, Z. Surface modifications by both anodic oxidation and ion beam implantation on electropolished titanium substrates. Appl. Surf. Sci. 2019, 487, 1111–1120. [Google Scholar] [CrossRef]
- Lin, Z.; Li, S.J.; Sun, F.; Ba, D.C.; Li, X.C. Surface characteristics of a dental implant modified by low energy oxygen ion implantation. Surf. Coat. Technol. 2019, 365, 208–213. [Google Scholar] [CrossRef]
- Gilabert-Chirivella, E.; Pérez-Feito, R.; Ribeiro, C.; Ribeiro, S.; Correia, D.M.; González-Martín, M.L.; Manero, J.M.; Lanceros-Méndez, S.; Ferrer, G.G.; Gómez-Ribelles, J.L. Chitosan patterning on titanium implants. Prog. Org. Coat. 2017, 111, 23–28. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Molaei, A.; Yari, M.; Afshar, M.R. Modification of electrophoretic deposition of chitosan-bioactive glass-hydroxyapatite nanocomposite coatings for orthopedic applications by changing voltage and deposition time. Ceram. Int. 2015, 41, 14537–14544. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Li, C.; Huang, Y.; Ding, Q.; Pang, X. Preparation and characterization of chitosan-silver/hydroxyapatite composite coatings onTiO 2 nanotube for biomedical applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Buga, C.; Hunyadi, M.; Gácsi, Z.; Hegedűs, C.; Hakl, J.; Schmidt, U.; Ding, S.J.; Csík, A. Calcium silicate layer on titanium fabricated by electrospray deposition. Mater. Sci. Eng. C 2019, 98, 401–408. [Google Scholar] [CrossRef]
- Szaraniec, B.; Pielichowska, K.; Pac, E.; Menaszek, E. Multifunctional polymer coatings for titanium implants. Mater. Sci. Eng. C 2018, 93, 950–957. [Google Scholar] [CrossRef]
- Araghi, A.; Hadianfard, M.J. Fabrication and characterization of functionally graded hydroxyapatite/TiO2 multilayer coating on Ti-6Al-4V titanium alloy for biomedical applications. Ceram. Int. 2015, 41, 12668–12679. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Murugan, N.; Karthikeyan, P.; Sathishkumar, S.; Anbarasu, G.; Rajavel, R. Development of antibacterial activity and corrosion resistance properties of electrodeposition of mineralized hydroxyapatite coated on titanium alloy for biomedical applications. Mater. Today Proc. 2017, 4, 12393–12400. [Google Scholar] [CrossRef]
- Grubova, I.Y.; Surmeneva, M.A.; Ivanova, A.A.; Kravchuk, K.; Prymak, O.; Epple, M.; Buck, V.; Surmenev, R.A. The effect of patterned titanium substrates on the properties of silver-doped hydroxyapatite coatings. Surf. Coat. Technol. 2015, 276, 595–601. [Google Scholar] [CrossRef]
- Jazdzewska, M.; Majkowska-Marzec, B. Hydroxyapatite Deposition on the Laser Modified Ti13Nb13Zr Alloy. Adv. Mater. Sci. 2017, 17, 5–13. [Google Scholar] [CrossRef]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Suchanek, K.; Hajdyła, M.; Maximenko, A.; Zarzycki, A.; Marszałek, M.; Jany, B.R.; Krok, F. The influence of nanoporous anodic titanium oxide substrates on the growth of the crystalline hydroxyapatite coatings. Mater. Chem. Phys. 2017, 186, 167–178. [Google Scholar] [CrossRef]
- Prem Ananth, K.; Nathanael, A.J.; Jose, S.P.; Oh, T.H.; Mangalaraj, D.; Ballamurugan, A.M. Controlled electrophoretic deposition of HAp/β-TCP composite coatings on piranha treated 316L SS for enhanced mechanical and biological properties. Appl. Surf. Sci. 2015, 353, 189–199. [Google Scholar] [CrossRef]
- Ročňáková, I.; Slámečka, K.; Montufar, E.B.; Remešová, M.; Dyčková, L.; Břínek, A.; Jech, D.; Dvořák, K.; Čelko, L.; Kaiser, J. Deposition of hydroxyapatite and tricalcium phosphate coatings by suspension plasma spraying: Effects of torch speed. J. Eur. Ceram. Soc. 2018, 38, 5489–5496. [Google Scholar] [CrossRef]
- Sayed, S.; Faruq, O.; Hossain, M.; Im, S.-B.; Kim, Y.-S.; Lee, B.-T. Thermal cycling effect on osteogenic differentiation of MC3T3-E1 cells loaded on 3D-porous Biphasic Calcium Phosphate (BCP) scaffolds for early osteogenesis. Mater. Sci. Eng. C 2019, 105, 110027. [Google Scholar] [CrossRef]
- Jo, I.H.; Ahn, M.K.; Moon, Y.W.; Koh, Y.H.; Kim, H.E. Novel rapid direct deposition of ceramic paste for porous biphasic calcium phosphate (BCP) scaffolds with tightly controlled 3-D macrochannels. Ceram. Int. 2014, 40, 11079–11084. [Google Scholar] [CrossRef]
- Bartmanski, M. The Properties of Nanosilver—Doped Nanohydroxyapatite Coating On the Ti13zr13Nb Alloy. Adv. Mater. Sci. 2017, 17, 18–28. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Majkowska-Marzec, B.; Strugala, G. Effects of solution composition and electrophoretic deposition voltage on various properties of nanohydroxyapatite coatings on the Ti13Zr13Nb alloy. Ceram. Int. 2018, 44, 19236–19246. [Google Scholar] [CrossRef]
- Bral, A.; Mommaerts, M.Y. In vivo biofunctionalization of titanium patient-specific implants with nano hydroxyapatite and other nano calcium phosphate coatings: A systematic review. J. Cranio-Maxillofac. Surg. 2016, 44, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Ahmad, Z.; Huang, J.; Edirisinghe, M.J.; Jayasinghe, S.N.; Ireland, D.C.; Brooks, R.A.; Rushton, N.; Bonfield, W.; Best, S.M. The role of surface wettability and surface charge of electrosprayed nanoapatites on the behaviour of osteoblasts. Acta Biomater. 2010, 6, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, R.M.G.; Wijesinghe, W.P.S.L.; Mantilaka, M.M.M.G.P.G.; Chathuranga Senarathna, K.G.; Herath, H.M.T.U.; Premachandra, T.N.; Ranasinghe, C.S.K.; Rajapakse, R.P.V.J.; Edirisinghe, M.; Mahalingam, S.; et al. Preparation of bone-implants by coating hydroxyapatite nanoparticles on self-formed titanium dioxide thin-layers on titanium metal surfaces. Mater. Sci. Eng. C 2016, 63, 172–184. [Google Scholar]
- Rau, J.V.; Cacciotti, I.; De Bonis, A.; Fosca, M.; Komlev, V.S.; Latini, A.; Santagata, A.; Teghil, R. Fe-doped hydroxyapatite coatings for orthopedic and dental implant applications. Appl. Surf. Sci. 2014, 307, 301–305. [Google Scholar] [CrossRef]
- Bose, S.; Vu, A.A.; Emshadi, K.; Bandyopadhyay, A. Effects of polycaprolactone on alendronate drug release from Mg-doped hydroxyapatite coating on titanium. Mater. Sci. Eng. C 2018, 88, 166–171. [Google Scholar] [CrossRef]
- Göncü, Y.; Geçgin, M.; Bakan, F.; Ay, N. Electrophoretic deposition of hydroxyapatite-hexagonal boron nitride composite coatings on Ti substrate. Mater. Sci. Eng. C 2017, 79, 343–353. [Google Scholar] [CrossRef]
- Długoń, E.; Niemiec, W.; Fra̧czek-Szczypta, A.; Jeleń, P.; Sitarz, M.; Błazewicz, M. Spectroscopic studies of electrophoretically deposited hybrid HAp/CNT coatings on titanium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 872–875. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2018, 45, 2852–2857. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram. Int. 2015, 41, 8878–8884. [Google Scholar] [CrossRef]
- Ruiz, G.C.M.; Cruz, M.A.E.; Faria, A.N.; Zancanela, D.C.; Ciancaglini, P.; Ramos, A.P. Biomimetic collagen/phospholipid coatings improve formation of hydroxyapatite nanoparticles on titanium. Mater. Sci. Eng. C 2017, 77, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yajing, Y.; Qiongqiong, D.; Yong, H.; Han, S.; Pang, X. Magnesium substituted hydroxyapatite coating on titanium with nanotublar TiO2intermediate layer via electrochemical deposition. Appl. Surf. Sci. 2014, 305, 77–85. [Google Scholar] [CrossRef]
- Karthika, A. Aliovalent ions substituted hydroxyapatite coating on titanium for improved medical applications. Mater. Today Proc. 2018, 5, 8768–8774. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Qiao, H.; Nian, X.; Zhang, X.; Wang, W.; Zhang, X.; Chang, X.; Han, S.; Pang, X. Anticorrosive effects and in vitro cytocompatibility of calcium silicate/zinc-doped hydroxyapatite composite coatings on titanium. Appl. Surf. Sci. 2015, 357, 1776–1784. [Google Scholar] [CrossRef]
- Peñaflor Galindo, T.G.; Kataoka, T.; Fujii, S.; Okuda, M.; Tagaya, M. Preparation of nanocrystalline zinc-substituted hydroxyapatite films and their biological properties. Colloids Interface Sci. Commun. 2016, 10–11, 15–19. [Google Scholar] [CrossRef]
- Chozhanathmisra, M.; Ramya, S.; Kavitha, L.; Gopi, D. Development of zinc-halloysite nanotube/minerals substituted hydroxyapatite bilayer coatings on titanium alloy for orthopedic applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 357–365. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chen, C.C.; Wang, J.B.; Wang, Y.C.; Lin, F.H. Flame sprayed zinc doped hydroxyapatite coating with antibacterial and biocompatible properties. Ceram. Int. 2017, 43, S829–S835. [Google Scholar] [CrossRef]
- Boanini, E.; Torricelli, P.; Sima, F.; Axente, E.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium and zoledronate hydroxyapatites graded composite coatings for bone prostheses. J. Colloid Interface Sci. 2015, 448, 1–7. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.; Li, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Li, X.; He, X.; Yu, X.; Wang, R.; et al. Strontium incorporation to optimize the antibacterial and biological characteristics of silver-substituted hydroxyapatite coating. Mater. Sci. Eng. C 2016, 58, 467–477. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, H.; Nian, X.; Zhang, X.; Zhang, X.; Song, G.; Xu, Z.; Zhang, H.; Han, S. Improving the bioactivity and corrosion resistance properties of electrodeposited hydroxyapatite coating by dual doping of bivalent strontium and manganese ion. Surf. Coat. Technol. 2016, 291, 205–215. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Copper substituted hydroxyapatite and fl uorapatite: Synthesis, characterization and antimicrobial properties. Ceram. Int. 2014, 40, 15655–15662. [Google Scholar] [CrossRef]
- Sikder, P.; Koju, N.; Ren, Y.; Goel, V.K.; Phares, T.; Lin, B.; Bhaduri, S.B. Development of single-phase silver-doped antibacterial CDHA coatings on Ti6Al4V with sustained release. Surf. Coat. Technol. 2018, 342, 105–116. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Huang, Y.; Ding, Q.; Pang, X. Antibacterial and bioactivity of silver substituted hydroxyapatite/TiO2 nanotube composite coatings on titanium. Appl. Surf. Sci. 2014, 314, 348–357. [Google Scholar] [CrossRef]
- Zhang, X.; Chaimayo, W.; Yang, C.; Yao, J.; Miller, B.L.; Yates, M.Z. Silver-hydroxyapatite composite coatings with enhanced antimicrobial activities through heat treatment. Surf. Coat. Technol. 2017, 325, 39–45. [Google Scholar] [CrossRef]
- Premphet, P.; Prasoetsri, M.; Boonyawan, D.; Supruangnet, R.; Udomsom, S.; Leksakul, K. Optimization of DC magnetron sputtering deposition process and surface properties of HA-TiO2 film. Mater. Today Proc. 2017, 4, 6372–6380. [Google Scholar] [CrossRef]
- Gopi, D.; Shinyjoy, E.; Kavitha, L. Influence of ionic substitution in improving the biological property of carbon nanotubes reinforced hydroxyapatite composite coating on titanium for orthopedic applications. Ceram. Int. 2015, 41, 5454–5463. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Krause, B.; Baumbach, T.; Ignatov, V.P.; Tyurin, A.I.; Loza, K.; Epple, M.; Surmenev, R.A. Hybrid biocomposites based on titania nanotubes and a hydroxyapatite coating deposited by RF-magnetron sputtering: Surface topography, structure, and mechanical properties. Appl. Surf. Sci. 2017, 426, 229–237. [Google Scholar] [CrossRef]
- Strąkowska, P.; Beutner, R.; Gnyba, M.; Zielinski, A.; Scharnweber, D. Electrochemically assisted deposition of hydroxyapatite on Ti6Al4V substrates covered by CVD diamond films—Coating characterization and first cell biological results. Mater. Sci. Eng. C 2016, 59, 624–635. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrokhi-Rad, M.; Shahrabi, T.; Mahmoodi, S.; Khanmohammadi, S. Electrophoretic deposition of hydroxyapatite-chitosan-CNTs nanocomposite coatings. Ceram. Int. 2017, 43, 4663–4669. [Google Scholar] [CrossRef]
- Robertson, S.F.; Bandyopadhyay, A.; Bose, S. Titania nanotube interface to increase adhesion strength of hydroxyapatite sol-gel coatings on Ti-6Al-4V for orthopedic applications. Surf. Coatings Technol. 2019, 372, 140–147. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.C.; García-Moreno, F.; Torres, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Cruz, M.A.E.; Ruiz, G.C.M.; Faria, A.N.; Zancanela, D.C.; Pereira, L.S.; Ciancaglini, P.; Ramos, A.P. Calcium carbonate hybrid coating promotes the formation of biomimetic hydroxyapatite on titanium surfaces. Appl. Surf. Sci. 2016, 370, 459–468. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ciobanu, O. Investigation on the effect of collagen and vitamins on biomimetic hydroxyapatite coating formation on titanium surfaces. Mater. Sci. Eng. C 2013, 33, 1683–1688. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Surface characteristics of hydroxyapatite coatings on nanotubular Ti-25Ta-xZr alloys prepared by electrochemical deposition. Surf. Coat. Technol. 2014, 259, 274–280. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Sima, F.; Sima, L.E.; Mihailescu, C.N.; Luculescu, C.; Iordache, I.; Socol, M.; Socol, G.; Mihailescu, I.N. Hydroxyapatite thin films grown by pulsed laser deposition and matrix assisted pulsed laser evaporation: Comparative study. Appl. Surf. Sci. 2017, 418, 580–588. [Google Scholar] [CrossRef]
- Hidalgo-Robatto, B.M.; López-Álvarez, M.; Azevedo, A.S.; Dorado, J.; Serra, J.; Azevedo, N.F.; González, P. Pulsed laser deposition of copper and zinc doped hydroxyapatite coatings for biomedical applications. Surf. Coat. Technol. 2018, 333, 168–177. [Google Scholar] [CrossRef]
- Zieliński, A.; Sobieszczyk, S. Corrosion of Titanium Biomaterials, Mechanisms, Effects and Modelisation. Corros. Rev. 2008, 26, 1–22. [Google Scholar] [CrossRef]
- Koike, M.; Fujii, H. The corrosion resistance of pure titanium in organic acids. Biomaterials 2001, 22, 2931–2936. [Google Scholar] [CrossRef]
- Ling Feng, Q.; Nam Kim, T.; Wu, J.; Seo Park, E.; Ock Kim, J.; Young Lim, D.; Zhai Cui, F. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films 1998, 335, 214–219. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Webster, T.J.; Ueda, K.; Narushima, T.; Ergun, C. In vitro performance of Ag-incorporated hydroxyapatite and its adhesive porous coatings deposited by electrostatic spraying. Mater. Sci. Eng. C 2017, 77, 556–564. [Google Scholar] [CrossRef]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P. Electrophoretic deposition (EPD) of nanohydroxyapatite—Nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Radovanovic, Z.; Jokic, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Vladescu, A.; Padmanabhan, S.C.; Ak Azem, F.; Braic, M.; Titorencu, I.; Birlik, I.; Morris, M.A.; Braic, V. Mechanical properties and biocompatibility of the sputtered Ti doped hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2016, 63, 314–325. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Rózycka, D. Substituted hydroxyapatites with antibacterial properties. BioMed Res. Int. 2014, 2014, 178123. [Google Scholar] [CrossRef]
- Rau, J.V.; Wu, V.M.; Graziani, V.; Fadeeva, I.V.; Fomin, A.S.; Fosca, M.; Uskoković, V. The Bone Building Blues: Self-hardening copper-doped calcium phosphate cement and its in vitro assessment against mammalian cells and bacteria. Mater. Sci. Eng. C 2017, 79, 270–279. [Google Scholar] [CrossRef]
- Hadidi, M.; Bigham, A.; Saebnoori, E.; Hassanzadeh-Tabrizi, S.A.; Rahmati, S.; Alizadeh, Z.M.; Nasirian, V.; Rafienia, M. Electrophoretic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf. Coat. Technol. 2017, 321, 171–179. [Google Scholar] [CrossRef]
- Weerasuriya, D.R.K.; Wijesinghe, W.P.S.L.; Rajapakse, R.M.G. Encapsulation of anticancer drug copper bis(8-hydroxyquinoline) in hydroxyapatite for pH-sensitive targeted delivery and slow release. Mater. Sci. Eng. C 2017, 71, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Banerjee, S.; Bagchi, B.; Bhandary, S.; Kool, A.; Amin Hoque, N.; Thakur, P.; Das, S. A facile vacuum assisted synthesis of nanoparticle impregnated hydroxyapatite composites having excellent antimicrobial properties and biocompatibility. Ceram. Int. 2018, 44, 1066–1077. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Yu, W.W.; Li, Y.; Zhu, L.; Hu, C.Y. Migration of copper from nanocopper/polypropylene composite films and its functional property. Food Packag. Shelf Life 2009, 22, 100416. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Mohan, L.; Durgalakshmi, D.; Geetha, M.; Sankara Narayanan, T.S.N.; Asokamani, R. Electrophoretic deposition of nanocomposite (HAp + TiO 2) on titanium alloy for biomedical applications. Ceram. Int. 2012, 38, 3435–3443. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Ni, Y.-J.; Huang, J.-C. Fabrication and characterization of HAp /Al2O3 composite cating on titanium substrate. J. Biomed. Sci. Eng. 2008, 1, 190–194. [Google Scholar] [CrossRef] [Green Version]
- He, L.H.; Standard, O.C.; Huang, T.T.Y.; Latella, B.A.; Swain, M.V. Mechanical behaviour of porous hydroxyapatite. Acta Biomater. 2008, 4, 577–586. [Google Scholar] [CrossRef]
- Singh, G.; Singh, S.; Prakash, S. Surface characterization of plasma sprayed pure and reinforced hydroxyapatite coating on Ti6Al4V alloy. Surf. Coat. Technol. 2011, 205, 4814–4820. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.C.; Qu, S.X.; Zhang, X.D. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials 2003, 24, 4663–4670. [Google Scholar] [CrossRef]
- Gross, K.A.; Babovic, M. Influence of abrasion on the surface characteristics of thermally sprayed hydroxyapatite coatings. Biomaterials 2002, 23, 4731–4737. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.H. Ion implantation of titanium based biomaterials. Prog. Mater. Sci. 2011, 56, 1137–1177. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zitelli, J.P.; TenHuisen, K.S.; Yu, X.; Libera, M.R. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials 2011, 32, 951–960. [Google Scholar] [CrossRef]
- Drevet, R.; Faur, J.; Sayen, S.; Marle-Spiess, M.; El Btaouri, H.; Bernhaoune, H. Electrodeposition of biphasic calcium phosphate coatings with improved dissolution properties. Mater. Chem. Phys. 2019, 236, 21797. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2002, 58, 570–592. [Google Scholar] [CrossRef]
- He, Y.H.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Microstructure evolution and enhanced bioactivity of Ti-Nb-Zr alloy by bioactive hydroxyapatite fabricated: Via spark plasma sintering. RSC Adv. 2016, 6, 100939–100953. [Google Scholar] [CrossRef]
- Roy, P.; Sailaja, R.R.N. Mechanical, thermal and bio-compatibility studies of PAEK-hydroxyapatite nanocomposites. J. Mech. Behav. Biomed. Mater. 2015, 49, 1–11. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Bartmański, M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef] [Green Version]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Wei, M.; Ruys, A.J.; Milthorpe, B.K.; Sorrell, C.C. Precipitation of hydroxyapatite nanoparticles: Effects of precipitation method on electrophoretic deposition. J. Mater. Sci. Mater. Med. 2005, 16, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Kocourek, T.; Remsa, J.; Weiserová, M.; Jurek, K.; Miksovsky, J.; Strnad, J.; Galandakova, A.; Ulrichova, J. Antibacterial, cytotoxicity and physical properties of laser—Silver doped hydroxyapatite layers. Mater. Sci. Eng. C 2013, 33, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Drevet, R.; Ben Jaber, N.; Fauré, J.; Tara, A.; Ben Cheikh Larbi, A.; Benhayoune, H. Electrophoretic deposition (EPD) of nano-hydroxyapatite coatings with improved mechanical properties on prosthetic Ti6Al4V substrates. Surf. Coat. Technol. 2015, 301, 94–99. [Google Scholar] [CrossRef]
- Sidane, D.; Chicot, D.; Yala, S.; Ziani, S.; Khireddine, H.; Iost, A.; Decoopman, X. Study of the mechanical behavior and corrosion resistance of hydroxyapatite sol-gel thin coatings on 316 L stainless steel pre-coated with titania film. Thin Solid Films 2015, 593, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Clèries, L.; Fernández-Pradas, J.; Morenza, J. Behavior in simulated body fluid of calcium phosphate coatings obtained by laser ablation. Biomaterials 2000, 21, 1861–1865. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef]
- Germano, F.; Bramanti, E.; Arcuri, C.; Cecchetti, F.; Cicciù, M. Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study results. Eur. J. Dent. 2013, 7, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]



| Element | Nb | Zr | Fe | C | N | O | H | Ti |
|---|---|---|---|---|---|---|---|---|
| wt.% | 13.5 | 13.5 | 0.05 | 0.04 | 0.013 | 0.11 | 0.04 | remainder |
| Specimen | Properties of Electrophoretic Deposition | ||||
|---|---|---|---|---|---|
| NanoHAp Content/100 mL of Ethanol (g) | Average Particle Size of NanoCu Powder (nm) | NanoCu Content/100 mL of Ethanol (g) | Voltage of Deposition (V) | Time of Deposition (min) | |
| nanoHAp | 0.1 | – | – | 30 | 2 |
| nanoHAp/nanoCu40 | 40 | 0.01 | |||
| nanoHAp/nanoCu40’ | 0.025 | ||||
| nanoHAp/nanoCu80 | 80 | 0.01 | |||
| nanoHAp/nanoCu80’ | 0.025 | ||||
| Specimen | Properties | |
|---|---|---|
| Thickness (µm) | Sa Parameters (µm) | |
| Reference Ti13Zr13Nb | – | 0.13 |
| nanoHAp | 4.67 ± 1.07 | 0.64 |
| nanoHAp/nanoCu40 | 6.27 ± 1.48 | 0.73 |
| nanoHAp/nanoCu40’ | 7.74 ± 1.45 | 0.86 |
| nanoHAp/nanoCu80 | 2.42 ± 0.34 | 0.76 |
| nanoHAp/nanoCu80’ | 3.28 ± 0.31 | 0.44 |
| Properties | Nanoindentation Properties | Nanoscratch Test Properties | ||||
|---|---|---|---|---|---|---|
| Specimen | Nanohardness (GPa) | Young’s Modulus, E (GPa) | Maximum Depth of Indentation (nm) | E3/h2 (GPa) | Critical Load, Lc (mN) | Critical Friction, Lf (mN) |
| nanoHAp | 0.032 ± 0.009 | 4.46 ± 0.91 | 2617.12 ± 359.26 | 86.64 | 106.77 ± 37.51 | 59.18 ± 20.46 |
| nanoHAp/nanoCu40 | 0.054 ± 0.020 | 10.27 ± 3.16 | 2084.71 ± 382.15 | 371.47 | 123.84 ± 52.46 | 59.14 ± 24.18 |
| nanoHAp/nanoCu40’ | 0.139 ± 0.050 | 17.82 ± 5.48 | 1287.28 ± 279.89 | 292.88 | 141.89 ± 13.09 | 78.99 ± 10.02 |
| nanoHAp/nanoCu80 | 0.051 ± 0.026 | 10.51 ± 3.63 | 1887.62 ± 479.95 | 446.34 | 155.24 ± 12.78 | 94.47 ± 9.73 |
| nanoHAp/nanoCu80’ | 0.059 ± 0.038 | 11.86 ± 6.09 | 2224.33 ± 757.61 | 479.24 | 128.73 ± 30.39 | 80.39 ± 21.01 |
| Specimen | Average Contact Angle (°) |
|---|---|
| Reference Ti13Zr13Nb | 53.7 ± 2.1 |
| nanoHAp | 35.8 ± 3.5 |
| nanoHAp/nanoCu40 | 22.6 ± 2.2 |
| nanoHAp/nanoCu40’ | 18.2 ± 1.9 |
| nanoHAp/nanoCu80 | 26.7 ± 2.8 |
| nanoHAp/nanoCu80’ | 48.3 ± 2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmański, M.; Pawłowski, Ł.; Strugała, G.; Mielewczyk-Gryń, A.; Zieliński, A. Properties of Nanohydroxyapatite Coatings Doped with Nanocopper, Obtained by Electrophoretic Deposition on Ti13Zr13Nb Alloy. Materials 2019, 12, 3741. https://doi.org/10.3390/ma12223741
Bartmański M, Pawłowski Ł, Strugała G, Mielewczyk-Gryń A, Zieliński A. Properties of Nanohydroxyapatite Coatings Doped with Nanocopper, Obtained by Electrophoretic Deposition on Ti13Zr13Nb Alloy. Materials. 2019; 12(22):3741. https://doi.org/10.3390/ma12223741
Chicago/Turabian StyleBartmański, Michał, Łukasz Pawłowski, Gabriel Strugała, Aleksandra Mielewczyk-Gryń, and Andrzej Zieliński. 2019. "Properties of Nanohydroxyapatite Coatings Doped with Nanocopper, Obtained by Electrophoretic Deposition on Ti13Zr13Nb Alloy" Materials 12, no. 22: 3741. https://doi.org/10.3390/ma12223741
APA StyleBartmański, M., Pawłowski, Ł., Strugała, G., Mielewczyk-Gryń, A., & Zieliński, A. (2019). Properties of Nanohydroxyapatite Coatings Doped with Nanocopper, Obtained by Electrophoretic Deposition on Ti13Zr13Nb Alloy. Materials, 12(22), 3741. https://doi.org/10.3390/ma12223741









