Improving the Mechanical and Surface Properties of Aramid Fiber by Grafting with 1,4-Dichlorobutane under Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Desizing
2.2.2. Treatment in scCO2
2.2.3. Characterization and Performance Study of Composites
3. Results and Discussion
3.1. Effect of Different Treatment Conditions on Surface Characteristic of Aramid Fiber
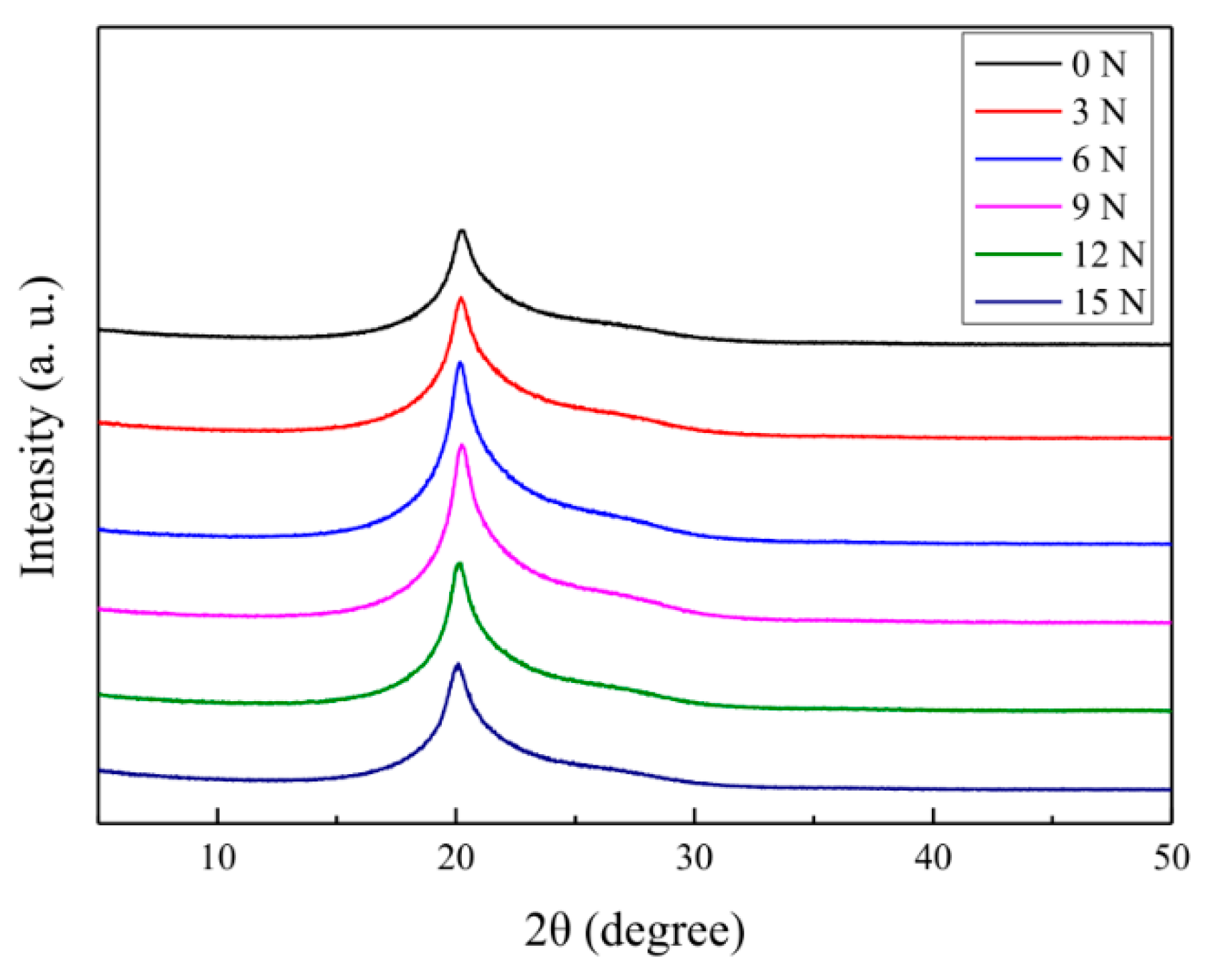
3.2. Effect of Different Stretching Conditions on Mechanical Properties of Aramid Fibers
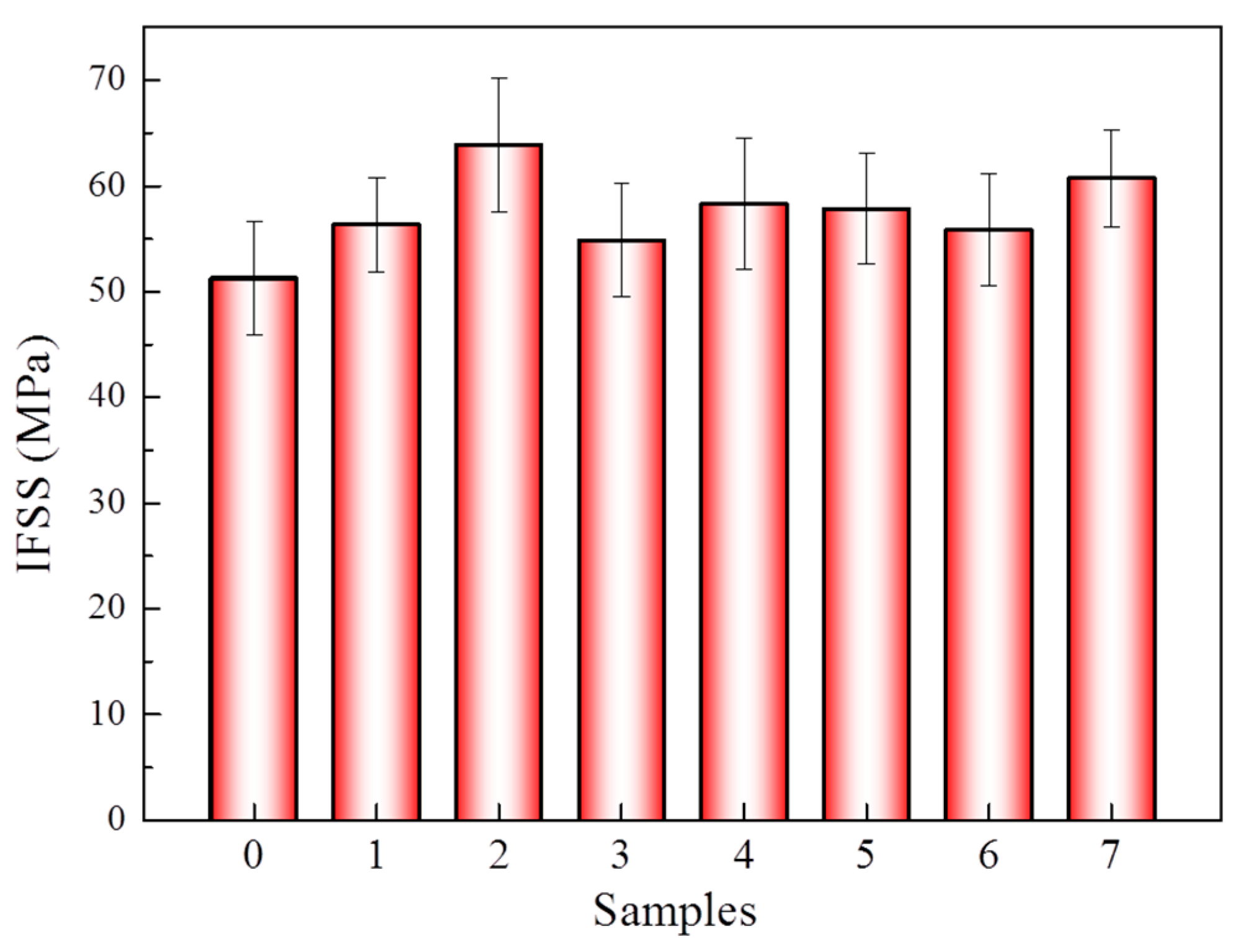
3.3. Effect of 1,4-Dichlorobutane in scCO2 on Mechanical Properties of Aramid Fibers and Aramid Fiber Enhanced Epoxy Resin Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Zong, M.; Zhang, X.; Wu, W. Surface modification mechanism of aramid fiber by AACH nanowire recasting and its enhanced modification of TPU. Mater. Res. Express 2019, 6, 075312. [Google Scholar] [CrossRef]
- Taloub, N.; Henniche, A.; Liu, L.; Li, J.; Rahoui, N.; Hegazy, M.; Huang, Y.D. Improving the mechanical properties, UV and hydrothermal aging resistance of PIPD fiber using MXene (Ti3C2(OH)2) nanosheets. Compos. Part B 2019, 163, 260–271. [Google Scholar] [CrossRef]
- Dobb, M.G.; Johnson, D.J.; Saville, B.P. Supramolecular structure of a high-modulus polyaromatic fiber (Kevlar 49). J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 2201–2211. [Google Scholar] [CrossRef]
- Morgan, R.J.; Pruneda, C.O.; Steele, W.J. The relationship between the physical structure and the microscopic deformation and failure processes of poly (p-phenylene terephthalamide) fibers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 1757–1783. [Google Scholar] [CrossRef]
- Roenbeck, M.R.; Sandoz-Rosado, E.J.; Cline, J.; Wu, V.; Moy, P.; Afshari, M.; Reichert, D.; Lustig, S.R.; Strawhecker, K.E. Probing the internal structures of Kevlar fibers and their impacts on mechanical performance. Polymer 2017, 128, 200–210. [Google Scholar] [CrossRef]
- Roenbeck, M.R.; Cline, J.; Wu, V.; Afshari, M.; Kellner, S.; Martin, P.; Londono, J.D.; Clinger, L.E.; Reichert, D.; Lustig, S.R.; et al. Structure–Property relationships of aramid fibers via X-ray scattering and atomic force microscopy. J. Mater. Sci. 2019, 54, 6668–6683. [Google Scholar] [CrossRef]
- Kanerva, M.; Korkiakoski, S.; Lahtonen, K.; Jokinen, J.; Sarlin, E.; Palola, S.; Iyer, A.; Laurikainen, P.; Liu, X.W.; Raappana, M.; et al. DLC-treated aramid-fibre composites: Tailoring nanoscale-coating for macroscale performance. Compos. Sci. Technol. 2019, 171, 62–69. [Google Scholar] [CrossRef]
- Qiu, M.; Miao, Y.W.; Li, Y.C.; Lu, J.J. Influence of ultrasonic modified liners on the adhesive and tribological performances of self-lubricating radial spherical plain bearings. Tribol. Int. 2016, 59, 655–662. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.X.; Yu, J.R.; Wang, Y.; Zhu, J.; Hu, Z.M. Cu (II) coordination modification of aramid fiber and effect on interfacial adhesion of composites. High Perform. Polym. 2019, 31, 1054–1061. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Li, H.; Li, W.; Wang, B.C.; Zhang, C.S.; Ren, N. Surface characteristic of poly(p-phenylene terephthalamide) fibers with oxygen plasma treatment. Surf. Interface Anal. 2008, 40, 1299–1303. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huang, Y.D.; Liu, L.; Cai, K.L. Effects of γ–ray radiation grafting on aramid fibers and its composites. Appl. Surf. Sci. 2008, 254, 3153–3161. [Google Scholar] [CrossRef]
- Xu, L.; Hu, J.T.; Ma, H.J.; Wu, G.Z. Electron-beam-induced post-grafting polymerization of acrylic acid onto the surface of Kevlar fibers. Radiat. Phys. Chem. 2018, 145, 74–79. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Zhao, Y.S.; Su, Z.P.; Zhang, M.Y.; Yang, B. The effect of phosphoric acid functionalization of para-aramid fiber on the mechanical property of para-aramid sheet. J. Eng. Fibers Fabr. 2018, 13, 14–22. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.H. Effect of surface treatment on the mechanical and tribological performance of kevlar pulp reinforced epoxy composites. Tribol. Lett. 2006, 24, 195–199. [Google Scholar] [CrossRef]
- Dunnigan, J.; Nadeau, D.; Paradis, D. Cyto-toxic effects of aramid fibers on rat pulmonary macrophages-comparison with chrysotile asbestos. Toxicol. Lett. 1984, 20, 277–282. [Google Scholar] [CrossRef]
- Dong, Z.X.; Liu, Z.M.; Han, B.X.; Pei, X.W.; Liu, L.L.; Yang, G.Y. Modification of isotactic polypropylene films by grafting methyl acrylate using supercritical CO2 as a swelling agent. J. Supercrit. Fluids 2004, 31, 67–74. [Google Scholar] [CrossRef]
- Muth, O.; Hirth, T.; Vogel, H. Polymer modification by supercritical impregnation. J. Supercrit. Fluids 2000, 17, 65–72. [Google Scholar] [CrossRef]
- Gooijer, J.M.; Ellmann, J.; Moller, M.; Koning, C.E. End group modification of polyamide-6 in supercritical and subcritical fluids: Part 3: Amine end group modification with diketene and diketene acetone adduct in CO2. J. Supercrit. Fluids 2004, 31, 75–87. [Google Scholar] [CrossRef]
- Qin, M.L.; Kong, H.J.; Yu, M.H. Improved Adhesion Performances of Aramid Fibers with Vinyl Epoxy via Supercritical Carbon Dioxide Modification. In Proceedings of the Global Conference on Polymer and Composite Materials, Guangzhou, China, 23–25 May 2017. [Google Scholar]
- Zheng, H.; Zhang, J.; Du, B. Effect of treatment pressure on structures and properties of PMIA fiber in supercritical carbon dioxide fluid. J. Appl. Polym. Sci. 2015, 132, 41756. [Google Scholar] [CrossRef]
- Kanbargi, N.; Lesser, A.J. Improving adhesion between aramid fibers and natural rubber through morphological and synthetic modification of the fibers. Appl. Polym. Sci. 2017, 135, 45520. [Google Scholar] [CrossRef]
- Kong, H.; Teng, C.; Liu, X. Simultaneously improving the tensile strength and modulus of aramid fiber by enhancing amorphous phase in supercritical carbon dioxide. RSC Adv. 2014, 4, 20599–20604. [Google Scholar] [CrossRef]
- Kong, H.J.; Yang, P.; Teng, C.Q.; Yu, M.H. Surface modification of poly(p-phenylene terephthalamide) fibers with HDI assisted by supercritical carbon dioxide. RSC Adv. 2015, 5, 58916–58920. [Google Scholar]

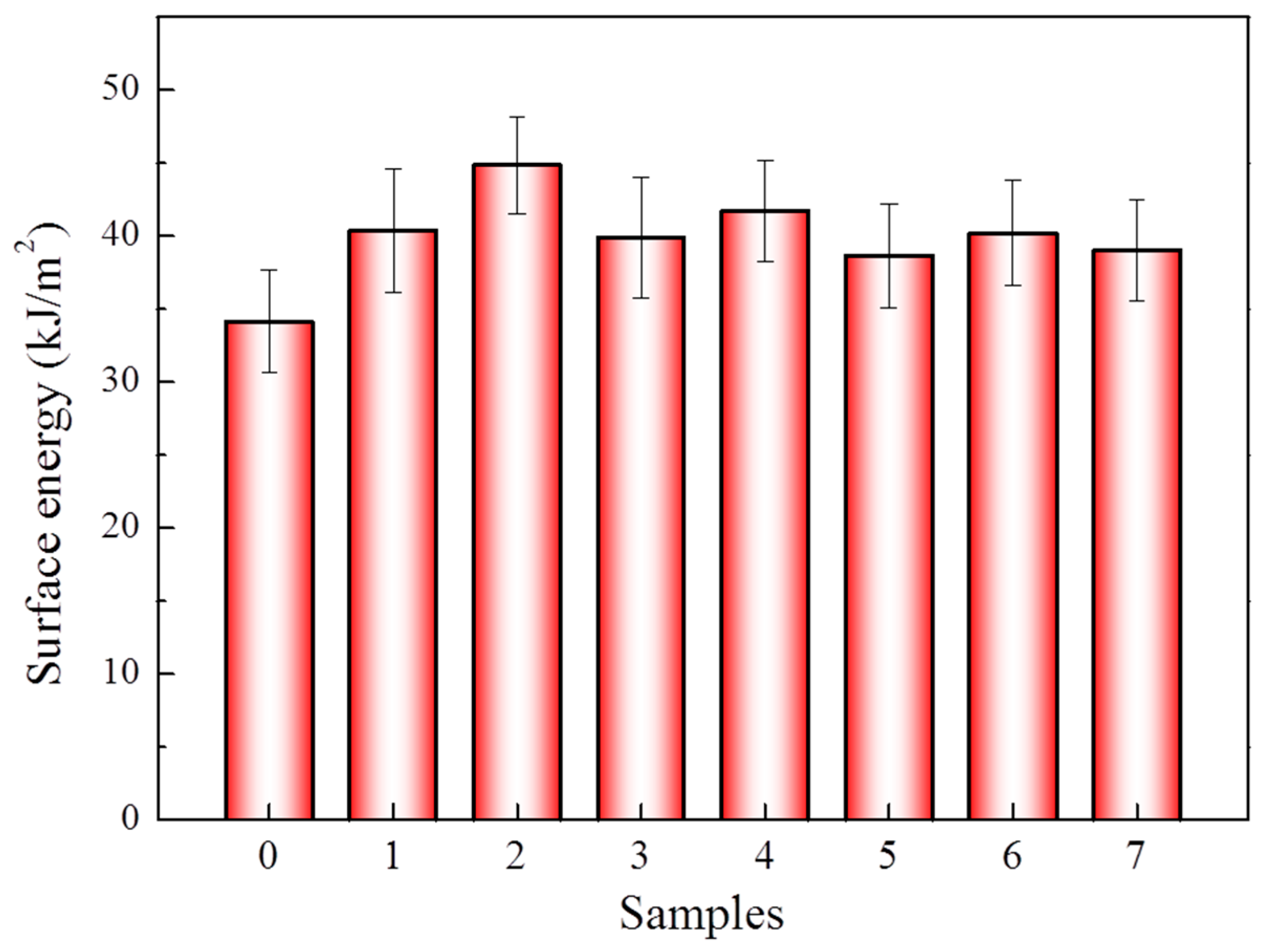
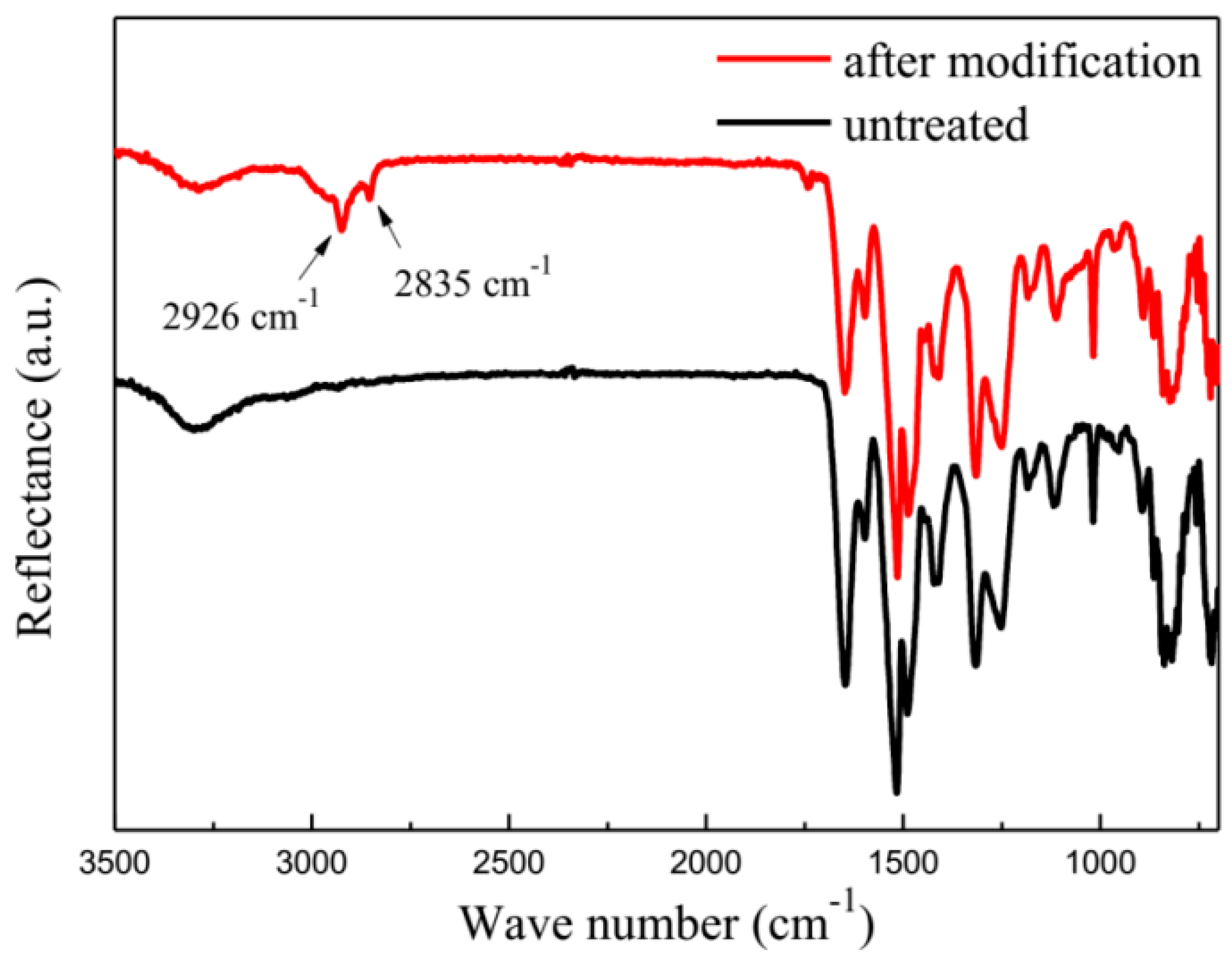
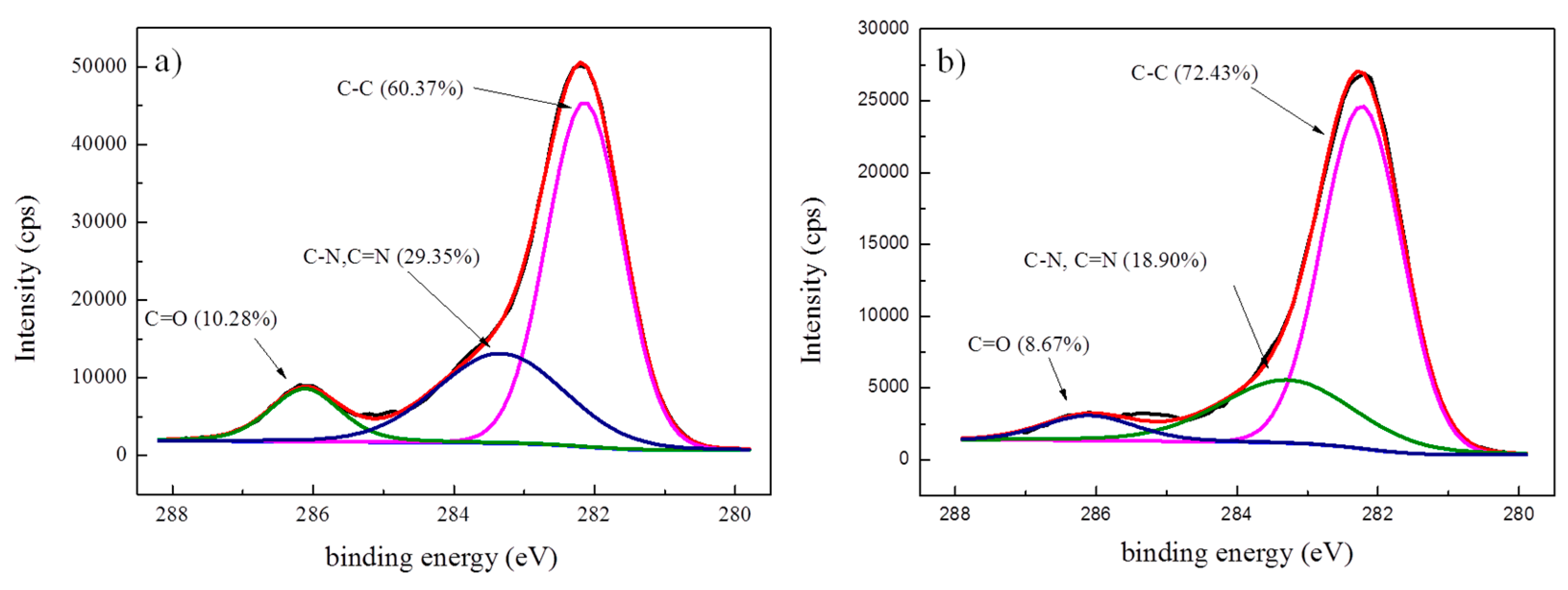
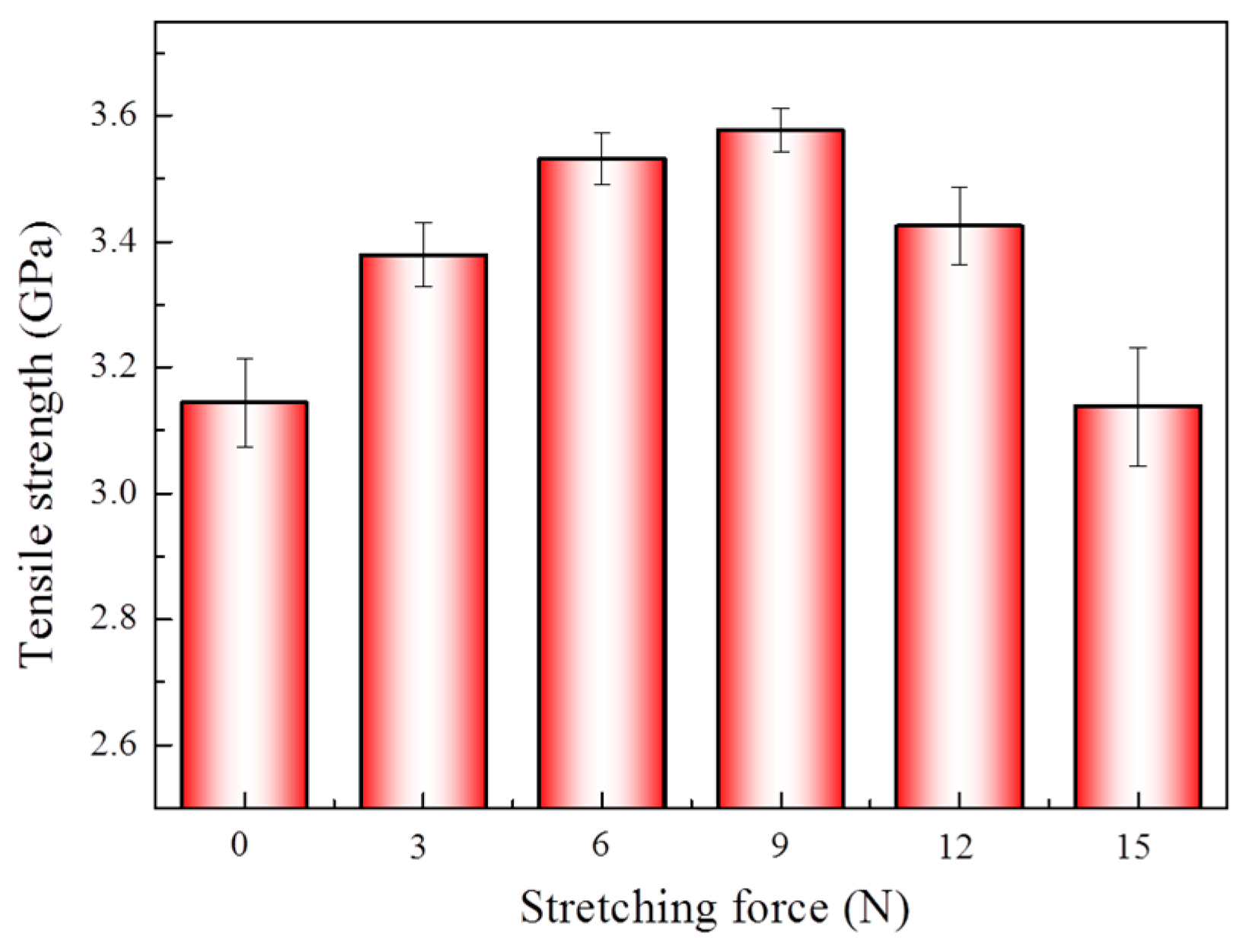


| Samples | Pressure (MPa) | Time (min) | Temperature (°C) |
|---|---|---|---|
| 1 | 9.0 | 90 | 40 |
| 2 | 9.0 | 90 | 60 |
| 3 | 9.0 | 90 | 80 |
| 4 | 7.5 | 90 | 60 |
| 5 | 10.0 | 90 | 60 |
| 6 | 9.0 | 40 | 60 |
| 7 | 9.0 | 60 | 60 |
| Classification | C–C | C–N, C=N | C=O |
|---|---|---|---|
| Untreated fibers | 60.37% | 29.35% | 10.28% |
| Modified fibers | 72.43% | 18.90% | 8.67% |
| Ion etched fibers | 64.72% | 26.45% | 8.83% |
| Classification | C/O (%) | Cl (%) |
|---|---|---|
| Untreated fibers | 4.80 | 0.04 |
| Modified fibers | 28.81 | 1.93 |
| Ion etched fibers | 25.27 | 1.45 |
| Stretching Force (N) | Crystallinity (%) | Interplanar Spacing (nm) | Grain Size (nm) |
|---|---|---|---|
| 0 | 80.71 | 4.394 | 6.622 |
| 3 | 80.93 | 4.383 | 6.758 |
| 6 | 81.43 | 4.357 | 6.846 |
| 9 | 84.52 | 4.346 | 6.971 |
| 12 | 83.68 | 4.356 | 6.912 |
| 15 | 83.07 | 4.362 | 6.882 |
| Stretching Force (N) | Crystallinity (%) | Interplanar Spacing (nm) | Grain Size (nm) |
|---|---|---|---|
| 0 | 80.71 | 4.394 | 6.622 |
| 3 | 80.87 | 4.372 | 6.788 |
| 6 | 82.01 | 4.355 | 6.899 |
| 9 | 84.49 | 4.344 | 7.012 |
| 12 | 83.60 | 4.349 | 6.935 |
| 15 | 82.97 | 4.365 | 6.901 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, C.; Yuan, C.; Ma, Z.; Du, Y.; Liu, L.; Huang, Y. Improving the Mechanical and Surface Properties of Aramid Fiber by Grafting with 1,4-Dichlorobutane under Supercritical Carbon Dioxide. Materials 2019, 12, 3766. https://doi.org/10.3390/ma12223766
Jia C, Yuan C, Ma Z, Du Y, Liu L, Huang Y. Improving the Mechanical and Surface Properties of Aramid Fiber by Grafting with 1,4-Dichlorobutane under Supercritical Carbon Dioxide. Materials. 2019; 12(22):3766. https://doi.org/10.3390/ma12223766
Chicago/Turabian StyleJia, Chuyuan, Chengce Yuan, Zhenyu Ma, Yunzhe Du, Li Liu, and Yudong Huang. 2019. "Improving the Mechanical and Surface Properties of Aramid Fiber by Grafting with 1,4-Dichlorobutane under Supercritical Carbon Dioxide" Materials 12, no. 22: 3766. https://doi.org/10.3390/ma12223766
APA StyleJia, C., Yuan, C., Ma, Z., Du, Y., Liu, L., & Huang, Y. (2019). Improving the Mechanical and Surface Properties of Aramid Fiber by Grafting with 1,4-Dichlorobutane under Supercritical Carbon Dioxide. Materials, 12(22), 3766. https://doi.org/10.3390/ma12223766




