CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis
2.2.2. Characterization
2.2.3. Photocatalytic Hydrogen Evolution
3. Results and Discussion
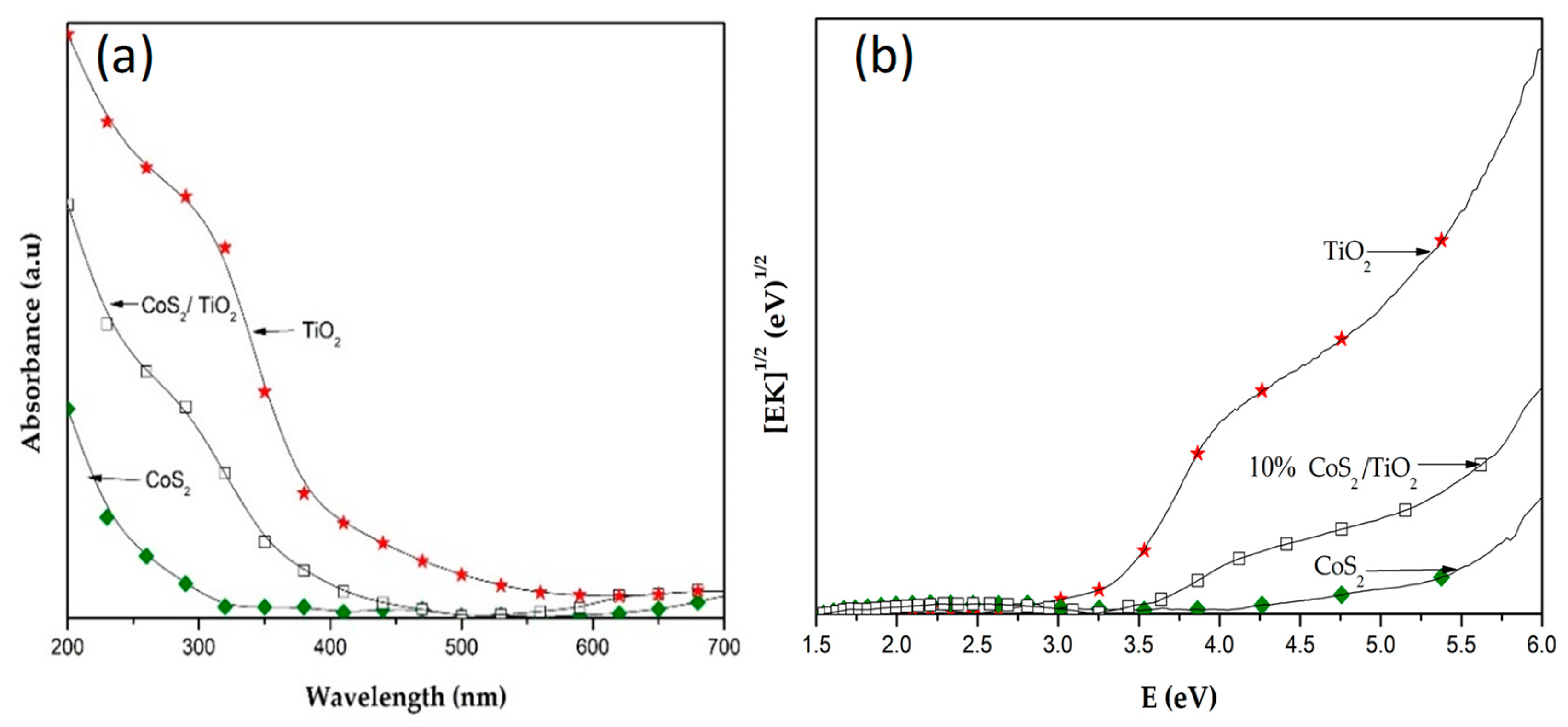
3.1. Characterization of Materials
3.2. Hydrogen Evolution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; Mckone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Yin, Y.; Jin, Z.; Hou, F. Enhanced solar water-splitting efficiency using core/sheath heterostructure CdS/TiO2 nanotube arrays. Nanotechnology 2007, 18, 495608. [Google Scholar] [CrossRef]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Matthews, R.W. Photooxidative degradation of coloured organics in water using supported catalysts. TiO2 on sand. Water Res. 1991, 25, 1169–1176. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Han, J.; Ma, G.; Yan, H.; Wu, G.; Li, C. Photocatalytic H2 evolution on CdS loaded with WS2 as cocatalyst under visible light irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chang, Y.H.; Hsu, C.L.; Wei, K.H.; Chiang, C.Y.; Li, L.J. Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int. J. Hydrog. Energy 2013, 38, 12302–12309. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Guo, Z.; Liu, H. Synthesis of tungsten disulfide (WS2) nanoflakes for lithium ion battery application. Electrochem. Commun. 2007, 9, 119–122. [Google Scholar] [CrossRef]
- Pirashanthan, A.; Murugathas, T.; Robertson, N.; Ravirajan, P.; Velauthapillai, D. A Quarterthiophene-Based Dye as an Efficient. Polymers 2019, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Photocatalytic degradation of diclofenac using TiO2–SnO2 mixed oxide catalysts. Environ. Technol. (UK) 2019, 40, 929–941. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, D.W.; Kim, J.; Kim, Y.G.; Lee, J.S. Highly donor-doped (110) layered perovskite materials as novel photocatalysts for overall water splitting. Chem. Commun. 1999, 2, 1077–1078. [Google Scholar] [CrossRef]
- Kanazawa, T.; Nozawa, S.; Lu, D.; Maeda, K. Structure and Photocatalytic Activity of PdCrOx Cocatalyst on SrTiO3 for Overall Water Splitting. Catalysts 2019, 9, 59. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, H.; Ji, Z.; Zhong, J.; Ding, M.; Chen, D.; Li, Y.; Tu, W.; Cao, D.; Yu, Z.; et al. Enhanced visible-light-induced hydrogen evolution from water in a noble-metal-free system catalyzed by ZnTCPP-MoSTiO2 assembly. Chem. Eng. J. 2015, 275, 8–16. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Shao-horn, Y.; Sheng, W.; Gasteiger, H.A.; Shao-horn, Y. Hydrogen Oxidation and Evolution Reaction Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar]
- Greeley, J.; Nørskov, J.K.; Kibler, L.A.; El-aziz, A.M.; Kolb, D.M. Hydrogen Evolution Over Bimetallic Systems: Understanding the Trends. Chem. Phys. Chem. 2006, 7, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, S. Hydrogen Overvoltage on Bright Platinum. J. Electrochem. Soc. 1952, 99, 488–494. [Google Scholar] [CrossRef]
- Hamidi, F. TiO2 -based Photocatalytic Cementitious Composites: Materials, Properties, Influential Parameters, and Assessment Techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.; Sun, H.; Ng, Y.H.; Zhang, K.Q.; Lai, Y. MoS2 Quantum Dots@TiO2 Nanotube Arrays: An Extended-Spectrum-Driven Photocatalyst for Solar Hydrogen Evolution. Chem. Sus. Chem. 2018, 11, 1708–1721. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, W.; Xiao, X. Preparation of titanium dioxide/tungsten disulfide composite photocatalysts with enhanced photocatalytic activity under visible light. Korean J. Chem. Eng. 2016, 33, 107–113. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.; Chen, P.; Wang, Y. Microwave-assisted solvothermal synthesis of 3D carnation-like SnS 2 nanostructures with high visible light photocatalytic activity. J. Mol. Catal. A Chem. 2013, 378, 285–292. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Moeinirad, S. Heterogeneous photocatalytic degradation of furfural using NiS-clinoptilolite zeolite. Desalination 2011, 273, 248–257. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Torres-Martínez, L.M.; Moctezuma, E.; Singh, A.P.; Wickman, B. Green synthesis of earth-abundant metal sulfides (FeS2, CuS, and NiS2) and their use as visible-light active photocatalysts for H2 generation and dye removal. J. Mater. Sci. Mater. Electron. 2018, 29, 11613–11626. [Google Scholar] [CrossRef]
- Fang, W.; Liu, D.; Lu, Q.; Sun, X.; Asiri, A.M. Nickel promoted cobalt disulfide nanowire array supported on carbon cloth: An efficient and stable bifunctional electrocatalyst for full water splitting. Electrochem. Commun. 2016, 63, 60–64. [Google Scholar] [CrossRef]
- Desheng, K.; Cha, J.J.; Wang, H.-T.; Hye, R.L.; Yi, C. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar]
- Susac, D.; Zhu, L.; Teo, M.; Sode, A.; Wong, K.C.; Wong, P.C.; Parsons, R.R.; Bizzotto, D.; Mitchell, K.A.R.; Campbell, S.A. Characterization of FeS2 -Based Thin Films as Model Catalysts for the Oxygen Reduction Reaction. J. Phys. Chem. C 2007, 111, 18715–18723. [Google Scholar] [CrossRef]
- Feng, Y.; He, T.; Alonso-vante, N. Electrochimica Acta Oxygen reduction reaction on carbon-supported CoSe2 nanoparticles in an acidic medium. Electrochim. Acta 2009, 54, 5252–5256. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Khan, Z.; Chetia, T.R.; Vardhaman, A.K.; Barpuzary, D.; Sastri, C.V.; Qureshi, M. Visible light assisted photocatalytic hydrogen generation and organic dye degradation by CdS-metal oxide hybrids in presence of graphene oxide. RSC Adv. 2012, 2, 12122–12128. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Yan, C.; Xue, X.; Zhang, W.; Li, X.; Liu, J.; Yang, S.; Hu, Y.; Chen, R.; Yan, Y.; Zhu, G.; et al. Well-designed Te/SnS2/Ag artificial nanoleaves for enabling and enhancing visible-light driven overall splitting of pure water. Nano Energy 2017, 39, 539–545. [Google Scholar] [CrossRef]
- Yuan, Y.P.; Cao, S.W.; Yin, L.S.; Xu, L.; Xue, C. NiS2 Co-catalyst decoration on CdLa2S4 nanocrystals for efficient photocatalytic hydrogen generation under visible light irradiation. Int. J. Hydrog. Energy 2013, 38, 7218–7223. [Google Scholar] [CrossRef]
- Chen, W.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-Evolution Catalysts Based on Non-Nobel Metal Nickel–Molybdenum Nitride Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 6131–6135. [Google Scholar] [CrossRef]
- Chen, W.-F.; Wang, C.-H.; Sasaki, K.; Marinkovic, N.; Xu, W.-Q.; Muckerman, J.; Zhu, Y.-M.; Adzic, R.R. Nanostructured Molybdenum Carbide as Pt-free Catalysts for Hydrogen Evolution. Electrochem. Soc. 2012, 1817. Available online: http://ma.ecsdl.org/content/MA2012-02/14/1817.short (accessed on 22 October 2019).
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 12703–12706. [Google Scholar] [CrossRef]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.J.; Zheng, G.; Yan, K.; Liu, N. MoSe2 and WSe2 Nano fi lms with Vertically Aligned Molecular Layers on Curved and Rough Surfaces. Nano Lett. 2013, 13, 3426–3433. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef]
- Merki, D.; Vrubel, H.; Rovelli, L.; Fierro, S.; Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 2515–2525. [Google Scholar] [CrossRef]
- Environ, E. Hydrogen evolution catalyzed by MoS3 and MoS2 particle. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef]
- Wu, L.; Dzade, N.Y.; Yu, M.; Mezari, B.; van Hoof, A.J.F.; Friedrich, H.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. Unraveling the Role of Lithium in Enhancing the Hydrogen Evolution Activity of MoS2: Intercalation versus Adsorption. ACS Energy Lett. 2019, 4, 1733–1740. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Sun, C.; Xi, P.; Peng, S.; Gao, D.; Xue, D. Accelerated Hydrogen Evolution Reaction in CoS2 by Transition-Metal Doping. ACS Energy Lett. 2018, 3, 779–786. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, W.; Zhang, F.; Zhang, Z.; Tang, B.; Li, J.; Wang, X. Theoretical Expectation and Experimental Implementation of in Situ Al-Doped CoS2 Nanowires on Dealloying-Derived Nanoporous Intermetallic Substrate as an Efficient Electrocatalyst for Boosting Hydrogen Production. ACS Catal. 2019, 9, 1489–1502. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Peng, S. Tunable photodeposition of MoS2 onto a composite of reduced graphene oxide and CdS for synergic photocatalytic hydrogen generation. J. Phys. Chem. C 2014, 118, 19842–19848. [Google Scholar] [CrossRef]
- Xing, J.; Li, Y.H.; Jiang, H.B.; Wang, Y.; Yang, H.G. The size and valence state effect of Pt on photocatalytic H2 evolution over platinized TiO2 photocatalyst. Int. J. Hydrog. Energy 2014, 39, 1237–1242. [Google Scholar] [CrossRef]
- Raut, P.; Li, S.; Chen, Y.M.; Zhu, Y.; Jana, S.C. Strong and Flexible Composite Solid Polymer Electrolyte Membranes for Li-Ion Batteries. ACS Omega 2019, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, H.; Zhang, Y.; Zheng, Y.; Xu, Z.; Liu, R. Structure and luminescent properties of electrodeposited Eu3 +-doped CaF2 thin films. Thin Solid Film. 2014, 562, 478–484. [Google Scholar] [CrossRef]
- Liu, E.; Chen, J.; Ma, Y.; Feng, J.; Jia, J.; Fan, J.; Hu, X. Fabrication of 2D SnS2 /g-C3N4 heterojunction with enhanced H2 evolution during photocatalytic water splitting. J. Colloid Interface Sci. 2018, 524, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, C.Y.; Ma, F.X.; Hu, S.P.; Zhang, Y.W.; Zhen, L. Monodisperse SnS2 nanosheets for high-performance photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 22370–22377. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Min, S.; Lu, G. Dye-sensitized NiSx catalyst decorated on graphene for highly efficient reduction of water to hydrogen under visible light irradiation. ACS Catal. 2014, 4, 2763–2769. [Google Scholar] [CrossRef]


| Material | Synthesis Method | Rate of Hydrogen Evolution | Sacrificial Agent | Reference | |
|---|---|---|---|---|---|
| 2D SnS2/g-C3N4 (5 wt.% SnS2/g-C3N4) | Hydrothermal method | 0.97 mmol h−1 g−1 | 10 vol% TEOA and 3 wt.% H2Pt2Cl6·6H2O | Enzhou Liu et al., 2018 | [53] |
| Te/SnS2/Ag | Hydrothermal method | 0.33 mmol h−1 | - | Changzeng Yan et al., 2017 | [36] |
| SnS2 Nanosheets | Solvothermal | 1.06 mmol h−1 g−1 | 0.1 M Na2S 0.1M Na2S2O3 | Jing yu et al., 2014 | [54] |
| CdS/ WS2 | Impregnation-sulfidation | 0.42 mmol h−1 | Latic acid solution | Zong et al., 2011 | [11] |
| Dye-Sensitized NiSx/ graphene (in EY/G) | Insitu chemical deposition method | 0.04 mmol h−1 | - | Chao Kong et al., 2014 | [55] |
| Dye-Sensitized NiSx/ graphene (in EY/NiSx/G) | Insitu chemical deposition method | 0.34 mmol h−1 | - | Chao Kong et al., 2014 | [55] |
| MoS2/ RGO and CdS (pH11-MoS2/rGO 1.5/CdS) | Photoreduction method | 0.10 mmol h−1 | 10 vol.% Latic acid solution | Yuexiang Li et al., 2014 | [49] |
| MoS2/Graphene | Hydrothermal | 1.80 mmol h−1 | Na2S-Na2S2O3 solution | Chang et al., 2014 | [35] |
| MoS2 quantum dots/TiO2 nanotube arrays | Electrodeposition | 0.07 mmol cm−2 h−1 0.05 mmol cm−2 h−1 0.02 mmoL cm−2 h−1 | - | Qun Wang et al., 2018 | [24] |
| ZnTCPP-MoS2 /TiO2 (1.00 wt.% MoS2 on TiO2) | Hydrothermal | 0.10 mmol h−1 | 0.2 M triethanolamine (TEOA) aqueous | Youngjun Yuan et al., 2015 | [19] |
| 10 wt.% CoS2/TiO2 | Hydrothermal | 2.55 mmol g−1 | Methanol | This work | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugaratnam, S.; Velauthapillai, D.; Ravirajan, P.; Christy, A.A.; Shivatharsiny, Y. CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation. Materials 2019, 12, 3882. https://doi.org/10.3390/ma12233882
Shanmugaratnam S, Velauthapillai D, Ravirajan P, Christy AA, Shivatharsiny Y. CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation. Materials. 2019; 12(23):3882. https://doi.org/10.3390/ma12233882
Chicago/Turabian StyleShanmugaratnam, Sivagowri, Dhayalan Velauthapillai, Punniamoorthy Ravirajan, Alfred Antony Christy, and Yohi Shivatharsiny. 2019. "CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation" Materials 12, no. 23: 3882. https://doi.org/10.3390/ma12233882
APA StyleShanmugaratnam, S., Velauthapillai, D., Ravirajan, P., Christy, A. A., & Shivatharsiny, Y. (2019). CoS2/TiO2 Nanocomposites for Hydrogen Production under UV Irradiation. Materials, 12(23), 3882. https://doi.org/10.3390/ma12233882








