Using Solubility Parameters to Model More Environmentally Friendly Solvent Blends for Organic Solar Cell Active Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Tests of N2200 and TQ1—Determining the HSP by Using the HSPiP Program
2.3. Active Layer Morphology Determined by AFM
3. Results and Discussion
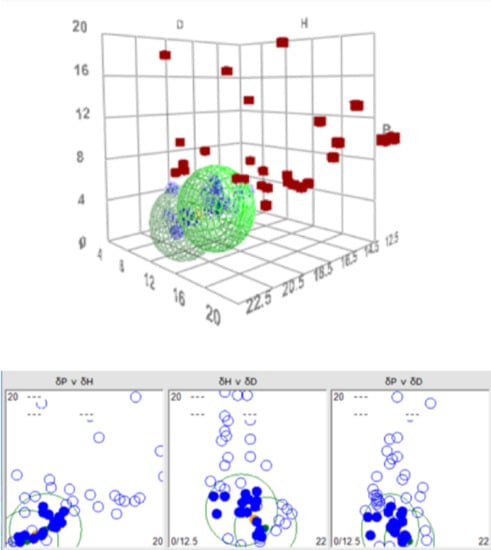
3.1. HSP for N2200 and TQ1-N2200 Double Sphere
3.2. Alternative Solvent Blends for TQ1 and N2200
3.3. TQ1 and N2200 Film Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Pan, X.; Bjuggren, J.M.; Gedefaw, D.; Xu, X.; Kroon, R.; Wang, E.; Campbell, J.A.; Lewis, D.A.; Andersson, M.R. Probing the Relationship between Molecular structures, Thermal Transitions, and Morphology in Polymer Semiconductors Using a Woven Glass-Mesh-Based DMTA Technique. Chem. Mater. 2019, 31, 6740–6749. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Li, D.; Xiao, Z.; Chen, Y.; Hua, Y.; Yang, S.; Ding, L. A Wide-Band Gap Copolymer Donor for Efficient Fullerene-Free Solar Cells. ACS Omega 2019, 4, 14800–14804. [Google Scholar] [CrossRef] [PubMed]
- Uranbileg, N.; Gao, C.; Yang, C.; Bao, X.; Han, L.; Yang, R. Amorphous electron donros with controllable morphology for non-fullerene polymer solar cells. J. Mater. Chem. C 2019, 7, 10881–10890. [Google Scholar] [CrossRef]
- Holmes, N.P.; Munday, H.; Barr, M.G.; Thomsen, L.; Marcus, M.A.; Kilcoyne, A.L.D.; Fahy, A.; van Stam, J.; Dastoor, P.C.; Moons, E. Unravelling donor-acceptor film morphology formation for environmentally-friendly OPV ink formulations. Green Chem. 2019, 21, 5090–5103. [Google Scholar] [CrossRef]
- Lindqvist, C.; Moons, E.; van Stam, J. Fullerene Aggregation in Thin Films of Polymer Blends for Solar Cell Applications. Materials 2018, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Cooling, N.A.; Barnes, E.F.; Almyahi, F.; Feron, K.; Al-Mudhafer, M.F.; Al-Ahmad, A.; Vaughan, B.; Andersen, T.R.; Griffith, M.J.; Hart, A.S.; et al. A low-cost mixed fullerene acceptor blend for printed electronics. J. Mater. Chem. A 2016, 4, 10274–10281. [Google Scholar] [CrossRef]
- Blazinic, V.; Ericsson, L.K.E.; Muntean, S.A.; Moons, E. Photo-degradation in air of spin-coated PC60BM and PC70BM films. Synth. Met. 2018, 241, 26–30. [Google Scholar] [CrossRef]
- Morvillo, P.; Bobeico, E.; Esposito, S.; Diana, R. Effect of the active layer thickness on the device performance of polymer solar cells having [60]PCBM and [70]PCBM as electron acceptor. Energy Procedia 2012, 31, 69–73. [Google Scholar] [CrossRef]
- Bruno, A.; Villani, F.; Grimaldi, I.A.; Loffredo, F.; Morvillo, P.; Diana, R.; Haque, S.; Minarini, C. Morphological and spectroscopic characterizations of inkjet-printed poly(3-hexylthiophene-2,5-diyl): Phenyl-C61-butyric acid methyl ester blends for organic solar cell applications. Thin Solid Film. 2014, 560, 14–19. [Google Scholar] [CrossRef]
- Morvillo, P.; Ricciardi, R.; Nenna, G.; Bobeico, E.; Diana, R.; Minarini, C. Elucidating the origin of the improved current output in inverted polymer solar cells. Sol. Energy Mater. Sol. Cells 2016, 152, 51–58. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.; Sin, D.H.; Park, J.H.; Cho, K. Recent Advances in Morphology Optimization for Organic Photovoltaics. Adv. Mater. 2018, 30, 1800453. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters. A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Machui, F.; Brabec, C.J. Solubility, Miscibility, and the Impact on Solid-State Morphology. In Semiconducting Polymer Composites: Principles, Morphologies, Properties and Applications, 1st ed.; Yang, X., Ed.; Wiley-VCH KGaA: Weinheim, Germany, 2012; pp. 1–38. [Google Scholar]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Agata, Y.; Yamamoto, H. Determination of Hansen solubility parameters of ionic liquids using double-sphere type of Hansen solubility sphere method. Chem. Phys. 2018, 513, 165–173. [Google Scholar] [CrossRef]
- Zhu, Q.-N.; Wang, Q.; Hu, Y.-B.; Abliz, X. Practical Determination of the Solubility Parameters of 1-Alkyl-3-methylimidazolium Bromide ([CnC1im]Br, n = 5, 6, 7, 8) Ionic Liquids by Inverse Gas Chromatography and the Hansen Solubility Parameter. Molecules 2019, 24, 1346. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-D.; Fleetham, T.A.; Li, J.; Vogt, B.D. High performance bulk-heterojunction organic solar cells fabricated with non-halogenated solvent processing. Org. Electron. 2011, 12, 1465–1470. [Google Scholar] [CrossRef]
- Duong, D.T.; Walker, B.; Lin, J.; Kim, C.; Love, J.; Purushothaman, B.; Anthony, J.E.; Nguyen, T.-C. Molecular Solubility and Hansen Solubility Parameters for the Analysis of Phase Separation in Bulk Heterojunctions. J. Polym. Sci. B Polym. Phys. 2012, 50, 1405–1413. [Google Scholar] [CrossRef]
- Burgués-Ceballos, I.; Machui, F.; Min, J.; Ameri, T.; Voigt, M.M.; Luponosov, Y.N.; Ponomarenko, S.A.; Lacharmoise, P.D.; Campoy-Quiles, M.; Brabec, C.J. Solubility Based Identification of Green Solvents for Small Molecule Organic Solar Cells. Adv. Funct. Mater. 2014, 24, 1449–1457. [Google Scholar] [CrossRef]
- Machui, F.; Langner, S.; Zhu, X.; Abbott, S.; Brabec, C.J. Determination of the P3HT:PCBM solubility parameters via a binary solvent gradient method: Impact of the solubility on the photovoltaic performance. Sol. Energy Mater. Sol. Cells 2012, 100, 138–146. [Google Scholar] [CrossRef]
- Hansen Solubility Parameters in Practice. Available online: https://www.hansen-solubility.com/contact.php (accessed on 1 October 2019).
- Royal Society of Chemistry. GSK Solvent Selection Guie 2009. Available online: http://www.rsc.org/suppdata/gc/c0/c0gc00918k/c0gc00918k.pdf (accessed on 1 October 2019).







| Solvent | Manufacturer | Grade | CAS |
|---|---|---|---|
| 1-Butanol | ACROS | 99% | 71-36-3 |
| 1-Methylnaphthalene | ACROS | 97% | 90-12-0 |
| 1-Octanol | Sigma–Aldrich | ≥99.5% | 111.87-5 |
| 2-Butanol | EMSURE | for analysis | 78-92-2 |
| 2-Methyl Tetrahydrofuran | Sigma–Aldrich | >99.5% | 96-47-9 |
| 2-Propanol (IPA) | VWR Chemicals | AnalaR | 67-63-0 |
| Acetone | VWR Chemicals | 100% | 67-64-1 |
| Amyl Acetate | Sigma–Aldrich | 99% | 628-63-7 |
| Benzaldehyde | Sigma–Aldrich | ≥99.5 | 100-52-7 |
| Chloroform | EMSURE | for analysis | 67-66-3 |
| Cyclohexane | Merck KGaA | ≥99.5% | 110-82-7 |
| Cyclohexanone | Sigma–Aldrich | ≥99% | 108-94-1 |
| Cyclopentyl Methyl Ether | Sigma–Aldrich | ≥99% | 5614-37-9 |
| Dimethyl Formamide (DMF) | Sigma–Aldrich | ≥99% | 68-12-02 |
| Dimethyl Sulfoxide (DMSO) | VWR Chemicals | 99% | 67-68-5 |
| Dipropyl Amine | Sigma–Aldrich | 99% | 142-84-7 |
| Dipropylene Glycol | Sigma–Aldrich | 99% | 25265-71-8 |
| Ethanol | VWR Chemicals | 96% | 64-17-5 |
| Ethyl Acetate | Sigma–Aldrich | ≥99.5% | 141-78-6 |
| Ethyl Benzene | Janssen Chimica | AnalaR Normapur | 100-41-4 |
| Formamide | Sigma–Aldrich | ≥99.5% | 75-12-07 |
| Glycerol | Sigma–Aldrich | ≥99.5% | 56-81-5 |
| Isobutyl Acetate | Sigma–Aldrich | 99% | 110-19-0 |
| Isopropyl Benzene (Cumene) | Sigma–Aldrich | 98% | 98-32-8 |
| Mesitylene | ACROS | 99% extra pure | 108-67-8 |
| Methyl Acetate | Merck KGaA | ≥99% | 79-20-9 |
| Methyl Cyclohexane | ACROS | 99% extra pure | 108-87-2 |
| n-Butyl Acetate | Sigma–Aldrich | ≥99.5% | 123-86-4 |
| N-Methyl Formamide | Sigma–Aldrich | 99% | 123-39-7 |
| o-Xylene | Alfa Aesar | 99% | 95-47-6 |
| Propylene Carbonate | Sigma–Aldrich | 99.7% | 108-32-7 |
| Propylene Glycol | ACROS | 99% | 57-55-6 |
| Tetrahydrofuran (THF) | Sigma–Aldrich | ≥99% | 109-99-9 |
| Tetrahydronaphthalene | Fischer Scientific | Lab. Reagent grade | 119-64-2 |
| Toluene | VWR Chemicals | AnalaR | 108-88-3 |
| Solvent | δD [(MPa)1/2] | δP [(MPa)1/2] | δH [(MPa)1/2] |
|---|---|---|---|
| 1-Butanol | 16.0 | 5.7 | 15.8 |
| 1-Octanol | 16.0 | 5.0 | 11.2 |
| 2-Butanol | 15.8 | 5.7 | 14.5 |
| 2-Methyl Tetrahydrofuran | 16.9 | 5.0 | 4.3 |
| 2-Propanol (IPA) | 15.8 | 6.1 | 16.4 |
| Acetone | 15.5 | 10.4 | 7.0 |
| Amyl Acetate | 15.8 | 3.3 | 6.1 |
| Cyclohexane | 16.8 | 0.0 | 0.2 |
| Cyclohexanone | 17.8 | 8.4 | 5.1 |
| Cyclopentyl Methyl Ether | 16.7 | 4.3 | 4.3 |
| Dimethyl Formamide (DMF) | 17.4 | 13.7 | 11.3 |
| Dimethyl Sulfoxide (DMSO) | 18.4 | 16.4 | 10.2 |
| Dipropyl Amine | 15.3 | 1.4 | 4.1 |
| Dipropylene Glycol | 16.5 | 10.6 | 17.7 |
| Ethanol | 15.8 | 8.8 | 19.4 |
| Ethyl Acetate | 15.8 | 5.3 | 7.2 |
| Ethyl Benzene | 17.8 | 0.6 | 1.4 |
| Formamide | 17.2 | 26.2 | 19.0 |
| Glycerol | 17.4 | 11.3 | 27.2 |
| Isobutyl Acetate | 15.1 | 3.7 | 6.3 |
| Isopropyl Benzene (Cumene) | 18.1 | 1.2 | 1.2 |
| Mesitylene | 18.0 | 0.6 | 0.6 |
| Methyl Acetate | 15.5 | 7.2 | 7.6 |
| Methyl Cyclohexane | 16.0 | 0.0 | 1.0 |
| n-Butyl Acetate | 15.8 | 3.7 | 6.3 |
| N-Methyl Formamide | 17.4 | 18.8 | 15.9 |
| o-Xylene | 18.0 | 2.6 | 2.8 |
| Propylene Carbonate | 20.0 | 18.0 | 4.1 |
| Propylene Glycol | 16.8 | 10.4 | 21.3 |
| Tetrahydrofuran (THF) | 16.8 | 5.7 | 8.0 |
| Tetrahydronaphthalene | 19.6 | 2.0 | 2.9 |
| Toluene | 18.0 | 1.4 | 2.0 |
| Solvent | δD | δP | δH | Score 1 h | Score 24 h | Score 48 h | Score 72 h | Score 96 h |
|---|---|---|---|---|---|---|---|---|
| Ethyl Benzene | 17.8 | 0.6 | 1.4 | 3 | 2 | 2 | 2 | 2 |
| Mesitylene | 18.0 | 0.6 | 0.6 | 3 | 2 | 2 | 2 | 1 |
| o-Xylene | 18.0 | 2.6 | 2.8 | 1 | 1 | 1 | 1 | 1 |
| Tetrahydrofuran (THF) | 16.8 | 5.7 | 8.0 | 4 | 3 | 3 | 3 | 3 |
| Tetrahydronaphthalene | 19.6 | 2.0 | 2.9 | 2 | 1 | 1 | 1 | 1 |
| Toluene | 18.0 | 1.4 | 2.0 | 2 | 2 | 2 | 2 | 2 |
| Test # | Solvent Blend | V% | Ra | RED | δD | δP | δH | Score | Score | Score |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/ml | mg/ml | mg/ml | ||||||||
| 1.0 | 5.0 | 10.0 | ||||||||
| A | Toluene | 62.0 | 1.2 | 0.69 | 18.6 | 1.3 | 2.0 | 1 | 1 | 1 |
| 1-Methyl Naphthalene | 38.0 | |||||||||
| B | Isopropyl Benzene (Cumene) | 72.0 | 1.1 | 0.64 | 18.5 | 2.9 | 2.3 | 3 | ||
| Benzaldehyde | 28.0 | |||||||||
| C | Tetrahydronaphthalene | 88.0 | 0.9 | 0.52 | 19.1 | 2.6 | 3.5 | 1 | 1 | 1 |
| Methyl Acetate | 12.0 | |||||||||
| D | Mesitylene | 65.0 | 1.1 | 0.68 | 18.5 | 3.0 | 2.2 | 3 | ||
| Benzaldehyde | 35.0 | |||||||||
| E | Tetrahydronaphthalene | 65.0 | 0.7 | 0.42 | 19.0 | 2.7 | 3.2 | 1 | 1 | 1 |
| o-Xylene | 35.0 | |||||||||
| F | Tetrahydronaphthalene | 77.0 | 0.6 | 0.36 | 18.4 | 2.6 | 2.0 | 1 | 1 | 1 |
| 2-Methyl Tetrahydrofuran | 23.0 | |||||||||
| G | Tetrahydronaphthalene | 85.0 | 0.6 | 0.34 | 18.9 | 2.3 | 3.4 | 2 | ||
| Isobutyl Acetate | 15.0 | |||||||||
| H | Isopropyl Benzene (Cumene) | 60.0 | 1.3 | 0.77 | 18.2 | 2.6 | 2.6 | 3 | ||
| 2-Methyl Anisole | 40.0 | |||||||||
| I | Ethyl Benzene | 53.0 | 1.6 | 0.92 | 18.0 | 2.5 | 3.0 | 3 | ||
| 2-Methyl Anisole | 47.0 | |||||||||
| J | Isopropyl Benzene (Cumene) | 72.0 | 1.6 | 0.93 | 18.0 | 2.1 | 2.8 | 2 | ||
| Anisole | 28.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalan, I.; Lundin, L.; van Stam, J. Using Solubility Parameters to Model More Environmentally Friendly Solvent Blends for Organic Solar Cell Active Layers. Materials 2019, 12, 3889. https://doi.org/10.3390/ma12233889
Jalan I, Lundin L, van Stam J. Using Solubility Parameters to Model More Environmentally Friendly Solvent Blends for Organic Solar Cell Active Layers. Materials. 2019; 12(23):3889. https://doi.org/10.3390/ma12233889
Chicago/Turabian StyleJalan, Ishita, Lisa Lundin, and Jan van Stam. 2019. "Using Solubility Parameters to Model More Environmentally Friendly Solvent Blends for Organic Solar Cell Active Layers" Materials 12, no. 23: 3889. https://doi.org/10.3390/ma12233889
APA StyleJalan, I., Lundin, L., & van Stam, J. (2019). Using Solubility Parameters to Model More Environmentally Friendly Solvent Blends for Organic Solar Cell Active Layers. Materials, 12(23), 3889. https://doi.org/10.3390/ma12233889