Use of Genetically Modified Bacteria to Repair Cracks in Concrete
Abstract
:1. Introduction
2. Bacteria Cultivation and Characterization
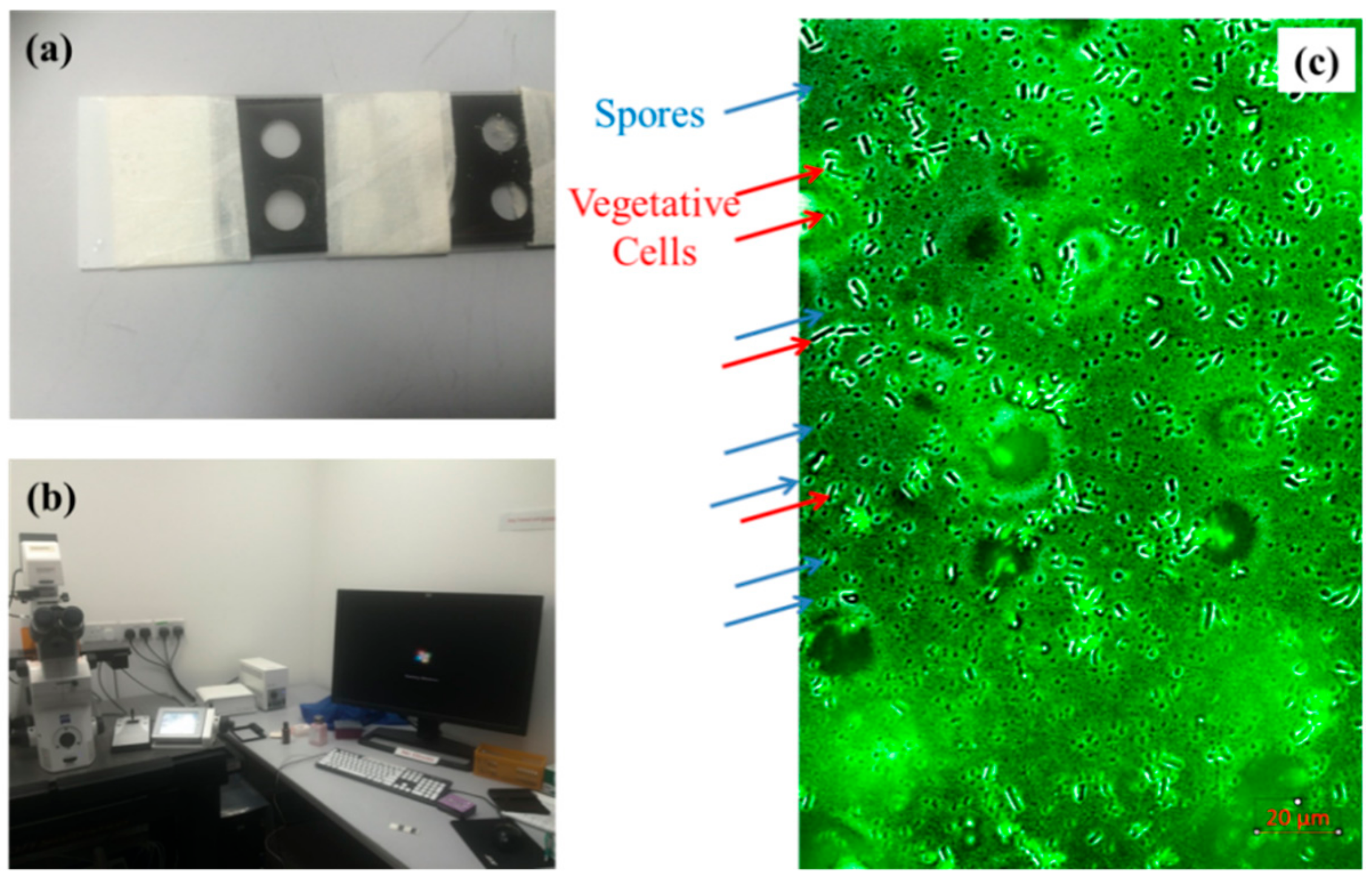
2.1. Bacterial Strains and Cultures Preparation
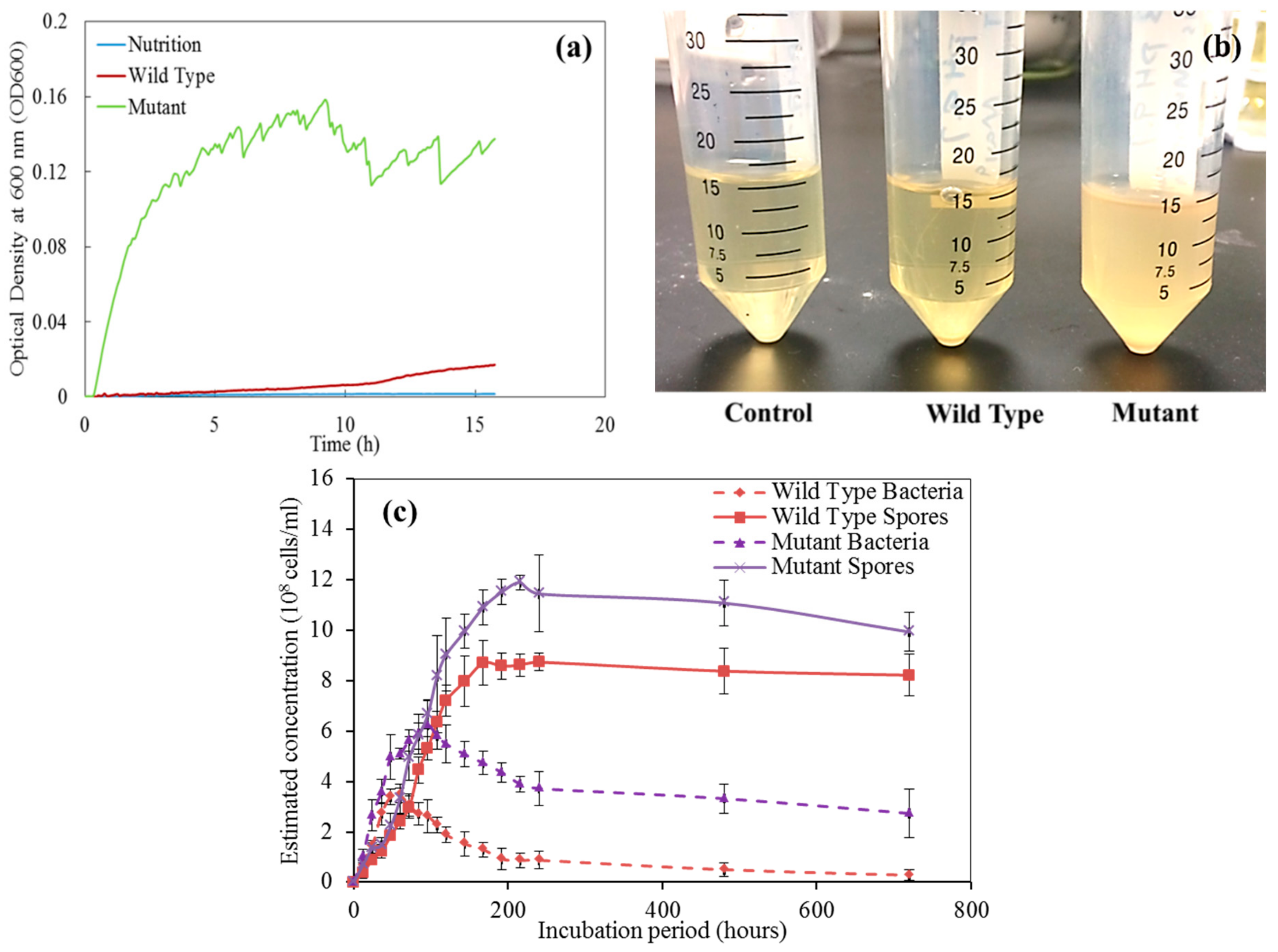
2.2. Characterization of Bacteria via the Growth Curve Measurement
3. Assessment of the Crack-Repairing Efficiency
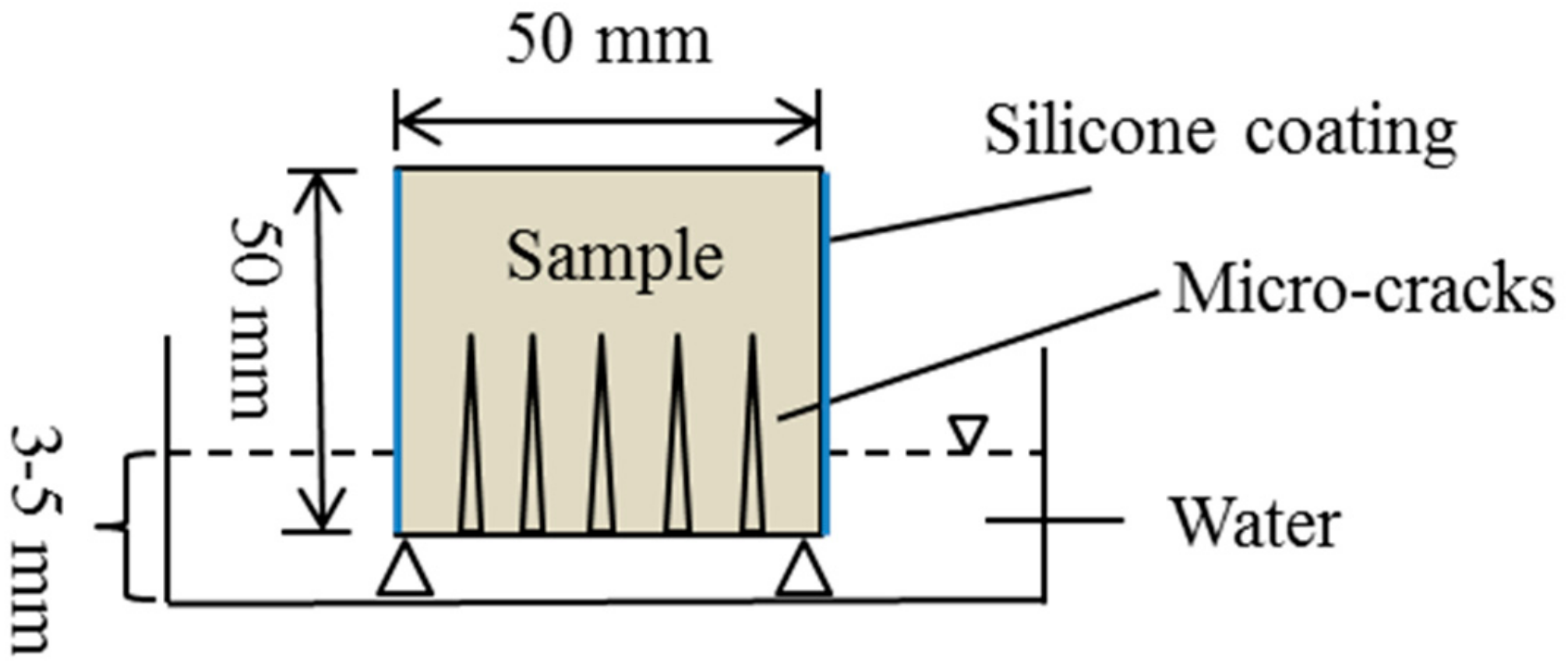
3.1. Specimen Preparation
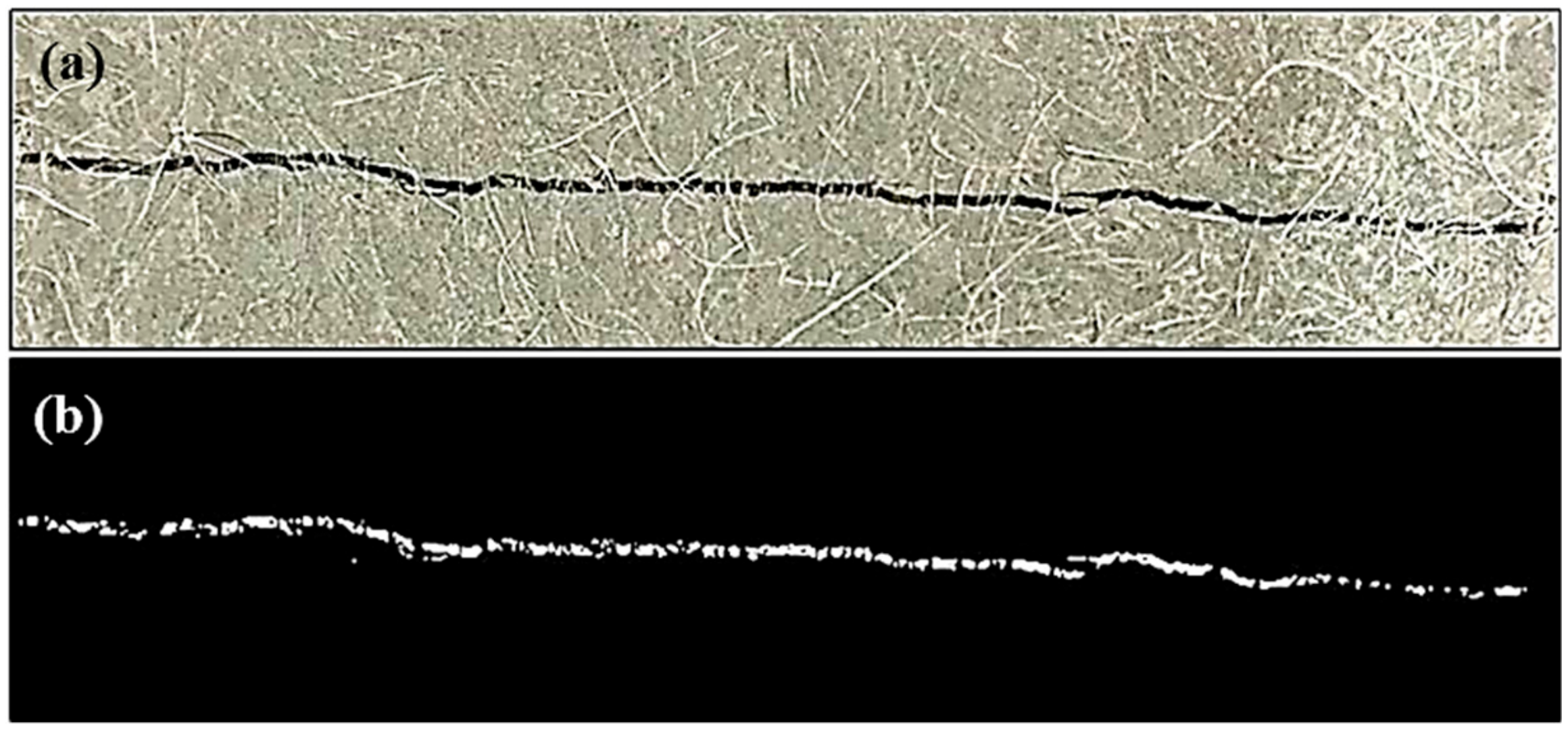
3.2. Image Analysis
3.3. X-ray CT Scanning
3.4. Sorptivity Test
4. Results and Discussion
4.1. The Growth of Bacteria
4.2. Crack Sealing Estimation by DIP Technique
4.3. Crack Sealing Phenomenon Observed by X-CT Scanning
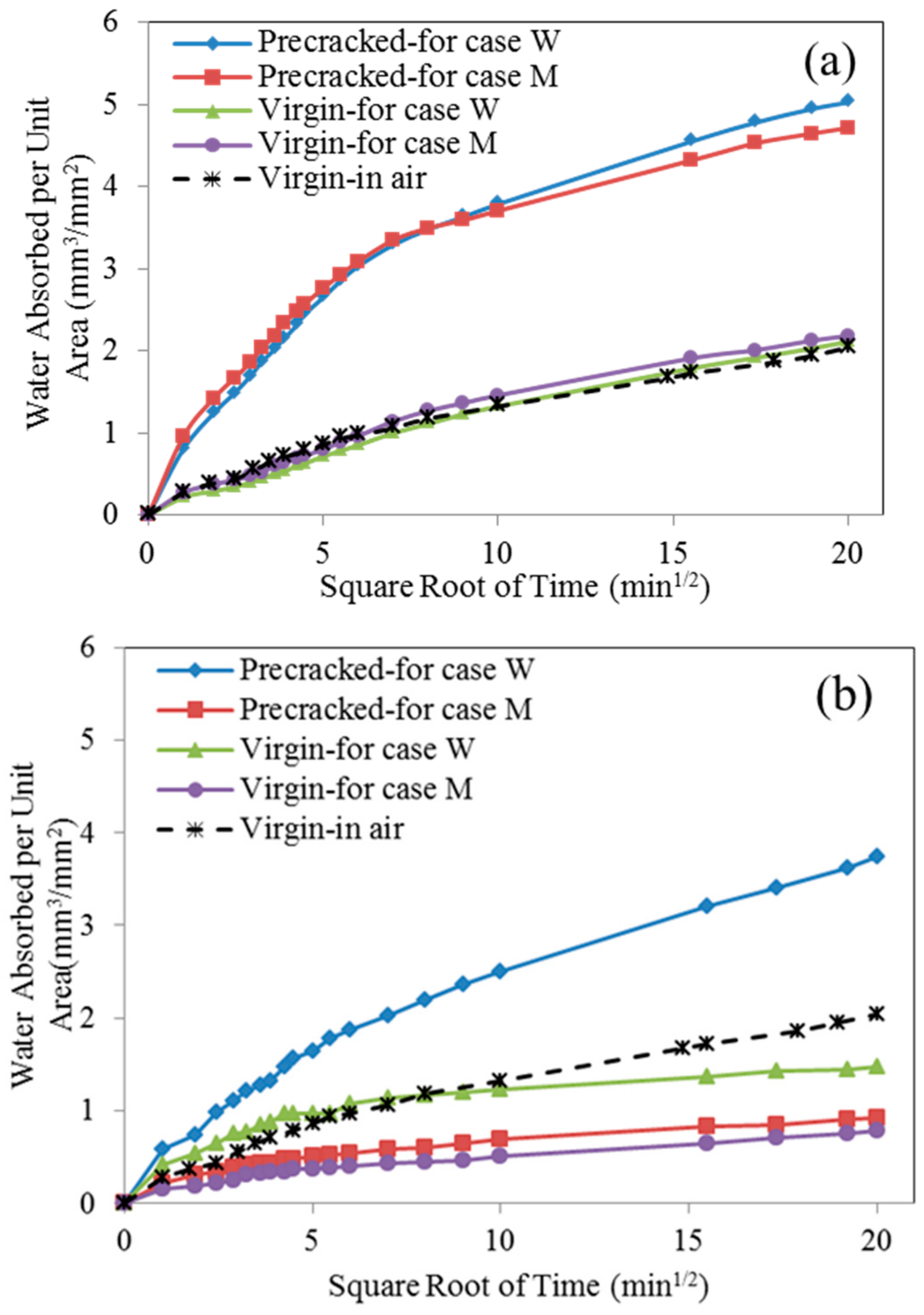
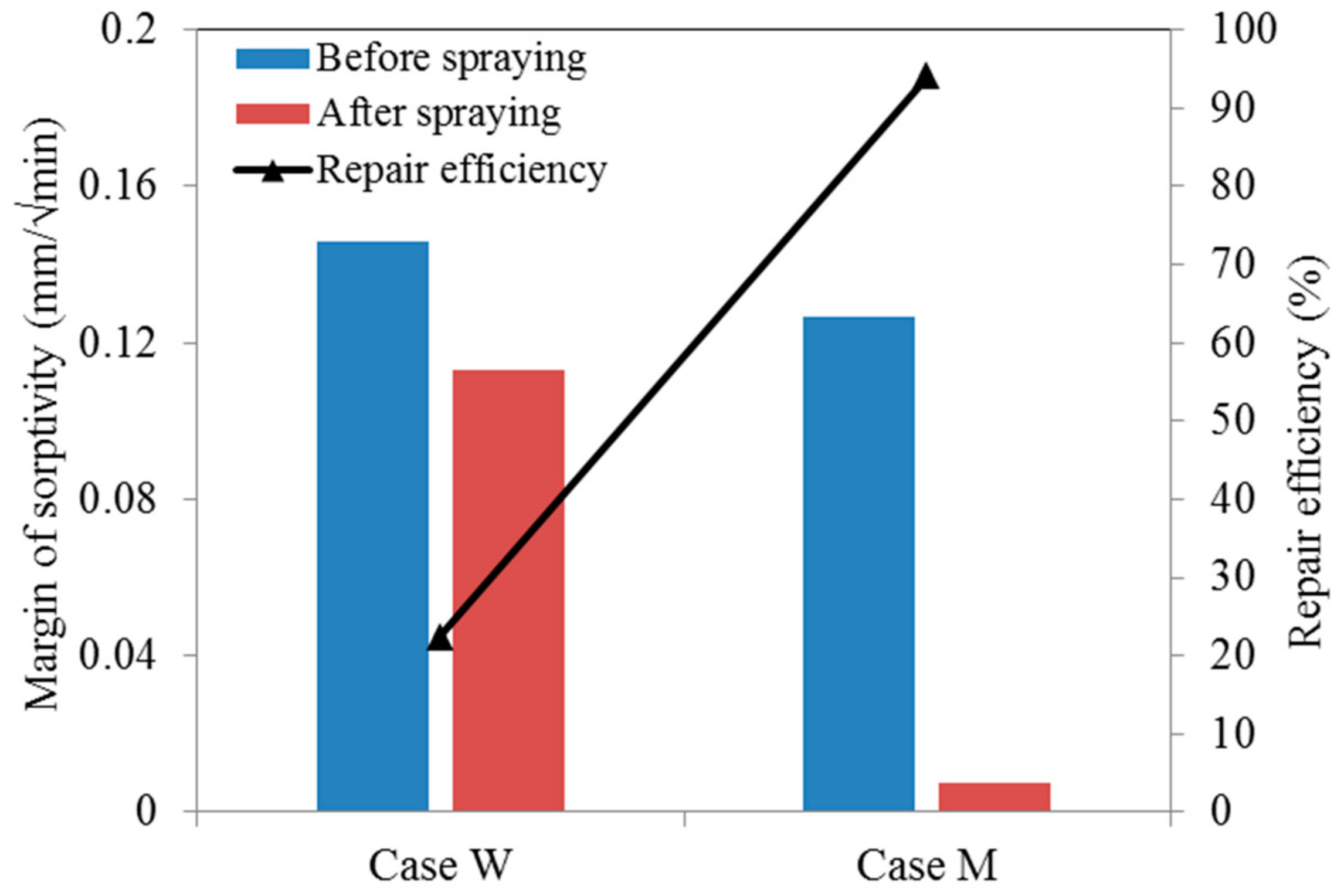
4.4. Sorptivity Test
5. Conclusions
- (1)
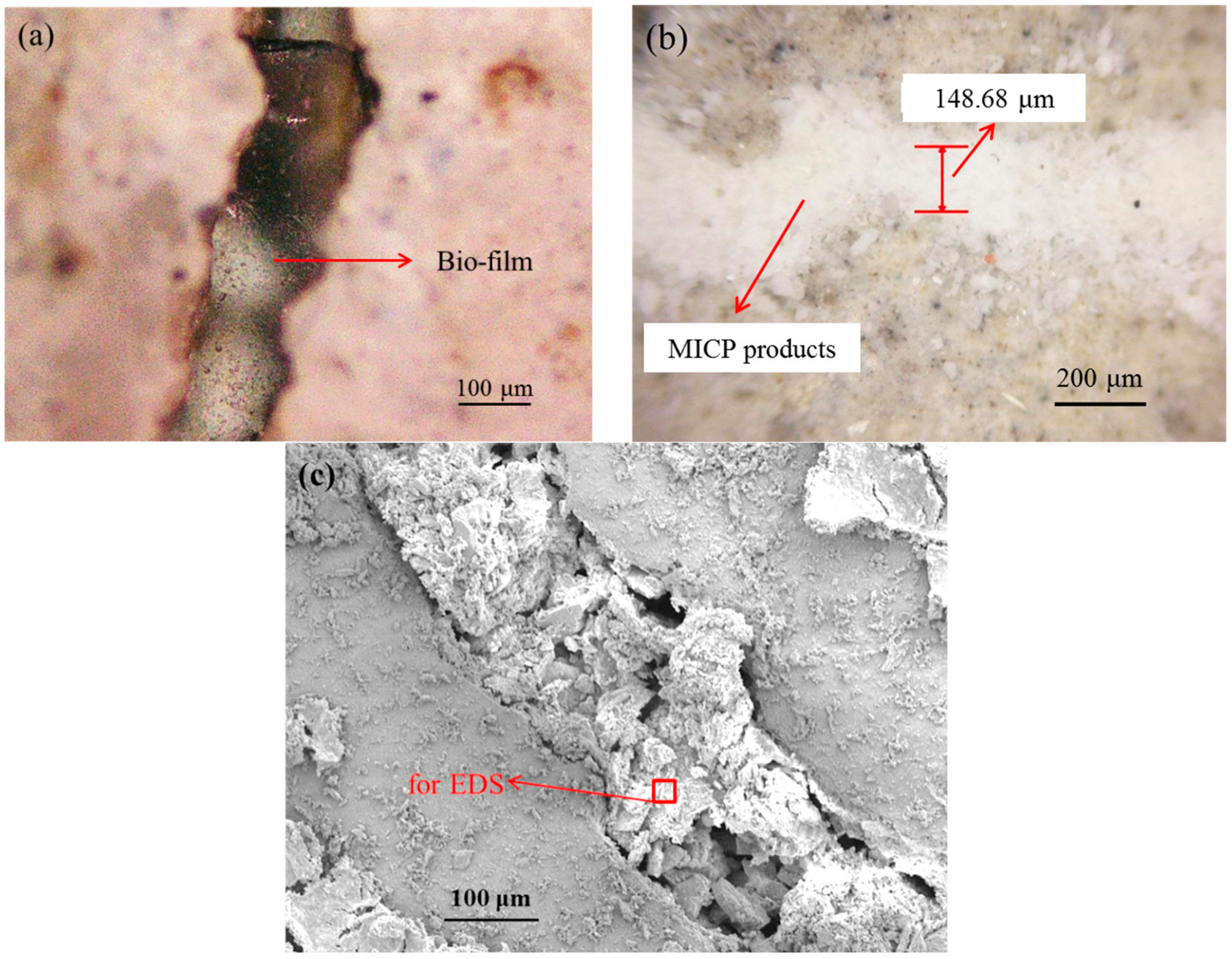
- The cracks in concrete with width ranging from 80 to 270 μm were sealed near completely by white residue—mutant bacterial metabolism induced products after only three times of spraying. On the other hand, no product was visible in crack even after 20 times of spraying with a wide type of bacterial liquid.
- (2)
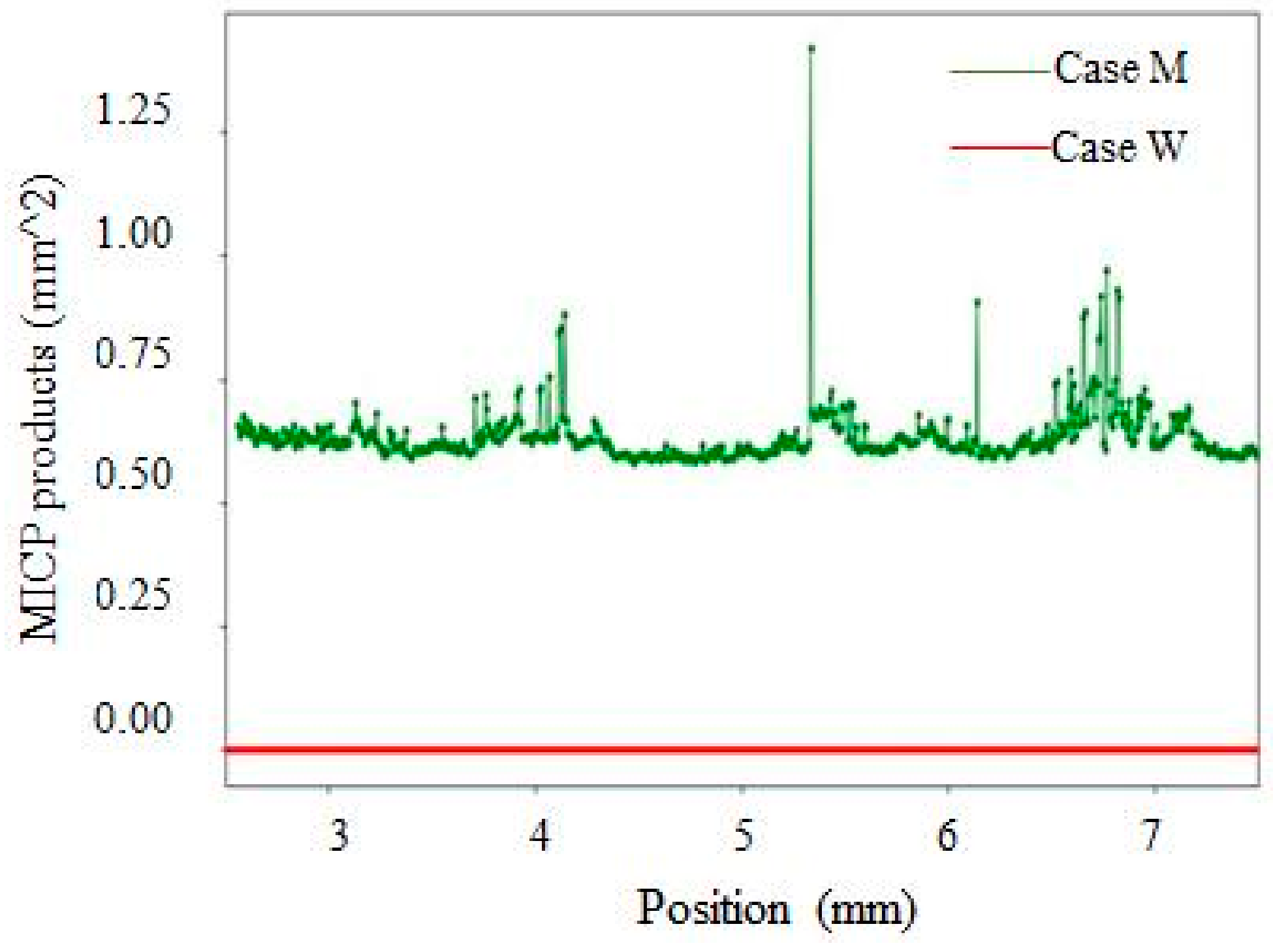
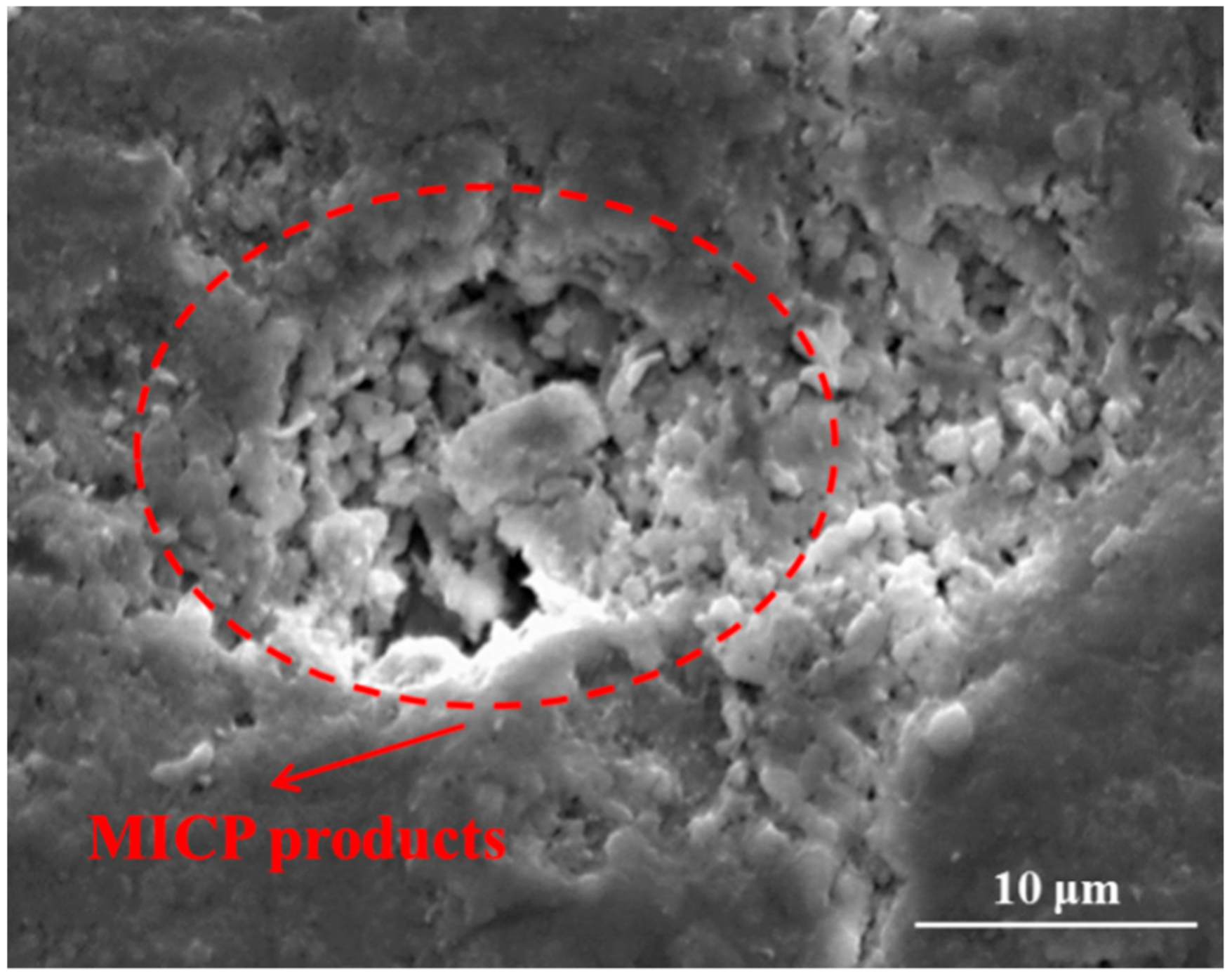
- By using X-ray CT scanning technology, a large amount of microbiologically induced calcite precipitation (MICP) products was found to be distributed along with the whole crack depth for the mutant bacterial case, while no products were observed along with the crack depth for wild type bacterial case.
- (3)
- The sorptivity of pre-cracked samples presented a much higher value than that of virgin ones due to the presence of cracks. After spraying mutant bacterial liquid for three times, MICP products, which are postulated to be CaCO3 by EDS analysis, were found to distribute within crack space and micro-pore on the surface of samples, and subsequently reduce the sorptivity significantly. For the case of wild type bacteria, a kind of bio-film instead of MICP products was found within a crack, which is plausibly due to its inability to catalyze the combination of Ca2+ and CO32− effectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, H.M.; Inoue, S.; Kwon, H.; Choi Lim, M. Effective Crack Control of Concrete by Self-Healing of Cementitious Composites Using Synthetic Fiber. Materials 2016, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Ryou, J.S. Crack Healing Performance of PVA-Coated Granules Made of Cement, CSA, and NaCO(3) in the Cement Matrix. Materials 2016, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Ersan, Y.C.; Gruyaert, E.; Louis, G.; Lors, C.; De Belie, N.; Boon, N. Self-protected nitrate reducing culture for intrinsic repair of concrete cracks. Front. Microbiol. 2015, 6, 1228. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.H.; Kim, H.G.; Ryou, J.S. New Surface-Treatment Technique of Concrete Structures Using Crack Repair Stick with Healing Ingredients. Materials 2016, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Dry, C.; Corsaw, M.; Bayer, E. A comparison of internal self-repair with resin injection in repair of concrete. J. Adhes. Sci. Technol. 2003, 17, 79–89. [Google Scholar] [CrossRef]
- Issa, C.A.; Debs, P. Experimental study of epoxy repairing of cracks in concrete. Constr. Build. Mater. 2007, 21, 157–163. [Google Scholar] [CrossRef]
- Van Breugel, K. Is there a market for self-healing cement-based materials. In Proceedings of the First International Conference on Self-Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007. [Google Scholar]
- Zhang, Z.G.; Zhang, Q.; Li, V.C. Multiple-scale Investigations on Self-healing Induced Mechanical Property Recovery of ECC. Cem. Concr. Compos. 2019, 103, 293–302. [Google Scholar] [CrossRef]
- Kan, L.L.; Shi, H.S.; Sakulich, A.R.; Li, V.C. Self-Healing Characterization of Engineered Cementitious Composite Materials. ACI Mater. J. 2010, 107, 617–624. [Google Scholar]
- Li, V.C.; Wang, S.; Wu, C. Tensile Strain-hardening Behavior of PVA-ECC. ACI Mater. J. 2001, 98, 483–492. [Google Scholar]
- Zhang, Z.G.; Zhang, Q. Matrix tailoring of Engineered Cementitious Composites (ECC) with non-oil-coated, low tensile strength PVA fiber. Constr. Build. Mater. 2018, 161, 420–431. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Qian, S.Z.; Liu, H.Z.; Li, V.C. Ductile concrete material with self-healing capacity for jointless concrete pavement use. Transport. Res. Rec. 2017, 2640, 78–83. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Qian, S.Z.; Ma, H. Investigating mechanical properties and self-healing behavior of micro-cracked ECC with different volume of fly ash. Constr. Build. Mater. 2014, 52, 17–23. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Wang, J.; Ersan, Y.C.; Boon, N.; De Belie, N. Application of microorganisms in concrete: A promising sustainable strategy to improve concrete durability. Appl. Microbiol. Biotechnol. 2016, 100, 2993–3007. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Available online: http://wwwbasiliskconcretecom/portfolio-items/parkeergarage-apeldoorn/?lang=en (accessed on 15 October 2019).
- Wang, J.Y.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Boone, C.D.; Gill, S.; Habibzadegan, A.; McKenna, R. Carbonic anhydrase: An efficient enzyme with possible global implications. Int. J. Chem. Eng. 2013, 2013, 813931. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Y.; Qian, S. Influence of bacterial incorporation on mechanical properties of engineered cementitious composites (ECC). Constr. Build. Mater. 2019, 196, 195–203. [Google Scholar] [CrossRef]
- Ding, Y.N.; Peng, Y.; Du, L.; Cao, B. Disruption of putrescine biosynthesis in Shewanella oneidensis enhances biofilm cohesiveness and performance in Cr(VI) immobilization. Appl. Environ. Microbiol. 2014, 80, 1498–1506. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.Z.; Hu, Y.D.; Cao, B.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhancing Bidirectional Electron Transfer of Shewanella oneidensis by a Synthetic Flavin Pathway. ACS Synth. Biol. 2015, 4, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, J.; Peng, Y.; Li, L.; Wang, W.; Wang, Q. Improvement of pyruvate production by Escherichia coli via pathway engineering and Tn5 transposon mediated mutagenesis. Sheng Wu Gong Cheng Xue Bao 2017, 33, 1913–1922. [Google Scholar] [PubMed]
- Lin, Z.; Dong, H.; Li, Y. Improvement of butanol production by Escherichia coli via Tn5 transposon mediated mutagenesis. Sheng Wu Gong Cheng Xue Bao 2015, 31, 1711–1719. [Google Scholar] [PubMed]
- Hemarajata, P.; Spinler, J.K.; Balderas, M.A.; Versalovic, J. Identification of a proton-chloride antiporter (EriC) by Himar1 transposon mutagenesis in Lactobacillus reuteri and its role in histamine production. Antonie Leeuwenhoek 2014, 105, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Yuvaraj, A.; Di, J.; Qian, S.Z. Matrix design of light weight, high strength, high ductility ECC. Constr. Build. Mater. 2019, 210, 188–197. [Google Scholar] [CrossRef]
- Mizushima, A.; Lu, R. An image segmentation method for apple sorting and grading using support vector machine and Otsu’s method. Comput. Electron. Agric. 2013, 94, 29–37. [Google Scholar] [CrossRef]
- ASTMC. Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes; ASTM C158; American Society for Testing and Materials: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Hall, C. Water sorptivity of mortars and concretes: A review. Mag. Concr. Res. 1989, 41, 51–61. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Schlangen, E. Self-healing of cracked concrete: A bacterial approach. Fract. Mech. Concr. Concr. Struct. 2007, 1–3, 1821–1826. [Google Scholar]
- Jonkers, H.M.; Schlangen, E. Development of a bacteria-based self healing concrete. Tailor Made Concr. Struct. 2008, 1, 425–430. [Google Scholar]
- Jonkers, H.M.; Schlangen, E. A two component bacteria-based self-healing concrete. In Proceedings of the 2nd International Conference on Concrete Repair, Rehabilitation and Retrofitting, Cape Town, South Africa, 24–26 November 2008; pp. 119–120. [Google Scholar]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012, 14, 1913–1928. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Wang, S.W.; Wang, D.; Parsek, M.R.; Wozniak, D.J. The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Latasa, C.; Gil, C.; Toledo-Arana, A.; Solano, C.; Penades, J.R.; Lasa, I. Bap, a Biofilm Matrix Protein of Staphylococcus aureus Prevents Cellular Internalization through Binding to GP96 Host Receptor. PLoS Pathog. 2012, 8, e1002843. [Google Scholar] [CrossRef] [PubMed]
- Denyer, S.P.; Gorman, S.P.; Sussman, M. Microbial Biofilms: Formation and Control; Blackwell Scientific Publications: Oxford, UK; Boston, MA, USA, 1993. [Google Scholar]
- Ruan, S.; Qiu, J.; Weng, Y.; Yang, Y.; Yang, E.H.; Chu, J.; Unluer, C. The use of microbial induced carbonate precipitation in healing cracks within reactive magnesia cement-based blends. Cem. Concr. Res. 2019, 115, 176–188. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Genet. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Aggarwal, S.; Stewart, P.; Hozalski, R. Biofilm Cohesive Strength as a Basis for Biofilm Recalcitrance: Are Bacterial Biofilms Overdesigned? Microbiol. Insights 2016, 8 (Suppl. 2), 29–32. [Google Scholar] [CrossRef]
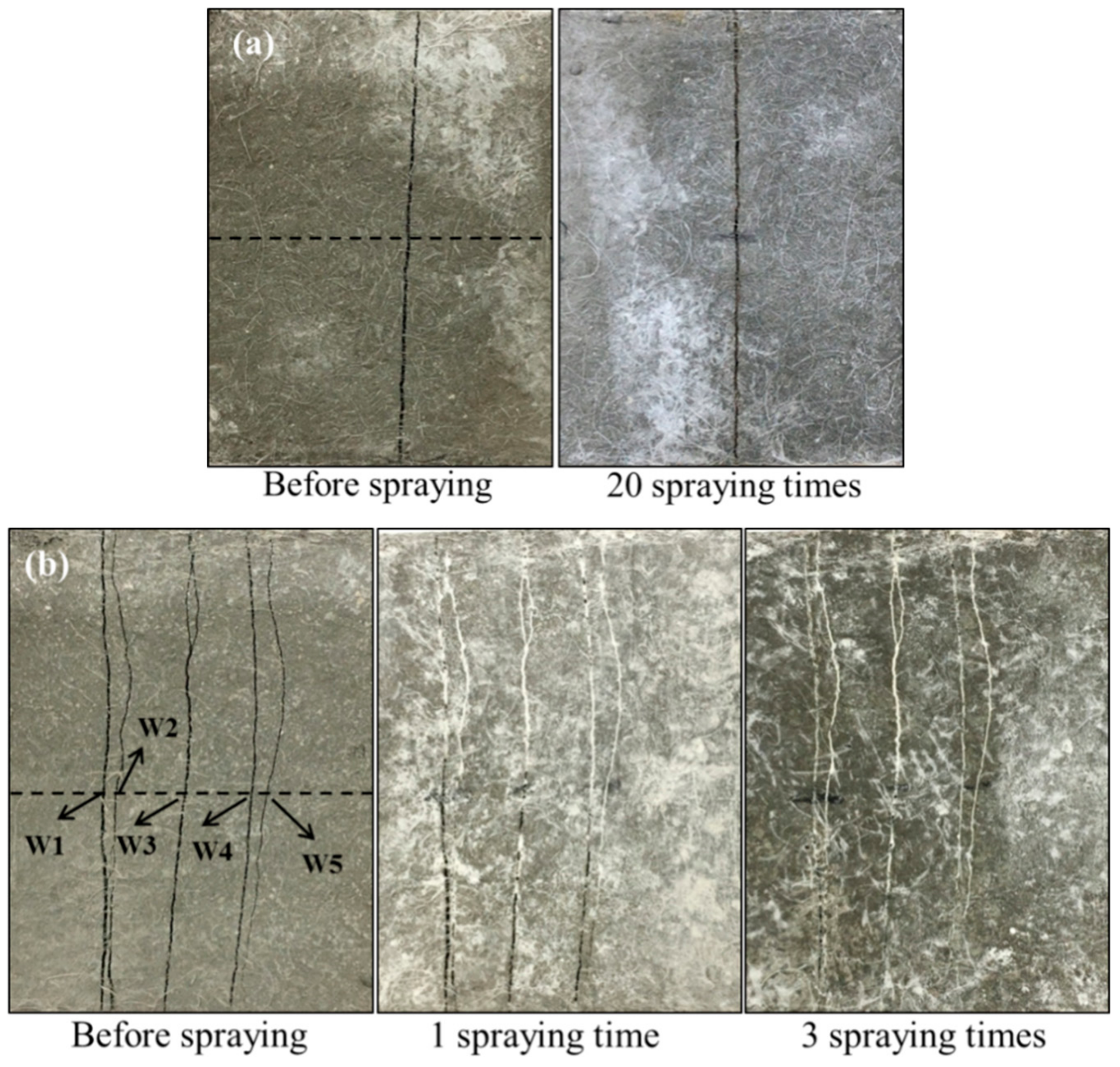

| Cement (C) | Fly Ash (FA/C) | Silica Sand (S/CM) | Water (W/CM) | Water Reducer (WR/C) | PE Fiber (by Volume) |
|---|---|---|---|---|---|
| 1.0 | 1.2 | 0.36 | 0.25 | 0.03 | 0.02 |
| SiO2 | SiO2 + Al2O3 + Fe2O3 | Na2O | CaO | MgO | Fineness | Loss of Ignition | Moisture |
|---|---|---|---|---|---|---|---|
| 50–55 | 80–85 | <1.5 | 7–9 | <5 | 5.3 | <1 | <1 |
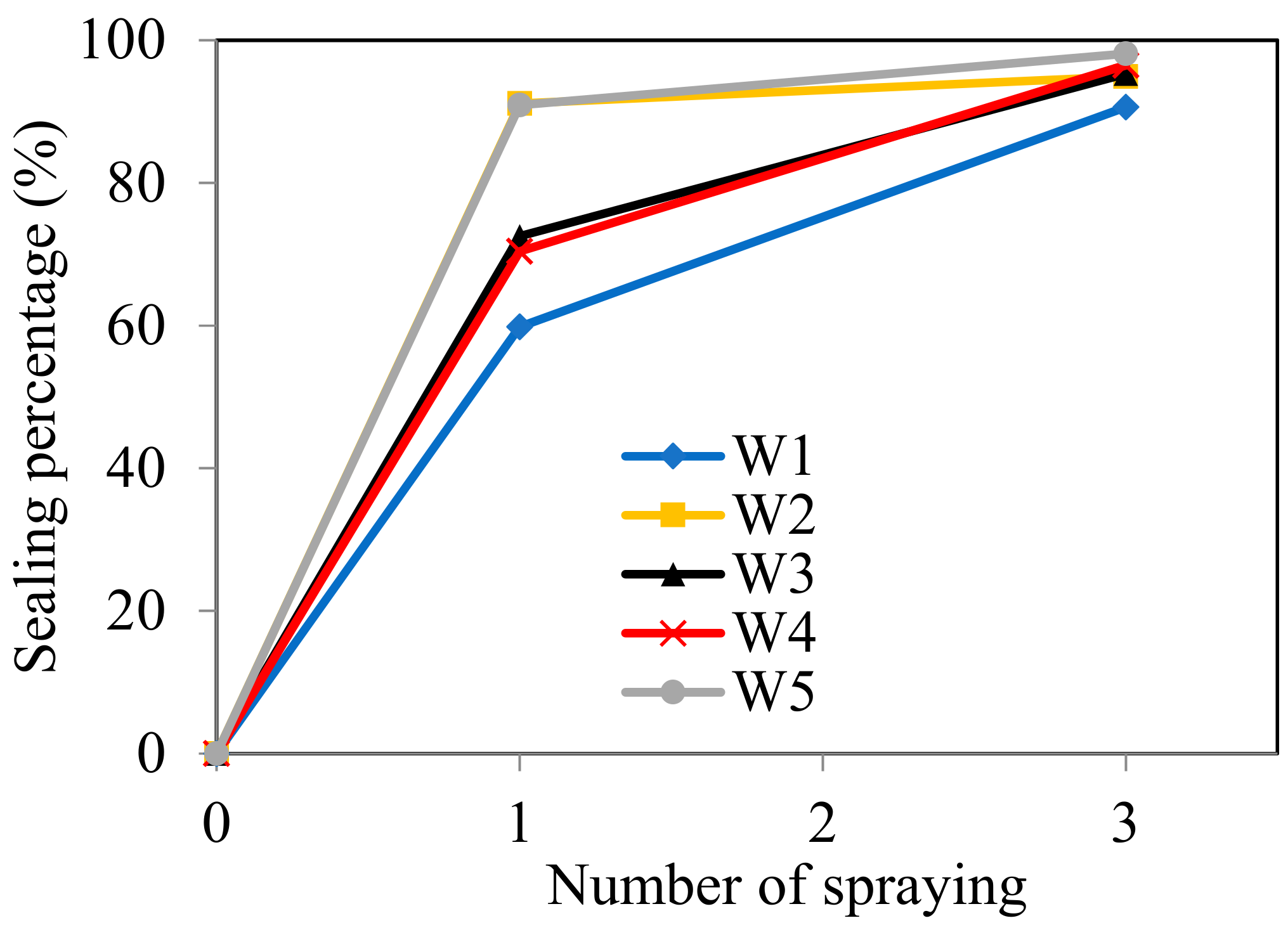
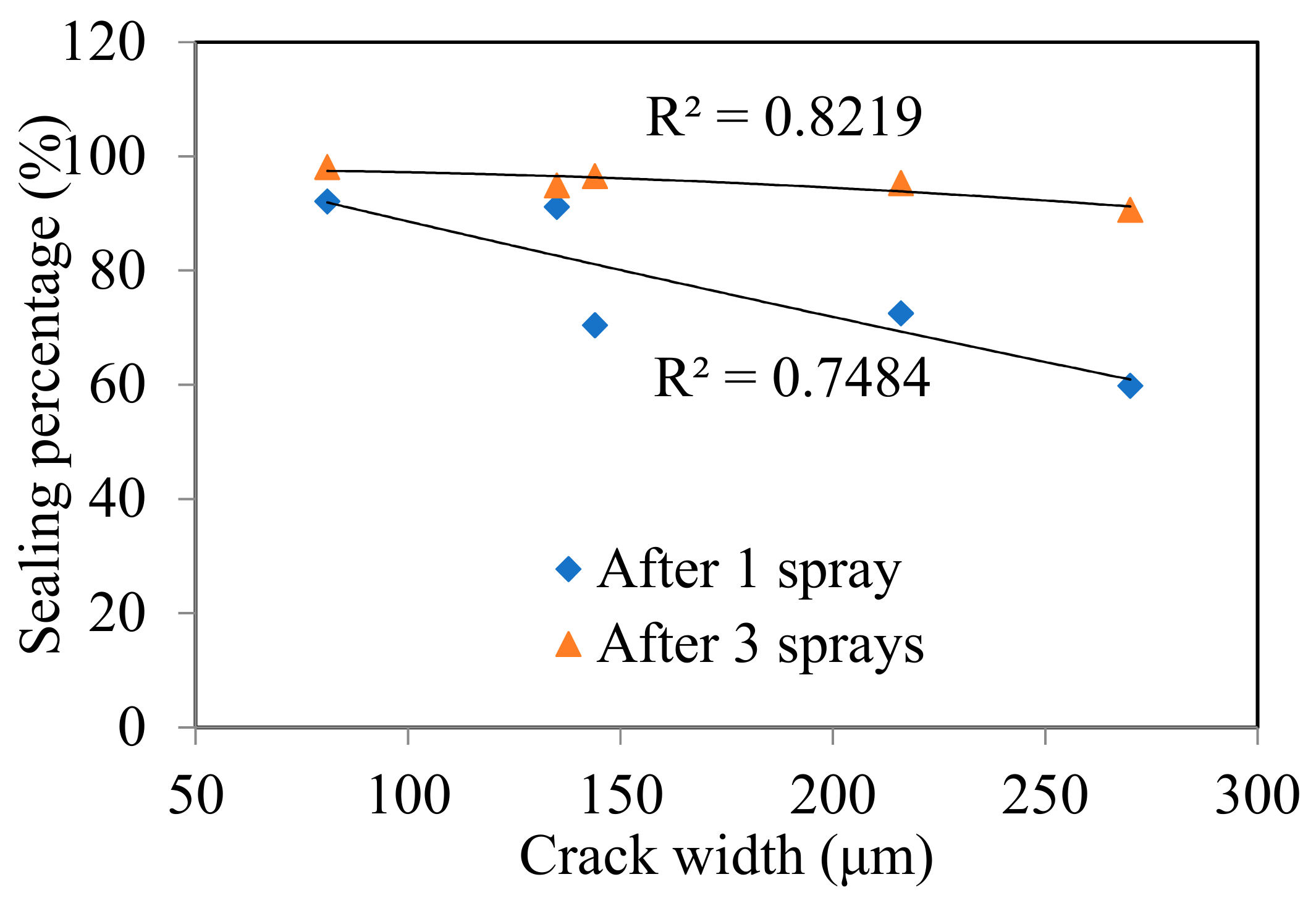
| Crack No. (Left-To-Right) | Crack Width (μm) | Crack Area (mm2)/Sealing Percentage α (%) | ||
|---|---|---|---|---|
| Before Spraying | 1 Spraying | 3 Spraying | ||
| W1 | 270 | 4.95 | 1.99/59.8% | 0.47/90.6% |
| W2 | 135 | 2.88 | 0.25/91.1% | 0.15/94.9% |
| W3 | 216 | 4.69 | 1.29/72.5% | 0.22/95.3% |
| W4 | 144 | 3.69 | 1.10/70.3% | 0.13/96.5% |
| W5 | 81 | 2.02 | 0.16/92.1% | 0.04/98.1% |
| Crack No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Crack width (μm) | 300 | 130 | 100 | 80 | 60 | 100 |
| Average crack width (μm) | 128 | |||||
| Element | C | O | Si | Ca |
|---|---|---|---|---|
| At % | 20.75 | 59.66 | 1.73 | 15.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Weng, Y.; Ding, Y.; Qian, S. Use of Genetically Modified Bacteria to Repair Cracks in Concrete. Materials 2019, 12, 3912. https://doi.org/10.3390/ma12233912
Zhang Z, Weng Y, Ding Y, Qian S. Use of Genetically Modified Bacteria to Repair Cracks in Concrete. Materials. 2019; 12(23):3912. https://doi.org/10.3390/ma12233912
Chicago/Turabian StyleZhang, Zhigang, Yiwei Weng, Yuanzhao Ding, and Shunzhi Qian. 2019. "Use of Genetically Modified Bacteria to Repair Cracks in Concrete" Materials 12, no. 23: 3912. https://doi.org/10.3390/ma12233912






