Using 445 nm and 970 nm Lasers on Dental Implants—An In Vitro Study on Change in Temperature and Surface Alterations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser Device
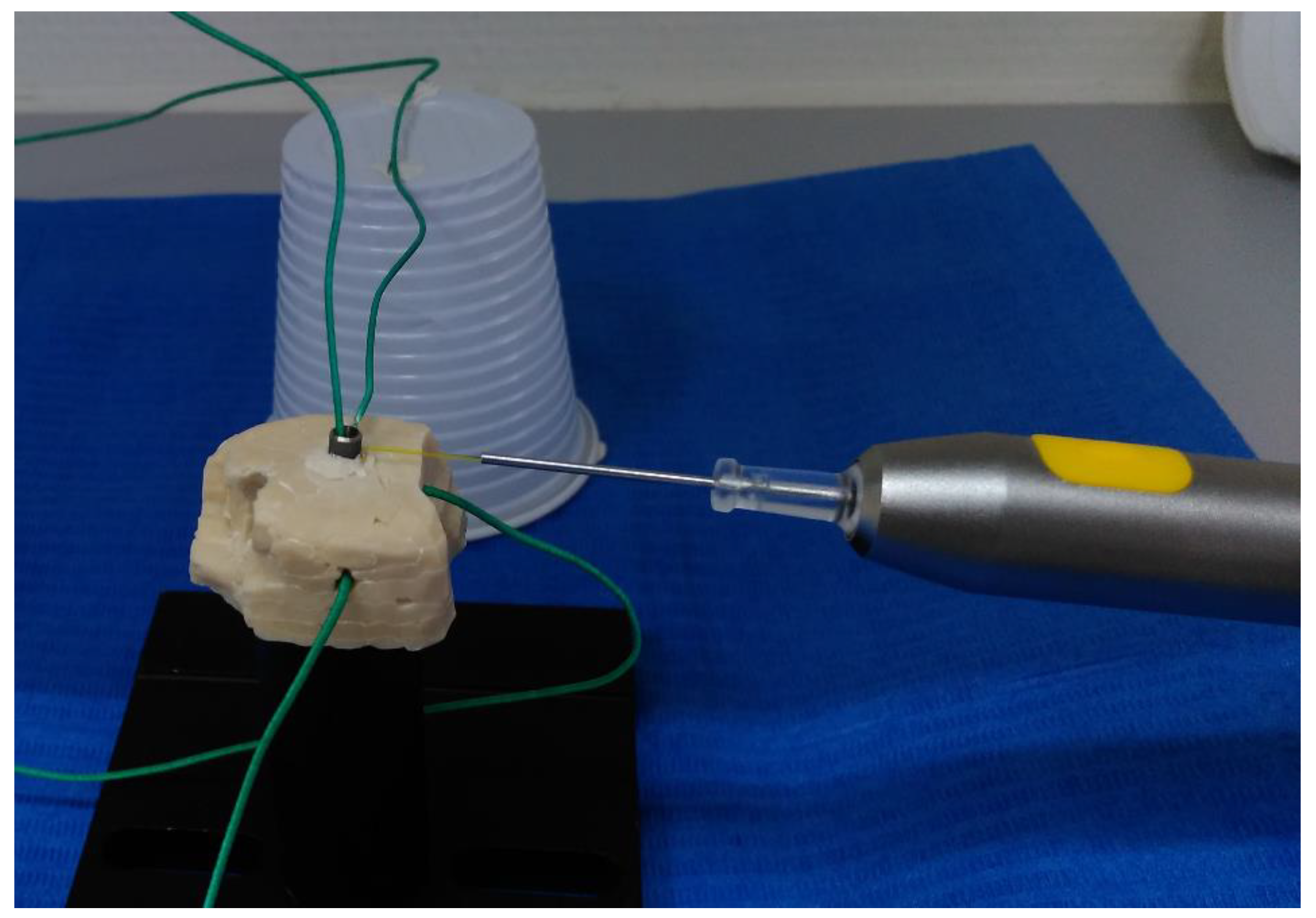
2.1.1. Glass Ionomer Cement Model
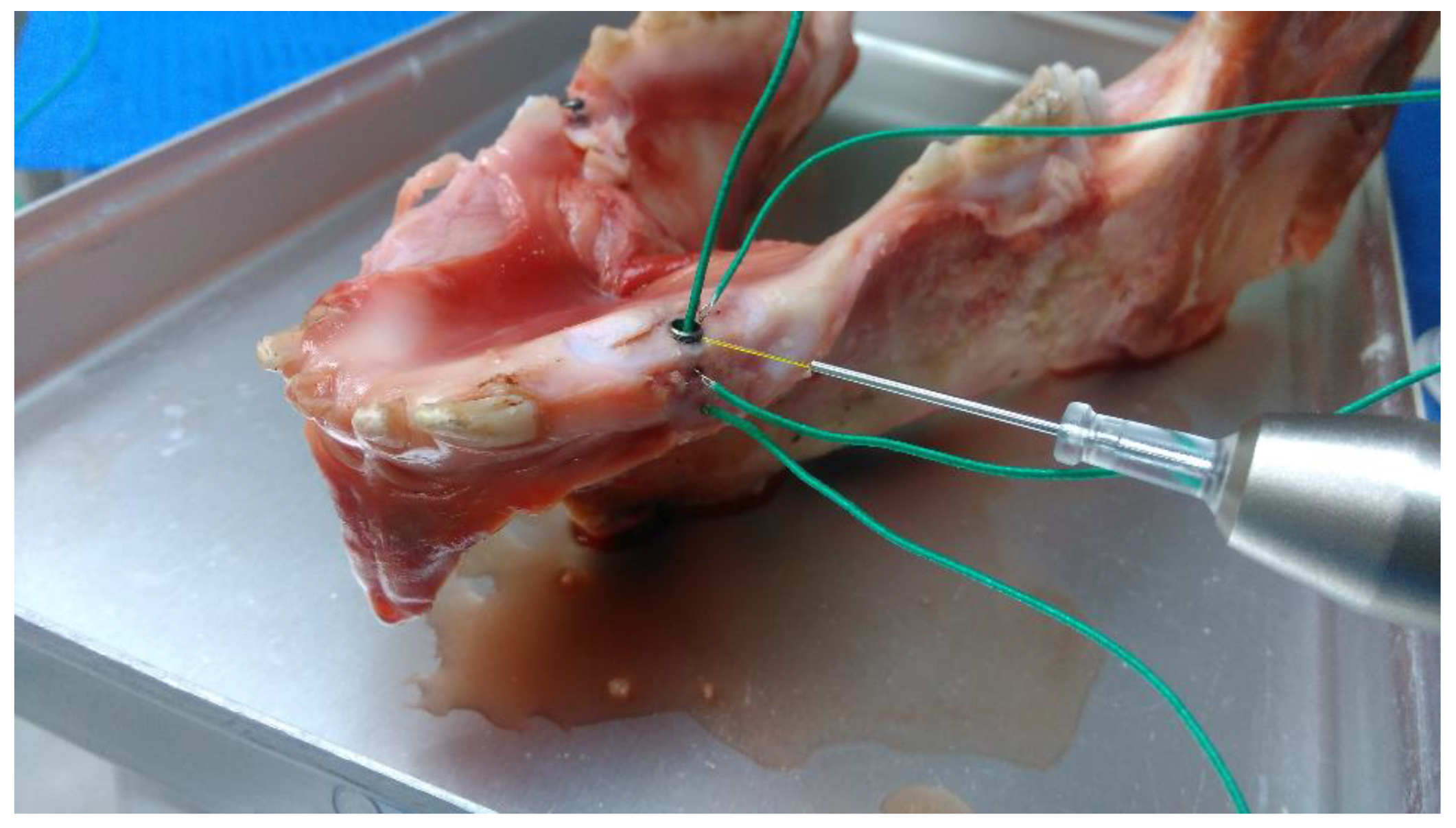
2.1.2. Pig Mandible Model
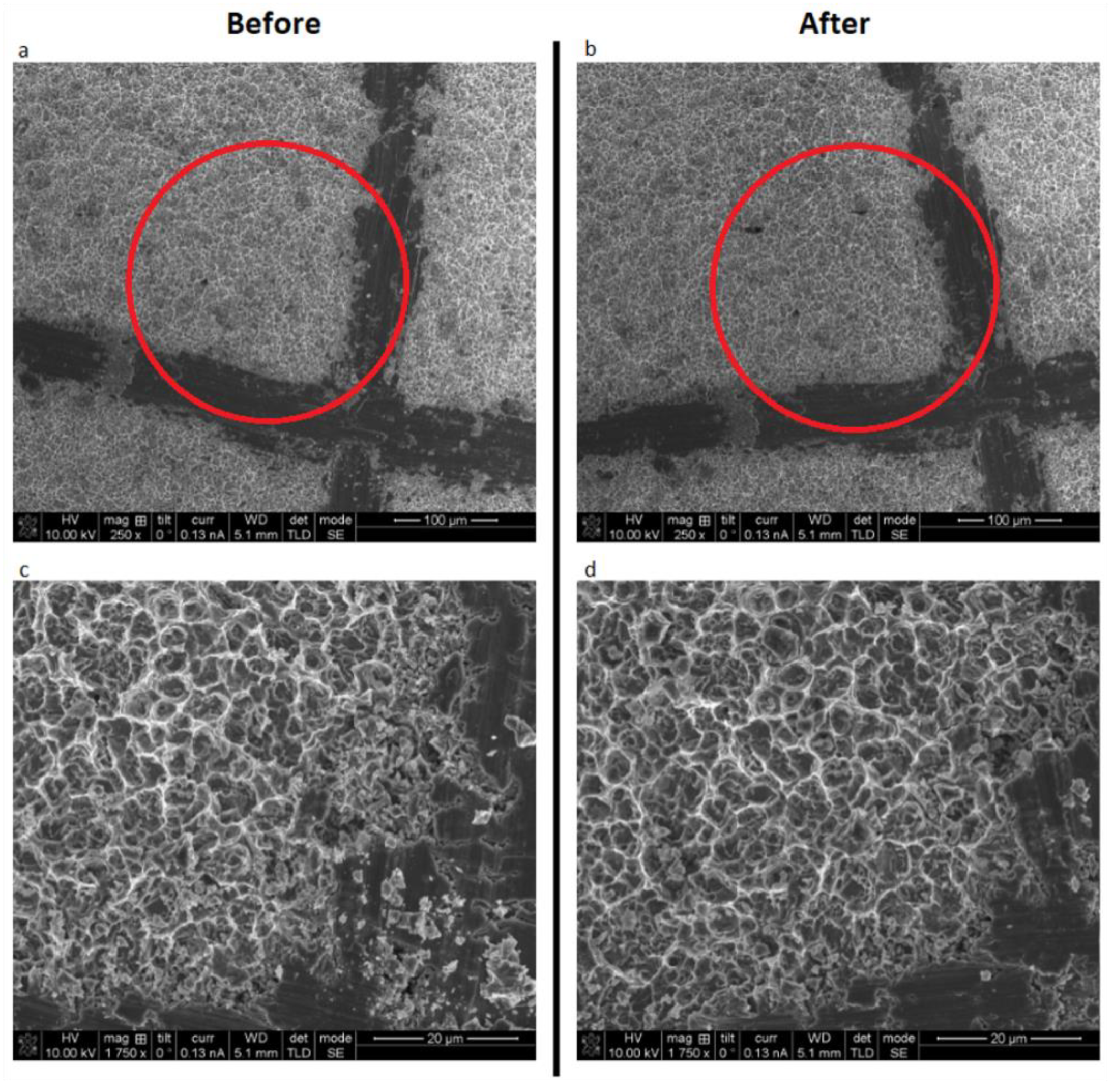
2.2. Surface Alteration Tests
2.3. Statistics
3. Results
3.1. Pig Mandible Model
3.2. Glass Ionomer Cement Model
3.3. Surface Alterations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. 1), S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Lundström, M.; Rosén, M.; Bertl, K.; Klinge, A.; Stavropoulos, A. Dental Implant Quality Register-A possible tool to further improve implant treatment and outcome. Clin. Oral Implants Res. 2018, 29 (Suppl. 18), 145–151. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef]
- Rodrigo, D.; Sanz-Sanchez, I.; Figuero, E.; Llodra, J.C.; Bravo, M.; Caffesse, R.G.; Vallcorba, N.; Guerrero, A.; Herrera, D. Prevalence and risk indicators of peri-implant diseases in Spain. J. Clin. Periodontol. 2018, 45, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Sculean, A. Nonsurgical therapy for teeth and implants-When and why? Periodontol. 2000 2019, 79, 15–21. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Treatment of pathologic peri-implant pockets. Periodontol. 2000 2018, 76, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol. 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of temperature on the dental implant osseointegration development in low-density bone: An in vivo histological evaluation. Implant Dent. 2015, 24, 96–100. [Google Scholar] [CrossRef]
- Valente, N.A.; Calascibetta, A.; Patianna, G.; Mang, T.; Hatton, M.; Andreana, S. Thermodynamic Effects of 3 Different Diode Lasers on an Implant-Bone Interface: An Ex-Vivo Study With Review of the Literature. J. Oral Implantol. 2017, 43, 94–99. [Google Scholar] [CrossRef]
- Leja, C.; Geminiani, A.; Caton, J.; Romanos, G.E. Thermodynamic effects of laser irradiation of implants placed in bone: An in vitro study. Lasers Med. Sci. 2013, 28, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Everts, H.; Nentwig, G.H. Effects of diode and Nd: YAG laser irradiation on titanium discs: A scanning electron microscope examination. J. Periodontol. 2000, 71, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Stubinger, S.; Etter, C.; Miskiewicz, M.; Homann, F.; Saldamli, B.; Wieland, M.; Sader, R. Surface alterations of polished and sandblasted and acid-etched titanium implants after Er: YAG, carbon dioxide, and diode laser irradiation. Int. J. Oral Maxillofac. Implants. 2010, 25, 104–111. [Google Scholar] [PubMed]
- Giannelli, M.; Lasagni, M.; Bani, D. Thermal effects of λ = 808 nm GaAlAs diode laser irradiation on different titanium surfaces. Lasers Med. Sci. 2015, 30, 2341–2352. [Google Scholar] [CrossRef]
- Fukase, Y.; Saitoh, M.; Kaketani, M.; Ohashi, M.; Nishiyama, M. Thermal coefficients of paste-paste type pulp capping cements. Dent. Mater. J. 1992, 11, 189–196. [Google Scholar] [CrossRef]
- El-Brawany, M.A.; Nassiri, D.K.; Terhaar, G.; Shaw, A.; Rivens, I.; Lozhken, K. Measurement of thermal and ultrasonic properties of some biological tissues. J. Med. Eng. Technol. 2009, 33, 249–256. [Google Scholar] [CrossRef]
- Feldmann, A.; Wili, P.; Maquer, G.; Zysset, P. The thermal conductivity of cortical and cancellous bone. Eur. Cell. Mater. 2018, 35, 25–33. [Google Scholar] [CrossRef]
- Braun, A.; Kettner, M.; Berthold, M.; Wenzler, J.S.; Heymann, P.G.B.; Frankenberger, R. Efficiency of soft tissue incision with a novel 445-nm semiconductor laser. Lasers Med. Sci. 2018, 33, 27–33. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Byarlay, M.R.; Reinhardt, R.A.; Marx, D.B.; Meinberg, T.A.; Kaldahl, W.B. Adjunctive Non-Surgical Therapy of Inflamed Periodontal Pockets During Maintenance Therapy Using Diode Laser: A Randomized Clinical Trial. J. Periodontol. 2015, 86, 1133–1140. [Google Scholar] [CrossRef]
- Stein, S.; Wenzler, J.; Hellak, A.; Schauseil, M.; Korbmacher-Steiner, H.; Braun, A. Intrapulpal Temperature Increases Caused by 445-nm Diode Laser-Assisted Debonding of Self-Ligating Ceramic Brackets During Simulated Pulpal Fluid Circulation. Photomed. Laser Surg. 2018, 36, 185–190. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kwon, Y.-H.; Herr, Y.; Shin, S.-I.; Chung, J.-H. Effect of erbium-doped: Yttrium, aluminium and garnet laser irradiation on the surface microstructure and roughness of sand-blasted, large grit, acid-etched implants. J. Periodontal. Implant Sci. 2011, 41, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sahm, N.; Iglhaut, G.; Becker, J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: A randomized controlled clinical study. J. Clin. Periodontol. 2011, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Roos Jansåker, A.-M.; Persson, G.R. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: A randomized clinical trial. J. Clin. Periodontol. 2011, 38, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bach, G.; Neckel, C.; Mall, C.; Krekeler, G. Conventional versus laser-assisted therapy of periimplantitis: A five-year comparative study. Implant Dent. 2000, 9, 247–251. [Google Scholar] [CrossRef] [PubMed]



| Wavelength | 1.0 W | 2.0 W |
|---|---|---|
| 445 nm | 1.14 (± 0.02) | 2.25 (± 0.03) |
| 970 nm | 1.14 (± 0.01) | 2.23 (± 0.02) |
| Wavelength and Variable | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|
| 445 nm, t to +10 °C | 9.00 (± 1.41) | 7.00 (± 1.41) | 24.00 (± 1.41) | 2.00 (± 0.00) |
| 970 nm, t to +10 °C | 8.00 (± 0.00) | 6.50 (± 0.71) | 24.50 (± 0.71) | 2.00 (± 0.00) |
| 445 nm, t to +20 °C | 27.00 (± 2.83) | 20.00 (± 1.41) | 75.00 (± 1.41) | 5.50 (± 1.41) |
| 970 nm, t to +20 °C | 23.50 (± 0.71) | 22.00 (± 1.41) | 82.50 (± 0.71) | 5.50 (± 0.71) |
| Wavelength and Variable | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|
| 445 nm, 15 s | 14.50 (± 1.56) | 17.30 (± 1.13) | 6.75 (± 0.64) | 28.55 (± 1.20) |
| 970 nm, 15 s | 16.00 (± 0.42) | 17.15 (± 0.49) | 6.70 (± 0.28) | 27.90 (± 0.71) |
| 445 nm, 30 s | 21.00 (± 0.99) | 23.55 (± 0.35) | 12.05 (± 0.21) | 34.00 (± 0.28) |
| 970 nm, 30 s | 22.50 (± 0.42) | 22.80 (± 0.71) | 11.70 (± 0.28) | 33.05 (± 0.49) |
| 445 nm, 60 s | 28.15 (± 2.33) | 29.05 (± 0.35) | 18.00 (± 0.28) | 39.70 (± 0.42) |
| 970 nm, 60 s | 28.50 (± 0.28) | 27.80 (± 0.28) | 17.20 (± 0.28) | 36.95 (± 0.35) |
| 445 nm, 120 s | 34.60 (± 2.12) | 34.05 (± 0.07) | 23.96 (± 0.07) | 44.80 (± 0.42) |
| 970 nm, 120 s | 34.70 (± 0.28) | 32.75 (± 0.35) | 23.05 (± 0.21) | 42.15 (± 0.64) |
| Wavelength and Variable | Model | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|---|
| 445 nm t to +10 °C | PM | 9.00 (± 1.41) | 7.00 (± 1.41) | 24.00 (± 1.41) | 2.00 (± 0.00) |
| GIC | 3.67 (± 0.58) | 11.67(± 0.58) | 25.00 (± 0.00) | 3.00 (± 0.00) | |
| 970 nm t to +10 °C | PM | 8.00 (± 0.00) | 6.50 (± 0.71) | 24.50 (± 0.71) | 2.00 (± 0.00) |
| GIC | 4.00 (± 0.00) | 12.00 (± 0.00) | 25.33 (± 0.58) | 3.33 (± 0.58) | |
| 445 nm Max Δ temp | PM | 34.90 (± 2.12) | 34.25 (± 0.07) | 24.25 (± 0.07) | 45.05 (± 0.35) |
| GIC | 87.83 (± 0.65) | 36.93 (0.25) | 27.07 (± 0.15) | 61.00 (± 0.36) | |
| 970 nm Max Δ temp | PM | 34.95 (± 0.35) | 32.95 (± 0.35) | 23.40 (± 0.28) | 42.20 (± 0.57) |
| GIC | 87.23 (± 0.12) | 36.77 (± 0.06) | 26.57 (± 0.06) | 58.63 (± 0.40) |
| Settings | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|
| 0.5 W CW | 45.97 (± 0.06) | 19.30 (± 0.00) | 13.90 (± 0.00) | 32.00 (± 0.36) |
| 3 W Pulsed 17% Duty Cycle 10 Hz (avg 0.51 W) | 51.60 (± 0.10) | 28.80 (± 0.00) | 13.93 (± 0.06) | 37.00 (± 0.20) |
| Wavelength | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|
| 445 nm | −10.3 | −5.9 | −3.5 | 2.5 |
| 970 nm | −5.4 | −3.1 | −1.8 | 2.1 |
| Power | Wavelength | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|---|
| 1.0 W | 445 nm | 37.68 (± 1.53) | 26.01 (± 0.50) | 30.72 (± 0.50) | 32.49 (± 2.61) |
| 970 nm | 42.02 (± 4.43) | 27.05 (± 0.72) | 33.96 (± 2.45) | 32.95 (± 3.86) | |
| 2.0 W | 445 nm | 52.45 (± 2.92) | 28.93 (± 1.02) | 39.96 (± 1.63) | 40.86 (± 3.50) |
| 970 nm | 61.89 (± 6.66) | 31.04 (± 1.09) | 46.00 (± 3.70) | 47.76 (± 3.81) |
| Wavelength | Amount of Water | Inside | Midway | Apex | Next to Irradiation Site |
|---|---|---|---|---|---|
| 445 nm | 5.0 mL | 82.92 (± 1.98) | 50.91 (± 7.67) | 61.74 (± 7.02) | 91.60 (± 2.19) |
| 2.5 mL | 61.72 (± 4.18) | 48.07 (± 4.69) | 38.62 (± 4.04) | 69.44 (± 4.83) | |
| 970 nm | 5.0 mL | 78.56 (± 1.92) | 63.74 (± 4.58) | 63.10 (± 4.23) | 89.76 (± 4.75) |
| 2.5 mL | 63.09 (± 8.01) | 49.16 (± 9.20) | 42.34 (± 7.13) | 70.43 (± 8.49) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malmqvist, S.; Liljeborg, A.; Qadri, T.; Johannsen, G.; Johannsen, A. Using 445 nm and 970 nm Lasers on Dental Implants—An In Vitro Study on Change in Temperature and Surface Alterations. Materials 2019, 12, 3934. https://doi.org/10.3390/ma12233934
Malmqvist S, Liljeborg A, Qadri T, Johannsen G, Johannsen A. Using 445 nm and 970 nm Lasers on Dental Implants—An In Vitro Study on Change in Temperature and Surface Alterations. Materials. 2019; 12(23):3934. https://doi.org/10.3390/ma12233934
Chicago/Turabian StyleMalmqvist, Sebastian, Anders Liljeborg, Talat Qadri, Gunnar Johannsen, and Annsofi Johannsen. 2019. "Using 445 nm and 970 nm Lasers on Dental Implants—An In Vitro Study on Change in Temperature and Surface Alterations" Materials 12, no. 23: 3934. https://doi.org/10.3390/ma12233934
APA StyleMalmqvist, S., Liljeborg, A., Qadri, T., Johannsen, G., & Johannsen, A. (2019). Using 445 nm and 970 nm Lasers on Dental Implants—An In Vitro Study on Change in Temperature and Surface Alterations. Materials, 12(23), 3934. https://doi.org/10.3390/ma12233934





