Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Material (Photo)-Activity
2.3. Analytical Procedures
3. Results and Discussion
3.1. MNP–BBS characterization
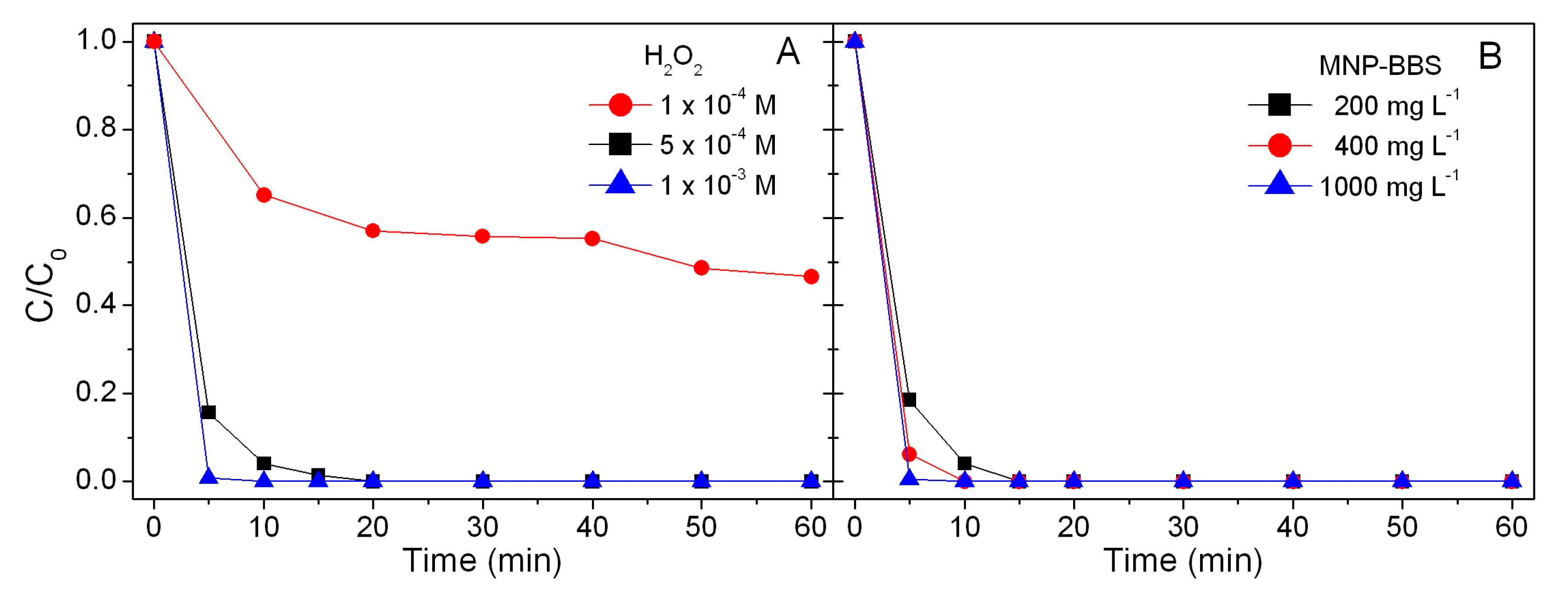
3.2. Phenol Abatement in the Dark through a Fenton-Like Process
3.3. Photo-Fenton Process: Influence of Irradiation
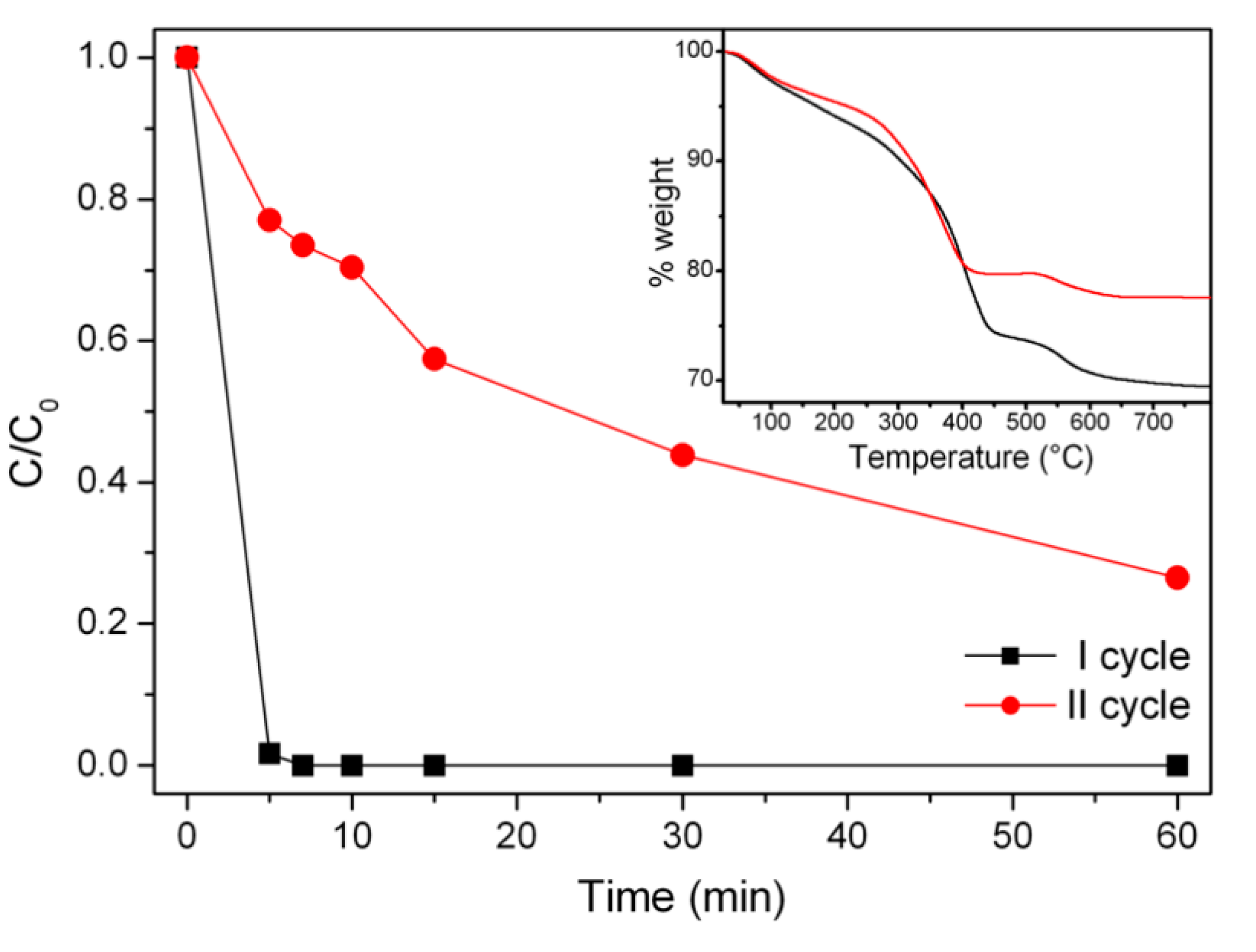
3.4. Stability and Re-Usability: Evaluation of Released Ions/Organic Matter
3.5. Release of Organic Matter
3.6. Release of Soluble Iron
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- United Nations Resolution 64/292: The Human Right to Water and Sanitation. Available online: https://www.unescwa.org/sites/www.unescwa.org/files/un_resolutions/a_res_64_292_e.pdf (accessed on 2 October 2018).
- United Nations Resolution 70/1: Transforming Our world: The 2030 Agenda for Sustainable Development. Available online: http://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (accessed on 2 October 2018).
- Laville, N.; Aït-Ässa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernández-Alba, A.R. Degradation of fifteen emerging contaminants at μg L-1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, M.; Zhu, X.; Xu, H.; Ma, S.; Zhi, Y.; Xia, H.; Liu, X.; Pan, J.; Tang, J.Y.; et al. 2020 roadmap on two-dimensional nanomaterials for environmental catalysis. Chin. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Minella, M.; Sordello, F.; Minero, C. Photocatalytic process in TiO2/Graphene hybrid materials. Evidence of charge separation by electron transfer from reduced graphene oxide to TiO2. Catal. Today 2017, 281, 29–37. [Google Scholar] [CrossRef]
- Gangu, K.; Maddila, S.; Jonnalagadda, S. A review on novel composites of MWCNTs mediated semiconducting materials as photocatalysts in water treatment. Sci. Total Environ. 2019, 646, 1398–1412. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts. Proc. R. Soc. Lond. Ser. A-Math. Phys. Sci. 1934, 147, 332–351. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Mestankova, H.; Mailhot, G.; Pilichowski, J.F.; Krýsa, J.; Jirkovský, J.; Bolte, M. Mineralisation of Monuron photoinduced by Fe(III) in aqueous solution. Chemosphere 2004, 57, 1307–1315. [Google Scholar] [CrossRef]
- Katsumata, H.; Kaneco, S.; Suzuki, T.; Ohta, K.; Yobiko, Y. Degradation of linuron in aqueous solution by the photo-Fenton reaction. Chem. Eng. J. 2005, 108, 269–276. [Google Scholar] [CrossRef]
- Lee, C.; Sedlak, D.L. A novel homogeneous Fenton-like system with Fe(III)-phosphotungstate for oxidation of organic compounds at neutral pH values. J. Mol. Catal. A Chem. 2009, 311, 1–6. [Google Scholar] [CrossRef]
- ElShafei, G.M.S.; Yehia, F.Z.; Dimitry, O.I.H.; Badawi, A.M.; Eshaq, G. Degradation of nitrobenzene at near neutral pH using Fe2+-glutamate complex as a homogeneous Fenton catalyst. Appl. Catal. B Environ. 2010, 99, 242–247. [Google Scholar] [CrossRef]
- Huang, W.; Brigante, M.; Wu, F.; Hanna, K.; Mailhot, G. Development of a new homogenous photo-Fenton process using Fe(III)-EDDS complexes. J. Photochem. Photobiol. A Chem. 2012, 239, 17–23. [Google Scholar] [CrossRef]
- Huang, W.; Brigante, M.; Wu, F.; Mousty, C.; Hanna, K.; Mailhot, G. Assessment of the Fe(III)-EDDS complex in Fenton-like processes: From the radical formation to the degradation of bisphenol A. Environ. Sci. Technol. 2013, 47, 1952–1959. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Passananti, M.; Brigante, M.; Dong, W.; Mailhot, G. Fe(III)–EDDS complex in Fenton and photo-Fenton processes: From the radical formation to the degradation of a target compound. Environ. Sci. Pollut. Res. 2014, 21, 12154–12162. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Luo, M.; Wei, C.; Wang, Y.; Hanna, K.; Mailhot, G. Enhanced heterogeneous photo-Fenton process modified by magnetite and EDDS: BPA degradation. Environ. Sci. Pollut. Res. 2017, 24, 10421–10429. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, D.; Dong, M.; Chai, Z.; Fu, G. Facile and Green Synthesis of Core-Shell Structured Magnetic Chitosan Submicrospheres and Their Surface Functionalization. Langmuir 2013, 29, 11770–11778. [Google Scholar] [CrossRef]
- Magnacca, G.; Allera, A.; Montoneri, E.; Celi, L.; Benito, D.E.; Gagliardi, L.G.; Gonzalez, M.C.; Mártire, D.O.; Carlos, L. Novel magnetite nanoparticles coated with waste-sourced biobased substances as sustainable and renewable adsorbing materials. ACS Sustain. Chem. Eng. 2014, 2, 1518–1524. [Google Scholar] [CrossRef]
- Cesano, F.; Fenoglio, G.; Carlos, L.; Nisticò, R. One-step synthesis of magnetic chitosan polymer composite films. Appl. Surf. Sci. 2015, 345, 175–181. [Google Scholar] [CrossRef]
- Palma, D.; Bianco Prevot, A.; Celi, L.; Fabbri, D.; Magnacca, G.; Nisticò, R.; Marin, M.; Chierotti, M. Isolation, Characterization, and Environmental Application of Bio-Based Materials as Auxiliaries in Photocatalytic Processes. Catalysts 2018, 8, 197. [Google Scholar] [CrossRef]
- Nisticò, R.; Bianco Prevot, A.; Magnacca, G.; Canone, L.; García-Ballesteros, S.; Arques, A. Sustainable Magnetic Materials (from Chitosan and Municipal Biowaste) for the Removal of Diclofenac from Water. Nanomaterials 2019, 9, 1091. [Google Scholar] [CrossRef]
- Aparicio, F.; Escalada, J.; De Gerónimo, E.; Aparicio, V.; García Einschlag, F.; Magnacca, G.; Carlos, L.; Mártire, D. Carbamazepine degradation mediated by light in the presence of humic substances-coated magnetite nanoparticles. Nanomaterials 2019, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Gomis, J.; Vercher, R.F.; Amat, A.M.; Mártire, D.O.; González, M.C.; Bianco Prevot, A.; Montoneri, E.; Arques, A.; Carlos, L. Application of soluble bio-organic substances (SBO) as photocatalysts for wastewater treatment: Sensitizing effect and photo-Fenton-like process. Catal. Today 2013, 209, 176–180. [Google Scholar] [CrossRef]
- Gomis, J.; Carlos, L.; Bianco Prevot, A.; Teixeira, A.C.S.C.; Mora, M.; Amat, A.M.; Vicente, R.; Arques, A. Bio-based substances from urban waste as auxiliaries for solar photo-Fenton treatment under mild conditions: Optimization of operational variables. Catal. Today 2015, 240, 39–45. [Google Scholar] [CrossRef]
- European Commission Circular Economy Strategy-Environment-Implementation of the Circular Economy Action Plan. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 3 October 2018).
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Mártire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Baino, F.; Fabbri, D.; Franzoso, F.; Magnacca, G.; Nisticò, R.; Arques, A. Urban biowaste-derived sensitizing materials for caffeine photodegradation. Environ. Sci. Pollut. Res. 2017, 24, 12599–12607. [Google Scholar] [CrossRef]
- Palma, D.; Bianco Prevot, A.; Fabbri, D.; Nisticò, R.; Brigante, M.; Richard, C.; Mailhot, G. New insights on the photodegradation of caffeine in the presence of bio-based substances-magnetite hybrid nanomaterials. Materials 2018, 11, 1084. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Perez-Gonzalez, T.; Rodriguez-Navarro, A.; Jimenez-Lopez, C. Inorganic magnetite precipitation at 25 °C: A low-cost inorganic coprecipitation method. J. Supercond. Nov. Magn. 2011, 24, 549–557. [Google Scholar] [CrossRef]
- Lim, Y.S.; Lai, C.W.; Hamid, S.B.A.; Julkapli, N.M.; Yehya, W.A.; Karim, M.Z.; Tai, M.F.; Lau, K.S. A study on growth formation of nano-sized magnetite Fe3O4 via co-precipitation method. Mater. Res. Innov. 2014, 18, S6-457–S6-461. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the catalytic activity of a doped SrFeO3 for water pollutants removal: Effect of light and temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Fortune, W.B.; Mellon, M.G. Determination of Iron with o-Phenanthroline: A Spectrophotometric Study. Ind. Eng. Chem. Anal. Ed. 1938, 10, 60–64. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol-gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Minella, M.; Marchetti, G.; De Laurentiis, E.; Malandrino, M.; Maurino, V.; Minero, C.; Vione, D.; Hanna, K. Photo-Fenton oxidation of phenol with magnetite as iron source. Appl. Catal. B Environ. 2014, 154–155, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Arques, A.; Bianco Prevot, A. Soluble Bio-Based Substances Isolated from Urban Wastes; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-14743-7. [Google Scholar]
- Miano, T.M.; Senesi, N. Synchronous excitation fluorescence spectroscopy applied to soil humic substances chemistry. Sci. Total Environ. 1992, 117, 41–51. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calza, P.; Di Sarro, J.; Magnacca, G.; Bianco Prevot, A.; Laurenti, E. Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants. Materials 2019, 12, 3942. https://doi.org/10.3390/ma12233942
Calza P, Di Sarro J, Magnacca G, Bianco Prevot A, Laurenti E. Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants. Materials. 2019; 12(23):3942. https://doi.org/10.3390/ma12233942
Chicago/Turabian StyleCalza, Paola, Jessica Di Sarro, Giuliana Magnacca, Alessandra Bianco Prevot, and Enzo Laurenti. 2019. "Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants" Materials 12, no. 23: 3942. https://doi.org/10.3390/ma12233942
APA StyleCalza, P., Di Sarro, J., Magnacca, G., Bianco Prevot, A., & Laurenti, E. (2019). Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants. Materials, 12(23), 3942. https://doi.org/10.3390/ma12233942







