A Facile Synthesis of Core-Shell SiO2@Cu-LBMS Nano-Microspheres for Drug Sustained Release Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Cu-LBMS
2.2.2. Preparation of SiO2 Microspheres
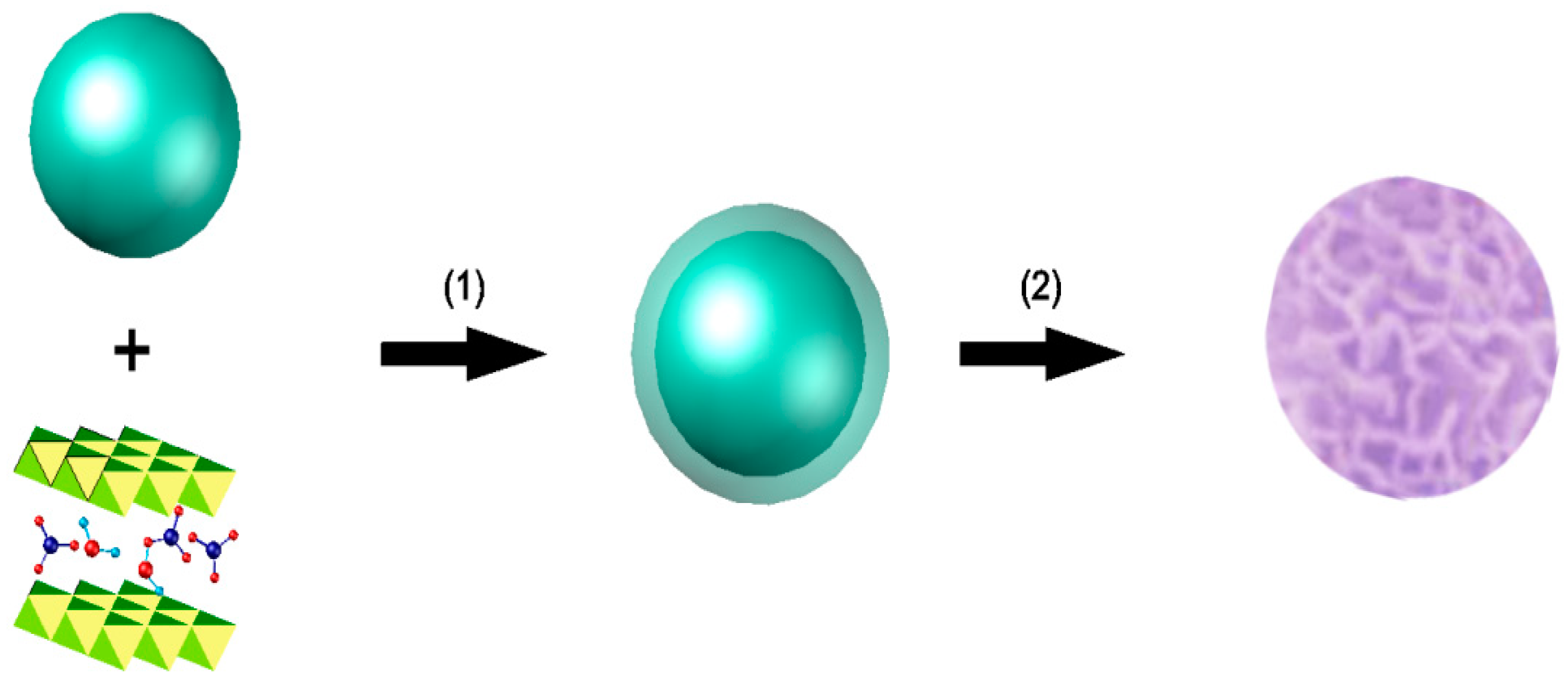
2.2.3. Hybrid Assembly of Cu-LBMS with SiO2 Microspheres
2.2.4. Preparation of the Drug-Loaded SiO2@Cu-LBMS and Drug-Loaded Cu-LBMS
2.2.5. In vitro Drug Cumulative Release Studies
2.2.6. Characterization
3. Results and Discussion
3.1. The Morphology of SiO2@Cu-LBMS
3.2. Hybrid Assembly Process of Cu-LBMS and SiO2
3.3. Preparation of SiO2@Cu-LBMS Nanocomposite
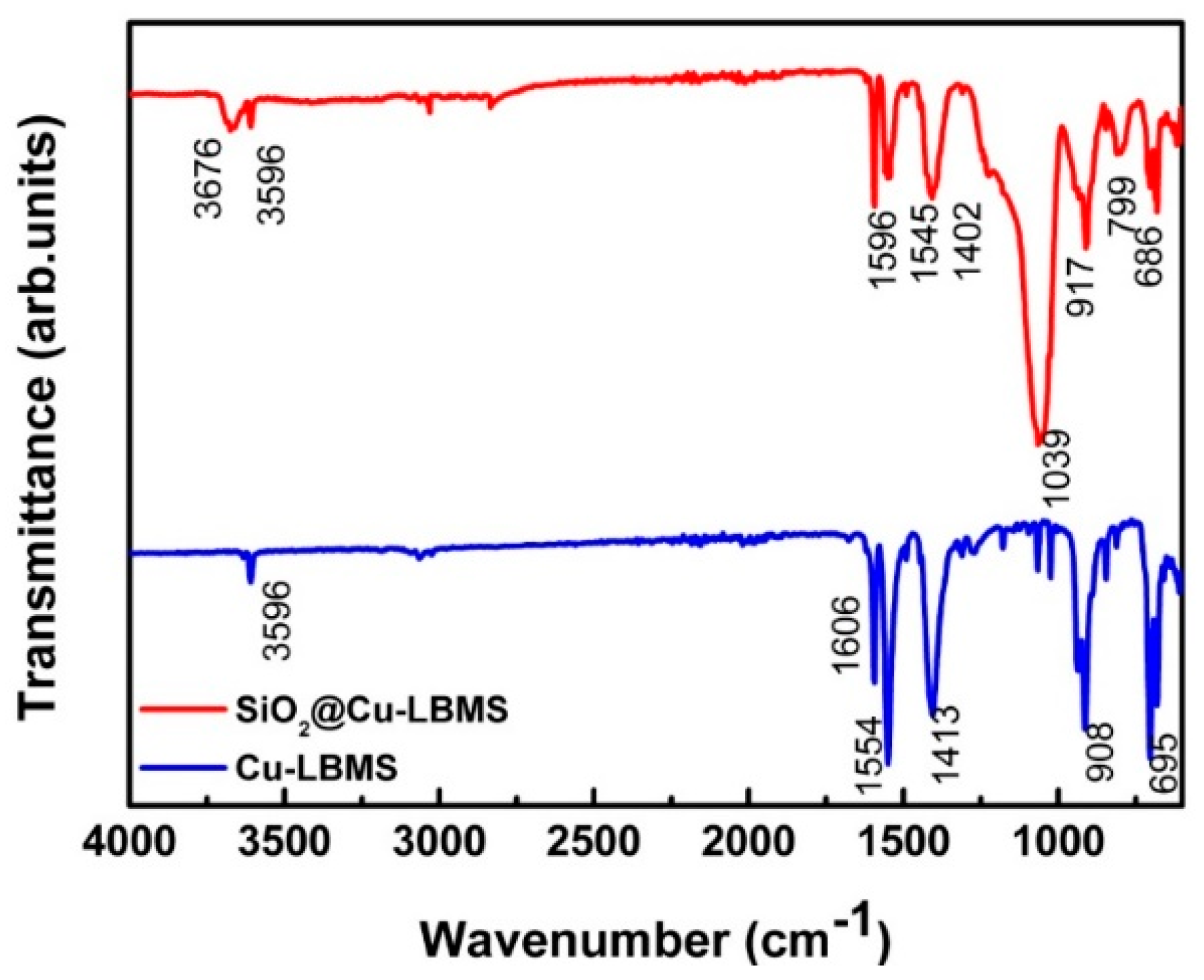
3.4. The Physicochemical Properties of SiO2@Cu-LBMS Microspheres
3.5. The Drug Loading Property of SiO2@Cu-LBMS Microspheres
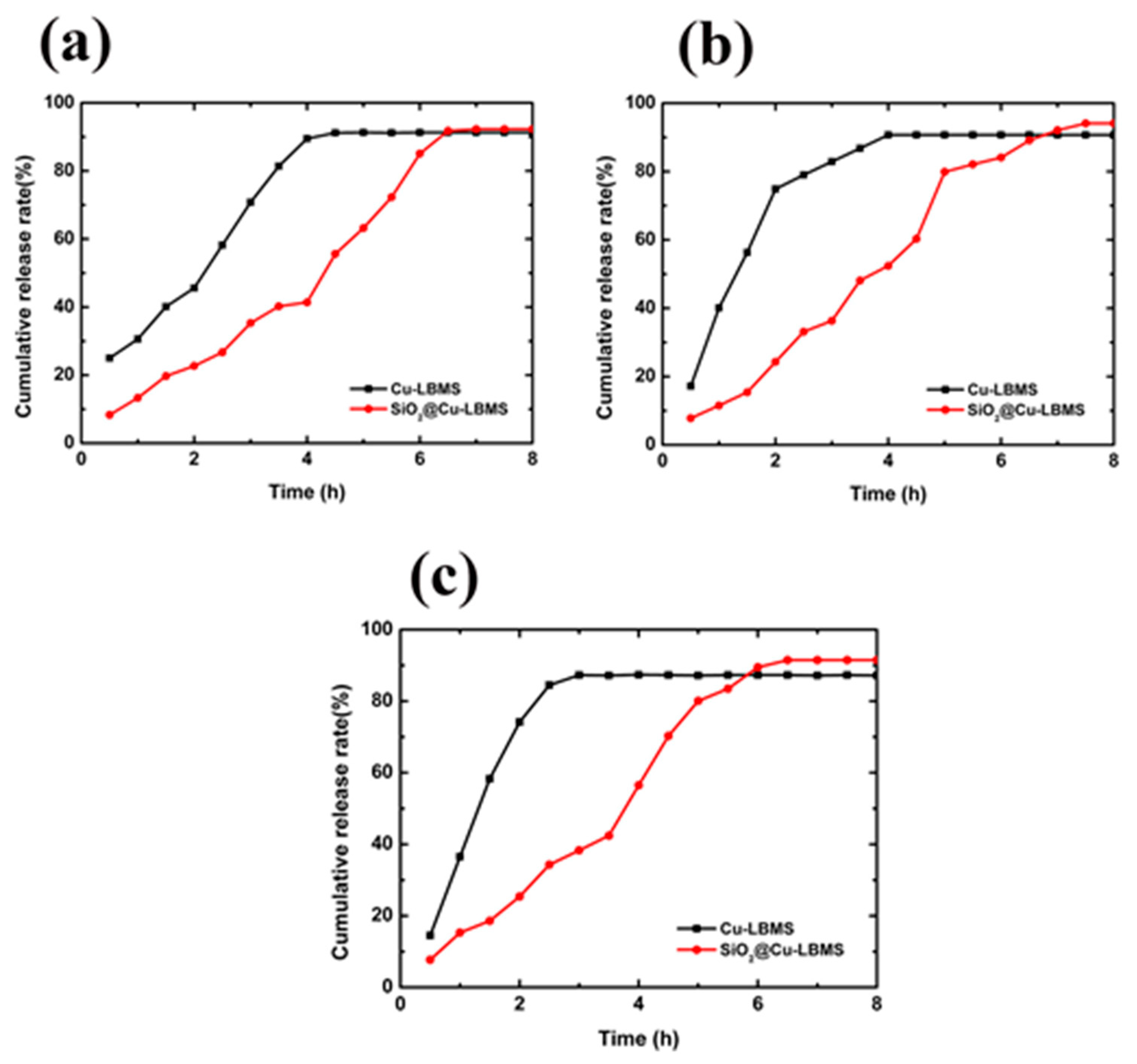
3.6. The Effects of SiO2@Cu-LBMS Microspheres on Drug Release Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Guo, W.; Yang, C.; Cui, L.; Lin, H.; Qu, F. An Enzyme-Responsive Controlled Release System of Mesoporous Silica Coated with Konjac Oligosaccharide. Langmuir 2014, 30, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Forsgren, J.; Strømme, M. Stabilisation of amorphous ibuprofen in Upsalite, a mesoporous magnesium carbonate, as an approach to increasing the aqueous solubility of poorly soluble drugs. Int. J. Pharm. 2014, 472, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.-S.; Yang, C. Thermo-Sensitive Poly(N-Isopropylacrylamide)/Mesoporous Silica Nanocomposites as Controlled Delivery Carriers: Loading and Release Behaviors for Drug Ibuprofen. J. Nanosci. Nanotechnol. 2011, 11, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zardán Gómez de la Torre, T.; Forsgren, J.; Bergström, C.A.S.; Strømme, M. Diffusion-Controlled Drug Release from the Mesoporous Magnesium Carbonate Upsalite®. J. Pharm. Sci. 2016, 105, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef]
- DeLeon, V.H.; Nguyen, T.D.; Nar, M.; D’Souza, N.A.; Golden, T.D. Polymer nanocomposites for improved drug delivery efficiency. Mater. Chem. Phys. 2012, 132, 409–415. [Google Scholar] [CrossRef]
- Wolfram, J.; Scott, B.; Boom, K.; Shen, J.; Borsoi, C.; Suri, K.; Grande, R.; Fresta, M.; Celia, C.; Zhao, Y.; et al. Hesperetin Liposomes for Cancer Therapy. Curr. Drug Deliv. 2016, 13, 711–719. [Google Scholar] [CrossRef]
- Scialabba, C.; Sciortino, A.; Messina, F.; Buscarino, G.; Cannas, M.; Roscigno, G.; Condorelli, G.; Cavallaro, G.; Giammona, G.; Mauro, N. Highly Homogeneous Biotinylated Carbon Nanodots: Red-Emitting Nanoheaters as Theranostic Agents toward Precision Cancer Medicine. ACS Appl. Mater. Interfaces 2019, 11, 19854–19866. [Google Scholar] [CrossRef]
- Carrasco, J.A.; Abellán, G.; Coronado, E. Influence of morphology in the magnetic properties of layered double hydroxides. J. Mater. Chem. C 2018, 6, 1187–1198. [Google Scholar] [CrossRef]
- Ansy, K.M.; Lee, J.-H.; Piao, H.; Choi, G.; Choy, J.-H. Stabilization of antioxidant gallate in layered double hydroxide by exfoliation and reassembling reaction. Solid State Sci. 2018, 80, 65–71. [Google Scholar] [CrossRef]
- Yang, W.; Xia, Y.; Liu, X.; Yang, J.; Liu, Y. Layered double hydroxides/reduced graphene oxide nanocomposites with enhanced barrier properties. Polym. Compos. 2018, 39, 3841–3848. [Google Scholar] [CrossRef]
- Xu, Z.P.; Gu, Z.; Cheng, X.; Rasoul, F.; Whittaker, A.K.; Lu, G.Q.M. Controlled release of ketorolac through nanocomposite films of hydrogel and LDH nanoparticles. J. Nanopart. Res. 2011, 13, 1253–1264. [Google Scholar] [CrossRef]
- Mahkam, M.; Davatgar, M.; Rezvani, Z.; Nejati, K. Preparation of pH-Sensitive Polymers/Layered Double Hydroxide Hybrid Beads for Controlled Release of Insulin. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 57–60. [Google Scholar] [CrossRef]
- Lai, Y.; Li, K.; Qiao, G.; Wang, F.; Zhao, H.; Yang, W.; Yang, J.; Du, H. Structure and magnetic properties of layered compounds RMn1.7Cr0.3Si2C (R = Nd, Sm, Dy). J. Alloys Compd. 2018, 746, 238–243. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Omidi-Ghallemohamadi, M. Preparation and characterization of poly(amide-imide)/Mg-Al LDH nanocomposites; effect of organo-modified LDH on thermal properties and morphology. Polym. Compos. 2018, 39, E1669–E1681. [Google Scholar] [CrossRef]
- Ma, B.; Fernandez-Martinez, A.; Grangeon, S.; Tournassat, C.; Findling, N.; Carrero, S.; Tisserand, D.; Bureau, S.; Elkaïm, E.; Marini, C.; et al. Selenite Uptake by Ca–Al LDH: A Description of Intercalated Anion Coordination Geometries. Environ. Sci. Technol. 2018, 52, 1624–1632. [Google Scholar] [CrossRef]
- Luo, S.; Hao, J.; Gao, Y.; Liu, D.; Cai, Q.; Yang, X. Pore size effect on adsorption and release of metoprolol tartrate in mesoporous silica: Experimental and molecular simulation studies. Mater. Sci. Eng. C 2019, 100, 789–797. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, P.; Bajnóczi, É.G.; Neagu, A.; Tai, C.-W.; Persson, I.; Strømme, M.; Cheung, O. Amorphous Calcium Carbonate Constructed from Nanoparticle Aggregates with Unprecedented Surface Area and Mesoporosity. ACS Appl. Mater. Interfaces 2018, 10, 21556–21564. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Benito, P.; Guinea, I.; Herrero, M.; Labajos, F.M.; Rives, V. Incidence of Microwave Hydrothermal Treatments on the Crystallinity Properties of Hydrotalcite-like Compounds. Z. Anorg. Allg. Chem. 2007, 633, 1815–1819. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Dash, B.; Pandey, S.; Mohanty, P.P. Antibacterial actions of silver nanoparticles incorporated Zn–Al layered double hydroxide and its spinel. J. Environ. Chem. Eng. 2013, 1, 1124–1130. [Google Scholar] [CrossRef]
- Ay, A.N.; Zümreoglu-Karan, B.; Temel, A.; Mafra, L. Layered double hydroxides with interlayer borate anions: A critical evaluation of synthesis methodology and pH-independent orientations in nano-galleries. Appl. Clay Sci. 2011, 51, 308–316. [Google Scholar] [CrossRef]
- Mandal, S.; Tripathy, S.; Padhi, T.; Sahu, M.K.; Patel, R.K. Removal efficiency of fluoride by novel Mg-Cr-Cl layered double hydroxide by batch process from water. J. Environ. Sci. 2013, 25, 993–1000. [Google Scholar] [CrossRef]
- Wang, L.Y.; Tong, D.S.; Zhao, L.Z.; Liu, F.G.; An, N.; Yu, W.H.; Zhou, C.H. Utilization of alum sludge for producing aluminum hydroxide and layered double hydroxide. Ceram. Int. 2014, 40, 15503–15514. [Google Scholar] [CrossRef]
- Zhao, Y.; He, S.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical films of layered double hydroxides by using a sol–gel process and their high adaptability in water treatment. Chem. Commun. 2010, 46, 3031–3033. [Google Scholar] [CrossRef]
- Chen, C.; Gunawan, P.; Xu, R. Self-assembled Fe3O4-layered double hydroxide colloidal nanohybrids with excellent performance for treatment of organic dyes in water. J. Mater. Chem. 2011, 21, 1218–1225. [Google Scholar] [CrossRef]
- Gong, J.; Liu, T.; Wang, X.; Hu, X.; Zhang, L. Efficient Removal of Heavy Metal Ions from Aqueous Systems with the Assembly of Anisotropic Layered Double Hydroxide Nanocrystals@Carbon Nanosphere. Environ. Sci. Technol. 2011, 45, 6181–6187. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Luo, T.; Jia, Y.; Xu, R.-X.; Gao, C.; Zhang, Y.-X.; Liu, J.-H.; Huang, X.-J. Three-dimensional hierarchical flower-like Mg–Al-layered double hydroxides: Highly efficient adsorbents for As(V) and Cr(VI) removal. Nanoscale 2012, 4, 3466–3474. [Google Scholar] [CrossRef]
- Xu, Y.; Kominami, K.; Ishikawa, Y.; Feng, Q. Layered hydroxide nickel benzoates: Hydrothermal synthesis, structure characterization, and exfoliation reaction. J. Colloid Interface Sci. 2012, 386, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Miao, J.; Iwasa, Y.; Feng, Q. Transformation of layered hydroxide zinc benzoate nanosheets into ZnO nanocrystals by electron beam irradiation. J. Ceram. Soc. Jpn. 2008, 116, 657–660. [Google Scholar] [CrossRef] [Green Version]
- Quites, F.J.; Germino, J.C.; da Silva Azevedo, C.K.; Moreto, J.A.; Faleiros, M.M.; Atvars, T.D.Z. Exfoliation of zinc-layered hydroxide by luminescent conjugate polyelectrolyte: Synthesis and photophysical aspects. J. Sol-Gel Sci. Technol. 2017, 83, 457–466. [Google Scholar] [CrossRef]
- Hussein, M.Z.; Hashim, N.; Yahaya, A.H.; Zainal, Z. Synthesis and characterization of [4-(2,4-dichlorophenoxybutyrate)-zinc layered hydroxide] nanohybrid. Solid State Sci. 2010, 12, 770–775. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Wang, Y.; Miao, J.; Feng, Q. Synthesis of Layered Hydroxide Zinc m-Aminobenzoate Compounds and Their Exfoliation Reactions. Chin. J. Chem. 2011, 29, 1837–1845. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Lim, T.-T.; Liu, L.; Liu, S.; Xu, R. A facile synthesis of monodispersed hierarchical layered double hydroxide on silica spheres for efficient removal of pharmaceuticals from water. J. Mater. Chem. A 2013, 1, 3877–3880. [Google Scholar] [CrossRef]
- Chen, C.; Felton, R.; Buffet, J.-C.; O’Hare, D. Core–shell SiO2@LDHs with tuneable size, composition and morphology. Chem. Commun. 2015, 51, 3462–3465. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Wang, Z.; Liang, P.; He, X.; Zhuang, X.; Huang, R.; Wang, M.; Wang, Q.; Qian, Y.; Wang, S. Efficient VEGF targeting delivery of DOX using Bevacizumab conjugated SiO2@LDH for anti-neuroblastoma therapy. Acta Biomater. 2017, 63, 163–180. [Google Scholar] [CrossRef]
- Mallakpour, S.; Naghdi, M. Polymer/SiO2 nanocomposites: Production and applications. Prog. Mater. Sci. 2018, 97, 409–447. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Yamada, A.; Noba, M.; Kobayashi, Y.; Konno, M.; Nagao, D. Electrolyte-Added One-Pot Synthesis for Producing Monodisperse, Micrometer-Sized Silica Particles up to 7 μm. Langmuir 2010, 26, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, X.; Wu, D.; Yu, J.; Zhou, Y.; Luo, Y.; Wei, K.; Ma, W. Synthesis of spherical mesoporous silica materials by pseudomorphic transformation of silica fume and its Pb2+ removal properties. Microporous Mesoporous Mater. 2016, 222, 192–201. [Google Scholar] [CrossRef]
- Cao, C.; Li, Z.; Li, Y.; Wang, G.; Yuan, S.; Li, H.; Guo, P.; Zhao, X.S. Synthesis, characterization and electrochemical applications of Ir@SiO2 composite microspheres. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 529, 979–984. [Google Scholar] [CrossRef]
- Alimunnisa, J.; Ravichandran, K.; Meena, K.S. Synthesis and characterization of Ag@SiO2 core-shell nanoparticles for antibacterial and environmental applications. J. Mol. Liq. 2017, 231, 281–287. [Google Scholar] [CrossRef]
- Zhao, B.; Tian, C.; Zhang, Y.; Tang, T.; Wang, F. Size control of monodisperse nonporous silica particles by seed particle growth. Particuology 2011, 9, 314–317. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Z.R.; Niu, H.W.; Wang, Z.Y.; Ba, J.; Qi, J.L.; Feng, J.C.; He, P.; Ma, J. The effect of crystal structure of SiO2 on the wettability of AgCuTiSiO2f/SiO2 system. Vacuum 2018, 157, 124–127. [Google Scholar] [CrossRef]
- Haraketi, M.; Hosni, K.; Srasra, E. Intercalation behavior of salicylic acid into calcined Cu-Al-layered double hydroxides for a controlled release formulation. Surf. Eng. Appl. Electrochem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Behzadi Nia, S.; Namazi, H. Green encapsulation of LDH(Zn/Al)-5-Fu with carboxymethyl cellulose biopolymer; new nanovehicle for oral colorectal cancer treatment. Int. J. Biol. Macromol. 2019, 139, 994–1001. [Google Scholar] [CrossRef]
- Miao, J.; Xue, M.; Itoh, H.; Feng, Q. Hydrothermal synthesis of layered hydroxide zinc benzoate compounds and their exfoliation reactions. J. Mater. Chem. 2006, 16, 474–480. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.; Zhang, Y.; Song, Y.; Zuo, X. Interactions of atomic hydrogen with amorphous SiO2. Phys. B Condens. Matter 2018, 533, 5–11. [Google Scholar] [CrossRef]
- Tolnai, G.; Csempesz, F.; Kabai-Faix, M.; Kálmán, E.; Keresztes, Z.; Kovács, A.L.; Ramsden, J.J.; Hórvölgyi, Z. Preparation and Characterization of Surface-Modified Silica-Nanoparticles. Langmuir 2001, 17, 2683–2687. [Google Scholar] [CrossRef]
- Godjevargova, T.; Velikova, M.; Vasileva, N.; Dimova, N.; Damyanov, D. Metallochelate immobilization of urease on to amorphous SiO2. Process Biochem. 2005, 40, 3045–3049. [Google Scholar] [CrossRef]





| Materials | Ibuprofen (mg/g) | Aspirin (mg/g) | Salicylic Acid(mg/g) |
|---|---|---|---|
| SiO2@Cu-LBMS | 263.16 | 380.14 | 362.23 |
| Cu-LBMS | 160.05 | 300.02 | 254.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, H.; Zhao, L. A Facile Synthesis of Core-Shell SiO2@Cu-LBMS Nano-Microspheres for Drug Sustained Release Systems. Materials 2019, 12, 3978. https://doi.org/10.3390/ma12233978
Wang H, Yang H, Zhao L. A Facile Synthesis of Core-Shell SiO2@Cu-LBMS Nano-Microspheres for Drug Sustained Release Systems. Materials. 2019; 12(23):3978. https://doi.org/10.3390/ma12233978
Chicago/Turabian StyleWang, Hui, Haifeng Yang, and Lifang Zhao. 2019. "A Facile Synthesis of Core-Shell SiO2@Cu-LBMS Nano-Microspheres for Drug Sustained Release Systems" Materials 12, no. 23: 3978. https://doi.org/10.3390/ma12233978
APA StyleWang, H., Yang, H., & Zhao, L. (2019). A Facile Synthesis of Core-Shell SiO2@Cu-LBMS Nano-Microspheres for Drug Sustained Release Systems. Materials, 12(23), 3978. https://doi.org/10.3390/ma12233978





