Linear Tuning of Phase-Matching Temperature in LiNbO3:Zr Crystals by MgO Co-Doping
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
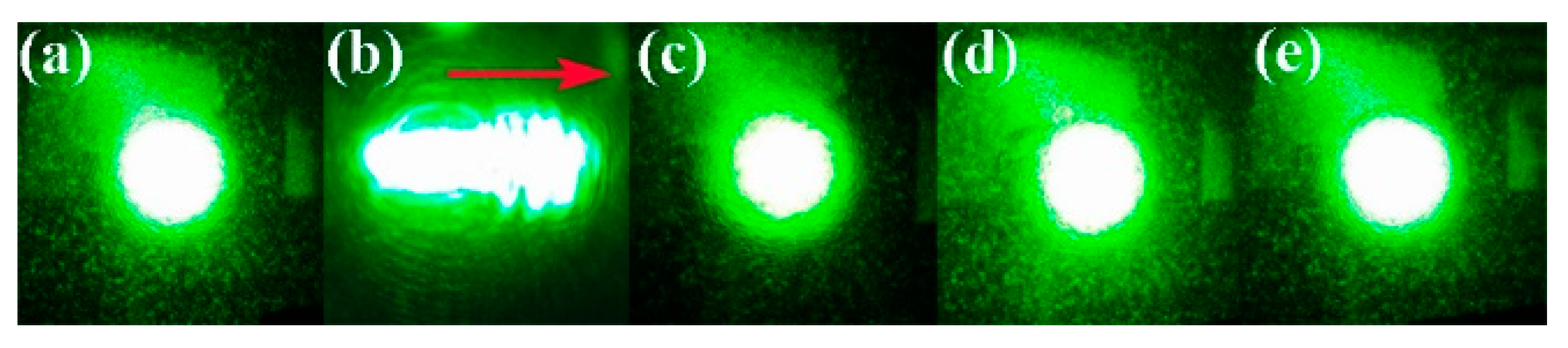
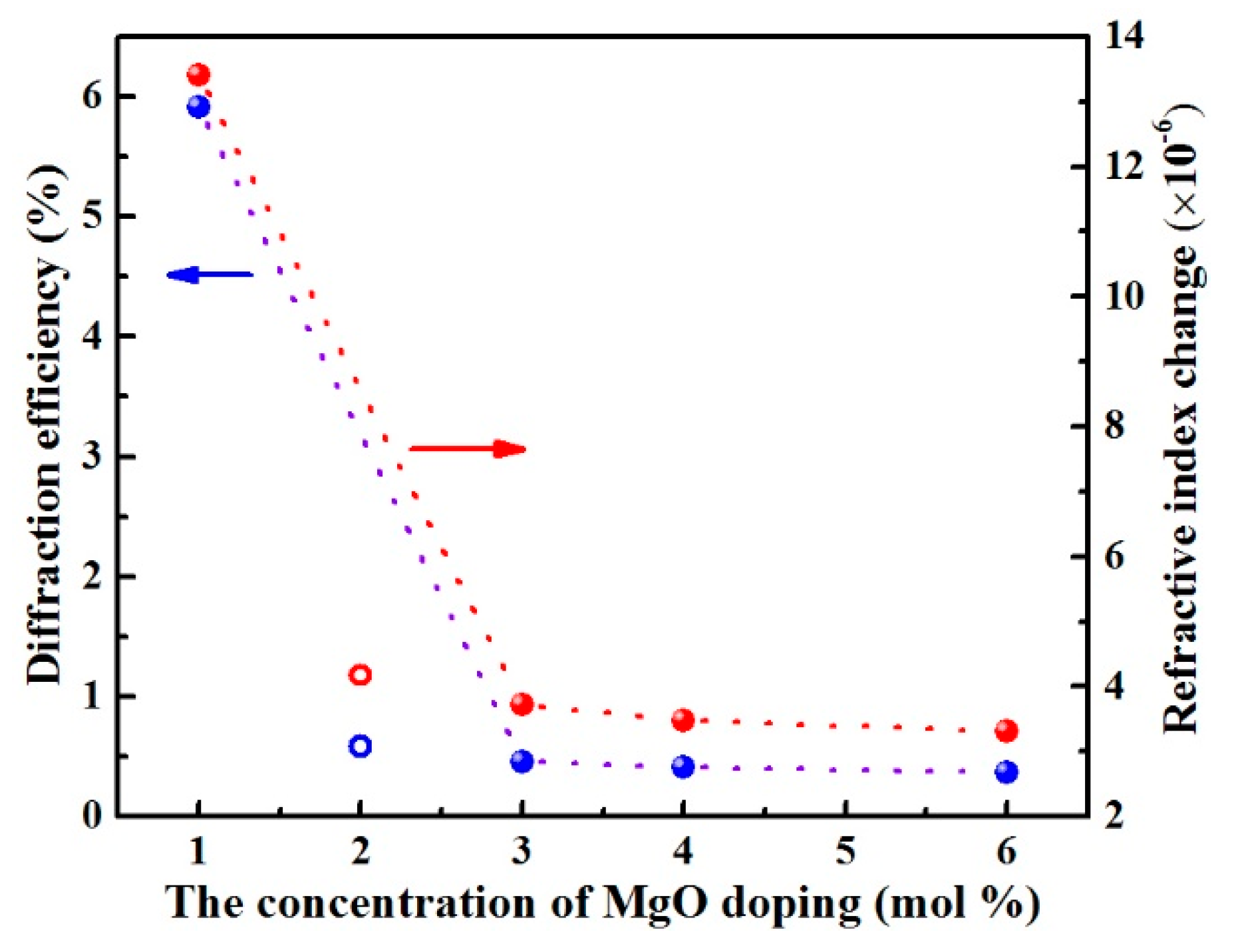
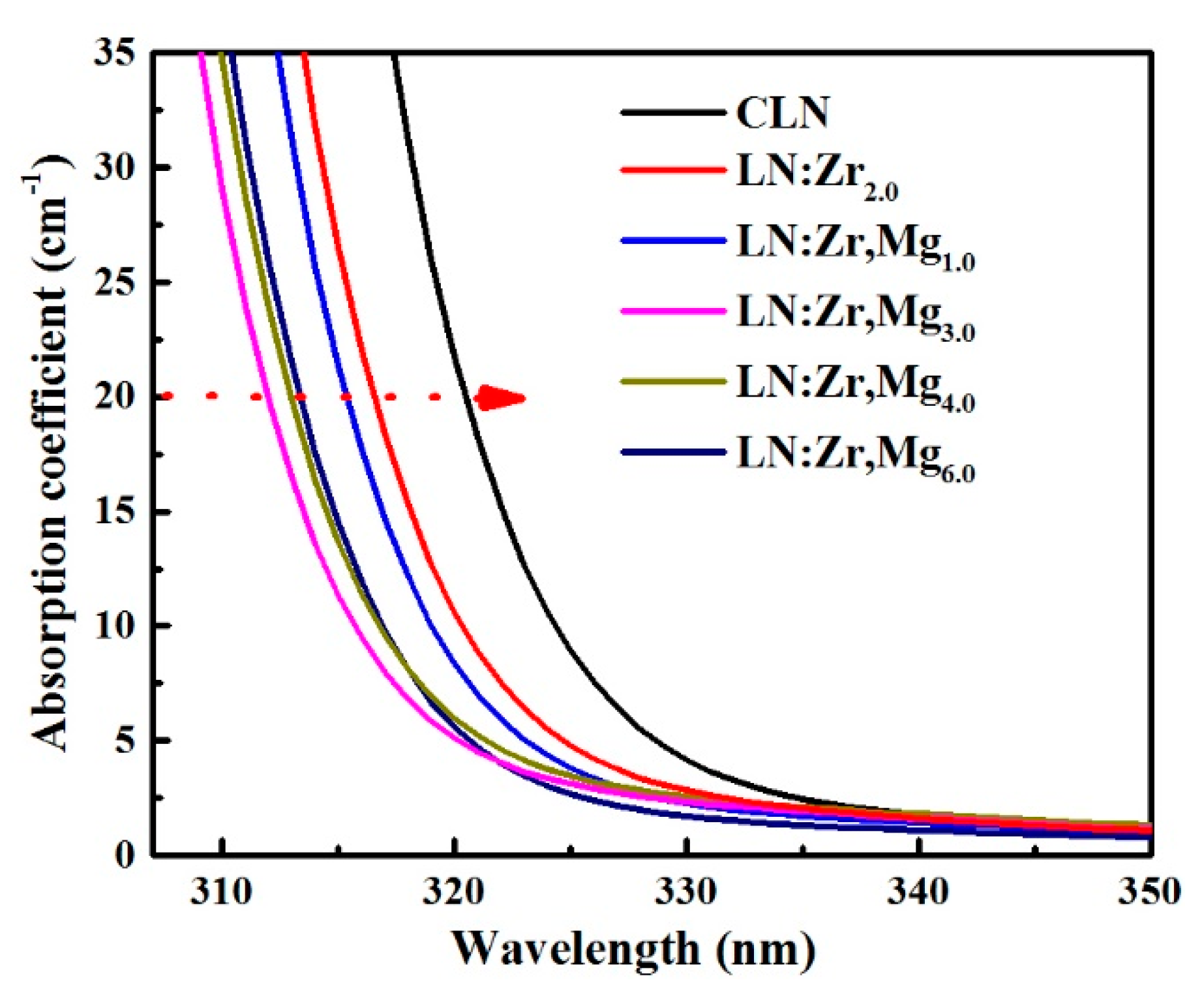
3.1. Optical Damage Resistance
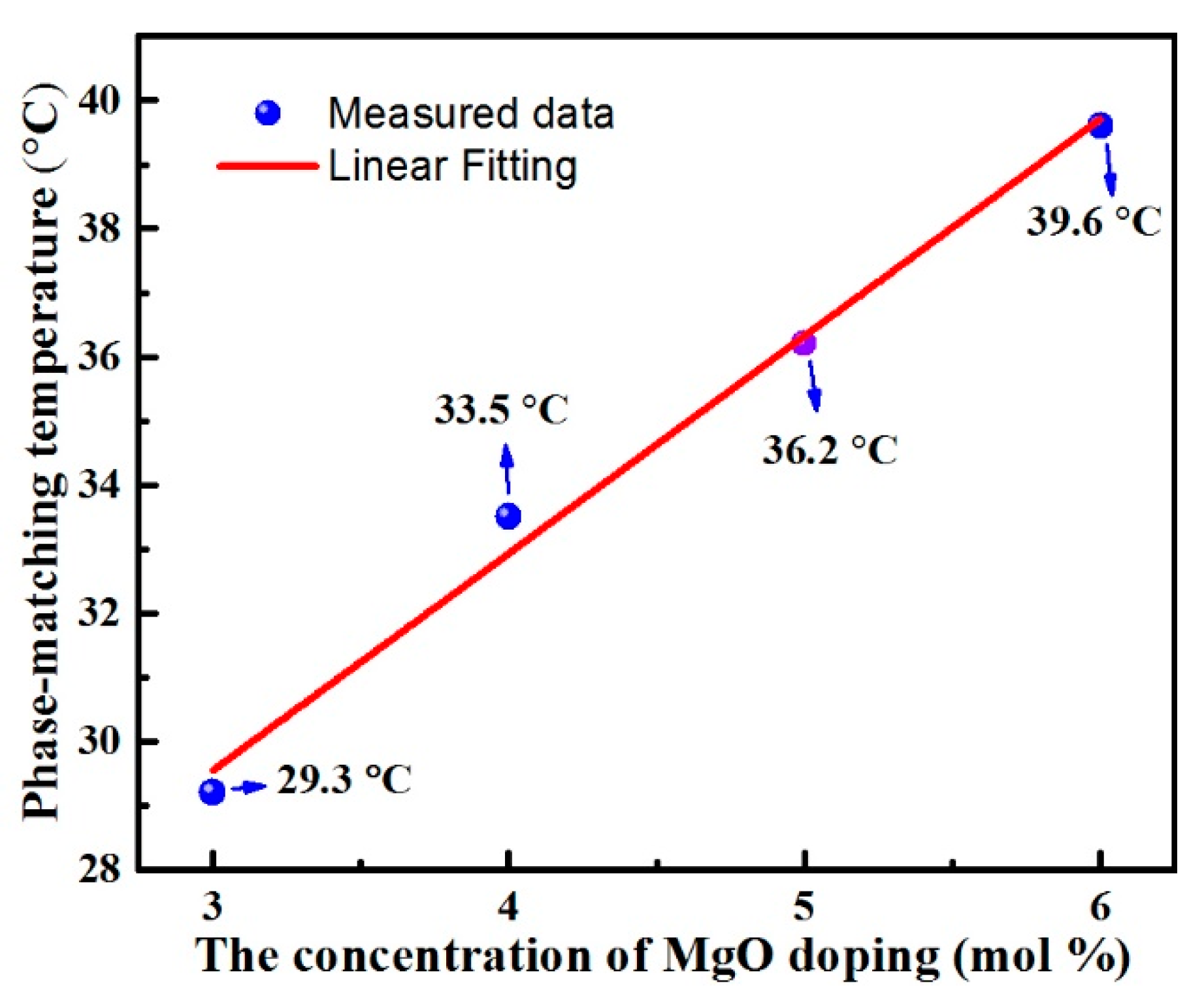
3.2. The Temperature for 90° Phase Matching
3.3. OH− Absorption Spectra
3.4. UV-Visible Absorption Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kong, Y.; Bo, F.; Wang, W.; Zheng, D.; Liu, H.; Zhang, G.; Rupp, R.; Xu, J. Recent progress in lithium niobate: Optical damage, defect simulation, and on-chip devices. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rota-Rodrigo, S.; Gouhier, B.; Dixneuf, C.; Antoni-Micollier, L.; Guiraud, G.; Leandro, D.; Lopez-Amo, M.; Traynor, N.; Santarelli, G. Watt-level green random laser at 532 nm by SHG of a Yb-doped fiber laser. Opt. Lett. 2018, 43, 4284–4287. [Google Scholar] [CrossRef] [PubMed]
- Fay, H. Dependence of second-harmonic phase-matching temperature in LiNbO3 crystals on melt composition. Appl. Phys. Lett. 1968, 12, 89. [Google Scholar] [CrossRef]
- Grabmaier, B.C.; Otto, F. Growth and investigation of MgO-doped LiNbO3. J. Cryst. Growth 1986, 79, 682–688. [Google Scholar] [CrossRef]
- Volk, T.R.; Pryalkin, V.I.; Rubinina, N.M. Optical-damage-resistant LiNbO3: Zn crystal. Opt. Lett. 1990, 15, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wen, J.; Wang, H. New doped lithium niobate crystal with high resistance to photorefraction-LiNbO3:In. Appl. Phys. Lett. 1995, 66, 280–281. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, S.; Zhao, Y.; Liu, H.; Chen, S.; Xu, J. Highly optical damage resistant crystal: Zirconium-oxide-doped lithium niobate. Appl. Phys. Lett. 2007, 91, 081908. [Google Scholar] [CrossRef]
- Kong, Y.F.; Liu, S.G.; Xu, J.J. Recent Advances in the photorefraction of doped Lithium Niobate Crystals. Materials 2012, 5, 1954–1971. [Google Scholar] [CrossRef] [Green Version]
- Ortega, T.A.; Pask, H.M.; Spence, D.J.; Lee, A.J. THz polariton laser using an intracavity Mg:LiNbO3 crystal with protective teflon coating. Opt. Express 2017, 25, 3991–3999. [Google Scholar] [CrossRef]
- Shukla, M.K.; Das, R. High-power single-frequency source in the mid-infrared using a singly resonant optical parametric oscillator pumped by Yb-fiber laser. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 17255125. [Google Scholar] [CrossRef]
- Zhao, J.; Jia, F.; Feng, Y.; Nilsson, J. Continuous-Wave 3.1–3.6 μm difference-frequency generation of dual wavelength-tunable fiber sources in PPMgLN-based rapid-tuning design. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 17088616. [Google Scholar] [CrossRef]
- Fan, T.Y.; Cordova-Plaza, A.; Digonnet, M.J.F.; Byer, R.L.; Shaw, H.J. Nd:MgO:LiNbO3 spectroscopy and laser devices. J. Opt. Soc. Am. B 1986, 3, 140–148. [Google Scholar] [CrossRef]
- Volk, T.; Rubinina, N.; Wöhlecke, M. Optical-damage-resistant impurities in lithium niobate. J. Opt. Soc. Am. B 1994, 11, 1681–1687. [Google Scholar] [CrossRef]
- Kong, T.; Liu, H.; Ge, X.; Qu, D.; Liu, S.; Chen, S.; Zhang, L.; Kong, Y.; Rupp, R.; Xu, J. Room temperature 90° phase-matching in zirconium and magnesium co-doped lithium niobate crystals. Sci. Rep. 2018, 8, 3865. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, W.; Wang, S.; Qu, D.; Liu, H.; Kong, Y.; Liu, S.; Chen, S.; Rupp, R.; Xu, J. Real-time dynamic holographic display realized by bismuth and magnesium co-doped lithium niobate. Appl. Phys. Lett. 2019, 114, 241903. [Google Scholar] [CrossRef]
- Li, M.H.; Xu, Y.H.; Xu, W.S.; Liu, C.X.; Zhang, W.L.; Shao, Z.S. Nonlinear optical effect of co-doped Mg, Zn: LiNbO3. Ferroelectrics 2001, 264, 273–278. [Google Scholar] [CrossRef]
- Liu, F.C.; Kong, Y.F.; Li, W.; Liu, H.D.; Liu, S.G.; Chen, S.L.; Zhang, X.Z.; Rupp, R.; Xu, J.J. High resistance against ultraviolet photorefraction in zirconium-doped lithium niobate crystals. Opt. Lett. 2010, 35, 10–12. [Google Scholar] [CrossRef]
- Liu, H.D.; Liang, Q.R.; Zhu, M.L.; Li, W.; Liu, S.G.; Zhang, L.; Chen, S.L.; Kong, Y.F.; Xu, J.J. An excellent crystal for high resistance against optical damage in visible-UV range: Near-stoichiometric zirconium-doped lithium niobate. Opt. Express 2011, 19, 1743–1748. [Google Scholar] [CrossRef]
- Kogelnik, H. Coupled Wave Theory for Thick Hologram Gratings. Bell System Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, W.; Wen, J.; Zhang, G.; Wang, H. Pulsed-laser-induced dark traces in LiNbO3:Zn and LiNbO3:Mg. Opt. Lett. 1994, 19, 933–935. [Google Scholar] [CrossRef]
- Kong, Y.; Deng, J.; Zhang, W.; Wen, J.; Zhang, G.; Wang, H. OH− absorption spectra in doped lithium niobate crystals. Phys. Lett. A 1994, 196, 128–132. [Google Scholar] [CrossRef]
- Xu, C.; Lei, Z.; Zhu, C.; Yang, C.; Zhang, C.; Sun, T.; Wang, R.; Xu, Y. Investigation on the OH− absorption and blue photorefractive properties in Zr:Ru:Fe:LiNbO3 crystals as a function of Li composition. J. Opt. Soc. Am. B 2012, 29, 2814–2818. [Google Scholar] [CrossRef]
- Kovács, L.; Szaller, Z.; Lengyel, K.; Péter, A.; Hajdara, I.; Mandula, G.; Pálfalvi, L.; Hebling, J. Photorefractive damage resistance threshold in stoichiometric LiNbO3:Zr crystals. Opt. Lett. 2013, 38, 2861–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.Q.; Tang, T. B, Mg-doping threshold effect and H-containing defects in LiNbO3. J. Phys. Condens. Matter 1993, 5, 2423–2430. [Google Scholar] [CrossRef]
- Volk, T.; Wöhlecke, M. Lithium Niobate. In Springer Series in Material Science; Springer: Berlin, Germany, 2008; Volume 115. [Google Scholar]
- Földvári, I.; Polgár, K.; Voszka, R.; Balasanyan, R.N. A simple method to determine the real composition of LiNbO3 crystals. Cryst. Res. Technol. 1984, 19, 1659–1661. [Google Scholar] [CrossRef]
- Redfield, D. Optical absorption edge of LiNbO3. J. Appl. Phys. 1974, 45, 4566. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Kong, Y.; Chen, S.; Huang, Z.; Wu, L.; Rupp, R.; Xu, J. Increased optical damage resistance in tin-doped lithium niobate. Opt. Lett. 2010, 35, 883–885. [Google Scholar] [CrossRef]
- Yang, X.; Xu, G.; Li, H.; Zhu, J.; Wang, X. Optical absorption edge of Mg+Zn: LiNbO3. Cryst. Res. Technol. 1996, 31, 521–527. [Google Scholar] [CrossRef]
- Xu, C.; Xu, Z.; Leng, X.; Yang, C.; Xu, Y. Investigation on the defect structure and light-induced scattering of LiNbO3:Fe:Mg:Zr crystals with various Li/Nb ratios. Mater. Chem. Phys. 2011, 130, 1203–1207. [Google Scholar] [CrossRef]
- Bhatt, R.; Bhaumik, I.; Ganesamoorthy, S.; Bright, R.; Soharab, M.; Karnal, A.; Gupta, P. Control of intrinsic defects in lithium niobate single crystal for optoelectronic applications. Crystals 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Kovács, L.; Rebouta, L.; Soares, J.C.; Silva, M.F.D.; Hage-Ali, M.; Stoquert, J.P.; Siffert, P.; Sanz-García, J.A.; Corradi, G.; Szaller, Z.; et al. On the lattice site of trivalent dopants and the structure of Mg2+-OH−-M3+ defects in LiNbO3:Mg crystals. J. Phys. Condens. Matter 1993, 5, 781. [Google Scholar] [CrossRef]

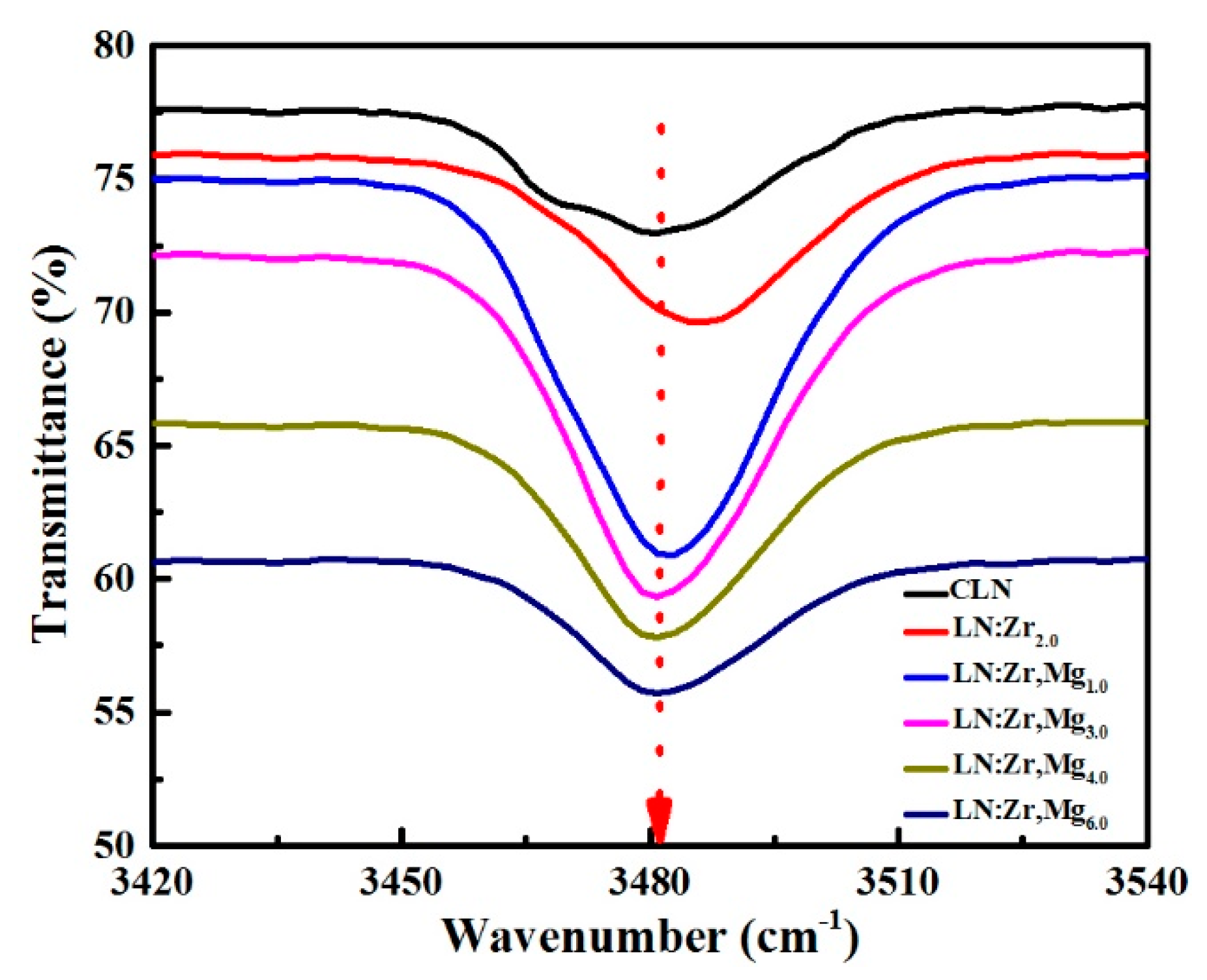
| Samples | Position of Peaks (cm−1) | ||
|---|---|---|---|
| CLN | 3468 | 3481 | 3490 |
| LN:Zr2.0 | 3475 | 3485 | 3495 |
| LN:Zr,Mg1.0 | 3470 | 3480 | 3490 |
| LN:Zr,Mg3.0 | 3473 | 3481 | 3491 |
| LN:Zr,Mg4.0 | 3474 | 3482 | 3493 |
| LN:Zr,Mg6.0 | 3475 | 3483 | 3495 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, T.; Liu, H.; Xue, L.; Wang, W.; Saeed, S.; Zheng, D.; Liu, S.; Chen, S.; Zhang, L.; Kong, Y.; et al. Linear Tuning of Phase-Matching Temperature in LiNbO3:Zr Crystals by MgO Co-Doping. Materials 2019, 12, 4155. https://doi.org/10.3390/ma12244155
Kong T, Liu H, Xue L, Wang W, Saeed S, Zheng D, Liu S, Chen S, Zhang L, Kong Y, et al. Linear Tuning of Phase-Matching Temperature in LiNbO3:Zr Crystals by MgO Co-Doping. Materials. 2019; 12(24):4155. https://doi.org/10.3390/ma12244155
Chicago/Turabian StyleKong, Tengfei, Hongde Liu, Liyun Xue, Weiwei Wang, Shahzad Saeed, Dahuai Zheng, Shiguo Liu, Shaolin Chen, Ling Zhang, Yongfa Kong, and et al. 2019. "Linear Tuning of Phase-Matching Temperature in LiNbO3:Zr Crystals by MgO Co-Doping" Materials 12, no. 24: 4155. https://doi.org/10.3390/ma12244155






