1. Introduction
Acousto-optic tunable filters (AOTFs) have attracted a great deal of attention as a core module of ultra-spectral image sensing systems. They comprise piezoelectric transducers and acousto-optic modulator (AOM) single crystals combined together. AOTF devices utilize the principle of changing and decomposing the frequency of light using soundwaves. The vibration of the piezoelectric transducer changes the diffraction characteristics of the light incident on the AOM material, which in turn only diffracts light of a certain wavelength. Thus, the unique acousto-optical properties allow the AOTF to selectively obtain a diffraction signal for each wavelength by modulating the frequency applied to the AOTF apparatus. AOTF devices have potential use for a variety of applications, such as measurements of a cell monolayer, mineral exploration, environmental monitoring, process control, and the identification of toxic biological agents [
1,
2,
3,
4,
5].
Specifically, toxic aerosols can be selectively identified, which enables superior performance in remote identification, detection, and tracking. Because the emission/absorption spectra corresponding to chemical agents and toxic substances are primarily found in the infrared region, with wavelengths of 8–12 μm, AOM crystal materials covering this range should be explored [
1,
3,
6,
7,
8]. Meanwhile, the figure of merit (
M2) index for evaluation of the optical properties of an AOM material is generally expressed by the following Equation:
where
ni is the refractive index of the incident light;
nd is the refractive index of the diffracted light;
is the effective photoelastic coefficient;
is the density of the AOM material; and
V is the speed of sound [
1]. Strong candidates for use as AOM materials include tellurium (Te), thallium arsenic selenide (Tl
3AsSe
3 or TAS), and mercurous halides (Hg
2Cl
2 and Hg
2Br
2) [
2,
4]. Although Te has the highest performance index (
M2) among these, it is easily activated by heat because of its small bandgap and relatively weak mechanical strength. Meanwhile, TAS is a very toxic material, making it difficult to handle, thus requiring large facility construction costs [
9]. However, the mercurous bromide (Hg
2Br
2) single-crystal material has advantages including a relatively wide optical transmission band (0.4–30 μm), wide birefringence, high nonlinear optical properties, and a high refractive index. Its figure of merit is also 2.5 times larger than that of the Hg
2Cl
2 single crystal with a similar lattice structure [
10,
11,
12]. Furthermore, this material has the unique characteristic of being easily decomposed into gas phases of Hg and HgBr
2 before melting. The physical properties make the physical vapor transport (PVT) process of the Hg
2Br
2 suitable [
1]. In this process, it is important to grow a high-quality AOM single crystal that can be applied to the fabrication of a high-performance AOTF device. For example, Kim et al. have reported the study showing the achievement of excellent AOTF performance with the high-quality AOM single crystals [
1,
3,
4,
13]. However, most of the corresponding research has focused on the integrated AOTF system rather than AOM crystal growth. There are very few studies that investigate the growth and evolution mechanism of the Hg
2Br
2 crystal in-depth, which is also essential for the realization of a high-performance AOTF system.
Herein, we investigate the crystal growth and evolution behavior of the Hg2Br2 crystal synthesized using PVT. The temperature profile conditions of the seed growth were optimized from the comparison between the experimental and simulation results. Two types of PVT-grown Hg2Br2 crystals (single and quasi-single) were characterized using various analysis techniques. The single-crystal Hg2Br2 offered a more uniform strain than that of the quasi-single crystal, as verified by X-ray diffraction (XRD). Meanwhile, the binding energy states of the as-synthesized Hg2Br2 crystals were similar regardless of the crystal type. Furthermore, Raman spectroscopy and transmission electron microscopy (TEM) analyses provided the comparison results concerning the atomic vibration mode and atomic structures of the two kinds of samples. Thus, this in-depth investigation of the growth mechanism of the Hg2Br2 crystals introduces a way toward potential acousto-optic tunable filter device applications.
3. Results and Discussion
Figure 1a shows the tetragonal lattice structure of the Hg
2Br
2 crystal with a space group of I4/mmm (139), which comprises strong covalent bonding along the
c-axis and relatively weak van der Waals bonds between the adjacent molecules along the
ab-plane [
15]. The lattice parameters a and c of Hg
2Br
2 are 4.65 and 11.10 Å, respectively [
12,
13]. This unique atomic structure causes an optically anisotropic property. The PVT process was selected to grow the Hg
2Br
2 single crystal because Hg
2Br
2 material can be sublimated at relatively low temperatures [
15,
16]. The PVT system designed for the Hg
2Br
2 crystal growth comprised three parts: the control unit for manipulating the temperature/time, the driving unit for moving the ampoule, and the chamber/heater unit that surrounded the ampoule. The ampoule partly surrounded by the heater could be divided into three zones: source, raw material, and growth zone (
Figure 1b). The pre-purified Hg
2Br
2 powder, prepared by a PVT-based purification process, was loaded on a ceramic membrane filter located in the center of the ampoule, for the passing of only small Hg
2Br
2 particles (<150 μm) to the bottom growth zone of the ampoule. The lower part of the growth zone had a conical-shaped tip while the upper source zone part had a cylindrical shape.
We checked the effects of the temperature gradient within the ampoule, the bottom heater temperature, and the ampoule position to explore the optimization condition of the formation and growth of the seed crystal. First, the different temperature gradient profiles within the ampoule were obtained experimentally and compared with the simulation results (
Figure 2a). When the temperature of the bottom heater was changed from 310 °C to 330 °C at a fixed upper heater temperature of 310 °C, different seed formation behaviors were obtained throughout the ampoule region. The experimental results show that the initial Hg
2Br
2 seed was observed in the upper wall of the ampoule at the temperature gradient of 0 °C. However, the seed was generated at the end of the tip for the temperature gradient of 20 °C, which was preferred for stable crystal growth thereafter. The simulation results show that Hg
2Br
2 nucleation occurred in a supersaturation area (light blue region in
Figure 2b). More specifically, as the temperature of the heater increased from 310 to 335 °C, the supersaturation area decreased (i.e., overlap between the supersaturation area and temperature profile decreased with increasing temperature of the heater). These results indicate that the temperature gradient profile determines the position of the initial seed formation. The simulation result is also highly consistent with the experimental result.
Secondly, we explored the effect of the bottom heater temperature on the step of crystal growth after the formation step of the seed, as shown in
Figure 2b. The rate of lowering the temperature of the bottom heater was carried out at 2 °C/day. We confirmed that the reduction of the bottom heater temperature caused the crystal including single- and polycrystalline crystal to have randomly oriented facets, as confirmed by the optical image of the crystal with opaque color (left of
Figure 2b). When the bottom heater temperature decreased from 330 to 310 °C in the simulation (right of
Figure 2b), the overall temperature of the seed portion decreased, but the temperature difference changed negligible (i.e., the total temperature of the seed portion was uniformly lowered). These results confirm that nucleation sites occurred on a relatively wide region of the wall, thereby facilitating polycrystalline growth. Finally, the effect of the ampoule position on the crystal growth mode was investigated (
Figure 2c). The ampoule position was lowered by 30 mm, and the descent speed of the ampoule was 2 mm/day. As validated by the optical image (left of
Figure 2c), a single crystal phase with high transparency was preferably formed by lowering the position of the ampoule by 30 mm under fixed temperatures of the heaters. In the simulation (right of
Figure 2c), the temperature difference was changed from 8.6 to 16.4 °C by lowering the ampoule position, which was more effective for causing a high temperature difference. Such an abrupt temperature difference of the seed crystal confines the region of the crystal growth to the end of the ampoule tip, narrowing the nucleation boundary and thereby leading to a stable growth mode.
We adopted the ampoule position change described above to grow the Hg
2Br
2 crystal.
Figure 3a displays an optical image of the as-grown Hg
2Br
2 crystal with two different growth regions (center and edge of the crystal) showing transparent and opaque colors (denoted as the single and quasi-single crystals). The XRD peak data of the Hg
2Br
2 samples exhibited the primary (110) and its family (220) planes, regardless of the kind of extracted sample (
Figure 3b,d) [
17]. The 2θ positions of the major planes in the two samples (crystal and quasi-crystal) were similar. Nevertheless, the single Hg
2Br
2 crystal exhibited relatively higher peak intensities for all planes, indicating more perfect crystallinity. In general, the full width at half maximum (FWHM) values in θ/2θ XRD measurements usually correspond to lattice distortion [
18]. The FWHM values of the (110) planes in the quasi-single and single crystals were calculated to be 0.13 and 0.09, respectively. The FWHM values usually correspond to lattice distortion. It is highly likely that a crystal with nonuniform strain in the lattice makes the FWHM comparatively broad. In our grown Hg
2Br
2 crystal, the FWHM of the single crystal was 1.4 times lower than that of the quasi-single crystal, which indicates a better crystal with no lattice distortion. The insets of
Figure 3b,d show the EBSD mapping results on the quasi-single and single crystals, showing a single Hg
2Br
2 crystal stacked with the (110) plane. This is strongly consistent with the XRD results. To identify the chemical compound states, the XPS data of the two crystals were obtained, showing that the peaks of Hg 4f5, 4f7, and Br 3d are exactly the same as the XPS data of the purified Hg
2Br
2 powder measured in the previous study [
19,
20]. The binding energies of Hg 4f7 and Br 3d in the quasi-single crystal were 100.8 eV and 68.9 eV, respectively, and the peak positions of the single crystal were observed at 100.7 eV and 68.7 eV. There was no remarkable difference in the XPS peak positions for the two samples. Even if there was lattice distortion in the quasi-single crystal, the chemical compound states remained the same regardless of the growth region.
To check the atomic vibration mode of the two crystals, Raman spectroscopy analysis was performed along the crystal plane direction (
Figure 4a,b). In the vibrational spectra of Hg
2Br
2, the four prominent peaks (35.5, 91, 136, and 221 cm
−1) follow the group theory of the selection rules: the two fully symmetric peaks with A
1g symmetry and two doubly degenerated peaks with
Eg symmetry modes. These symmetry modes indicate low-frequency vibrations (35.5 and 91 cm
−1) and high-frequency vibrations (136 and 221 cm
−1), respectively. The first vibration mode of E
g symmetry (35.5 cm
−1) is the librations of the linear molecules as a whole with respect to
Z-axis, while the second vibration of E
g symmetry (91 cm
−1) is the deformed zigzag ones. Meanwhile, the fully symmetric valence vibration A
1g mainly corresponds to Hg–Hg (136 cm
−1) and Br–Hg (221 cm
−1) displacements [
21,
22]. Information about the peak position of Hg
2Br
2 obtained experimentally and theoretically is described in
Table S1 in the supporting information. Overall, the peaks involving the vibration modes of Hg
2Br
2 (featured as star mark) were clearly observed, while extra peaks were detected as well, which are not consistent with the conventional Hg
2Br
2. Unfortunately, no Raman database can match the extra peaks. The origin of the corresponding extra peaks should be further explored.
Overall, the peaks of the Hg
2Br
2 vibrational mode on the (001) plane appeared almost the same regardless of a kind of crystal (quasi-single or single) as shown in
Figure 4a. There was no significant difference in Raman spectra between quasi-single and single Hg
2Br
2 crystals. The first vibration peak was negligible due to the resolution limit of Raman analysis equipment. However, the peaks involving the second and fourth vibration modes in single crystal showed higher intensity than that of quasi-single when the information of the Raman spectra was collected on the (110) plane. It is obvious that there was no strong plane dependency in the Raman spectra of the single crystal while the quasi-single crystal showed the interesting result of the Raman spectra strongly depending on the crystal plane. It might be due to the slight difference in van der Waals interaction in the intermolecular structure (right of
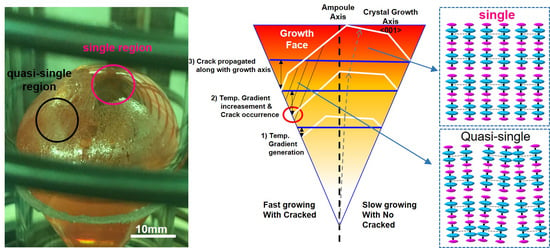
Figure 4c). Especially, quasi-single crystal has less intermolecular interaction due to the imperfect columnar-shaped structure of Hg
2Br
2, causing less vibration. For more detailed polarized vibrational analyses, we performed Raman mapping analysis on the large area of 1400 × 1400 μm
2 (
Figure S1 in supporting information). The large area mapping result was highly consistent with point data. Based on all the experimental and simulation results, the proposed model of the Hg
2Br
2 crystal growth illustrates the evolution of the crystal facets in the center and wall of the ampoule (
Figure 4c). It is reasonably acceptable that the Hg
2Br
2 crystal does not appear to grow in the perfectly vertical direction of the ampoule, but rather in a slightly tilted direction [
13]. When the Hg
2Br
2 phase was grown, individual faceted crystals were formed but the facet on the (001) plane was most preferable. This tends to be similar to (0001) facet growth of SiC based on the same PVT process [
23]. The crystal growth along the <001> direction is usually more preferable than that along the <110> direction [
24]. This occurs to reduce the total surface energy of the material system. A temperature gradient between the ampoule wall and growth surface of the Hg
2Br
2 crystal can be generated (red circle of
Figure 4b), causing cracks near the ampoule. These cracks expand toward the parallel direction of crystal growth with increasing time. Weak van der Waals bonds between adjacent Hg
2Br
2 molecules can facilitate the crack propagation, thus forming a quasi-single crystal with a slightly tilted columnar-shaped Hg
2Br
2 structure, unlike the perfect alignment of the Hg
2Br
2 lattice on the single crystal.
To verify the interatomic arrangements and lattice structures of the two samples, TEM measurements were performed.
Figure 5a shows a low-magnified TEM image of a single Hg
2Br
2 crystal. Layered atomic structures were clearly observed due to the unique lattice comprising covalent and van der Waals bonding. Meanwhile, the black region shows the intrinsic single-crystal region and the bright-colored region shows the empty region structurally collapsed. The highly magnified TEM image clearly shows the atomic arrangement of the Hg and Br elements (
Figure 5b). The inset of
Figure 5b, a selected area electron diffraction (SAED) pattern along the [001] zone axis, exhibits typical periodic dot patterns of [(110), (200), and (1
0)], representing a tetragonal structure [
25]. As shown in the TEM image of a single crystal, many stacking faults appeared due to the layer-type structure with relatively weak van der Waals bonds. Especially, more stacking faults existed and were propagated over the whole region of quasi-single crystals with relatively imperfect intermolecular bonding structure, causing irrecoverable damage on the preparation process of TEM specimen (
Figure S2 in supporting information). This might be because the symmetry was easily broken by the stacking faults and many phonons from all Brillouin zones became active [
26]. Thus, the structure of the quasi-single crystal sample was unstable and the limited area was analyzed. The high-resolution TEM image of the quasi-single crystal did not show a perfect single crystal, as supported by the SAED pattern with the mixed dot and ring patterns. The specimen of the quasi-single crystal was too vulnerable for even the relatively weak thermal energy of the room temperature, which easily dissociated the Hg
2Br
2 into individual molecules. Nevertheless, based on our knowledge, this is the first TEM observation of an Hg
2Br
2 crystal, which is a meaningful step for characterizing the van der Waals crystal with weak bonding strength.