The Effect of Potential on Surface Characteristic and Corrosion Resistance of Anodic Oxide Film Formed on Commercial Pure Titanium at the Potentiodynamic-Aging Mode
Abstract
:1. Introduction
2. Experimental
2.1. Specimen Preparation
2.2. Surface Characterization
2.3. Corrosion Behavior
3. Results and Discussion
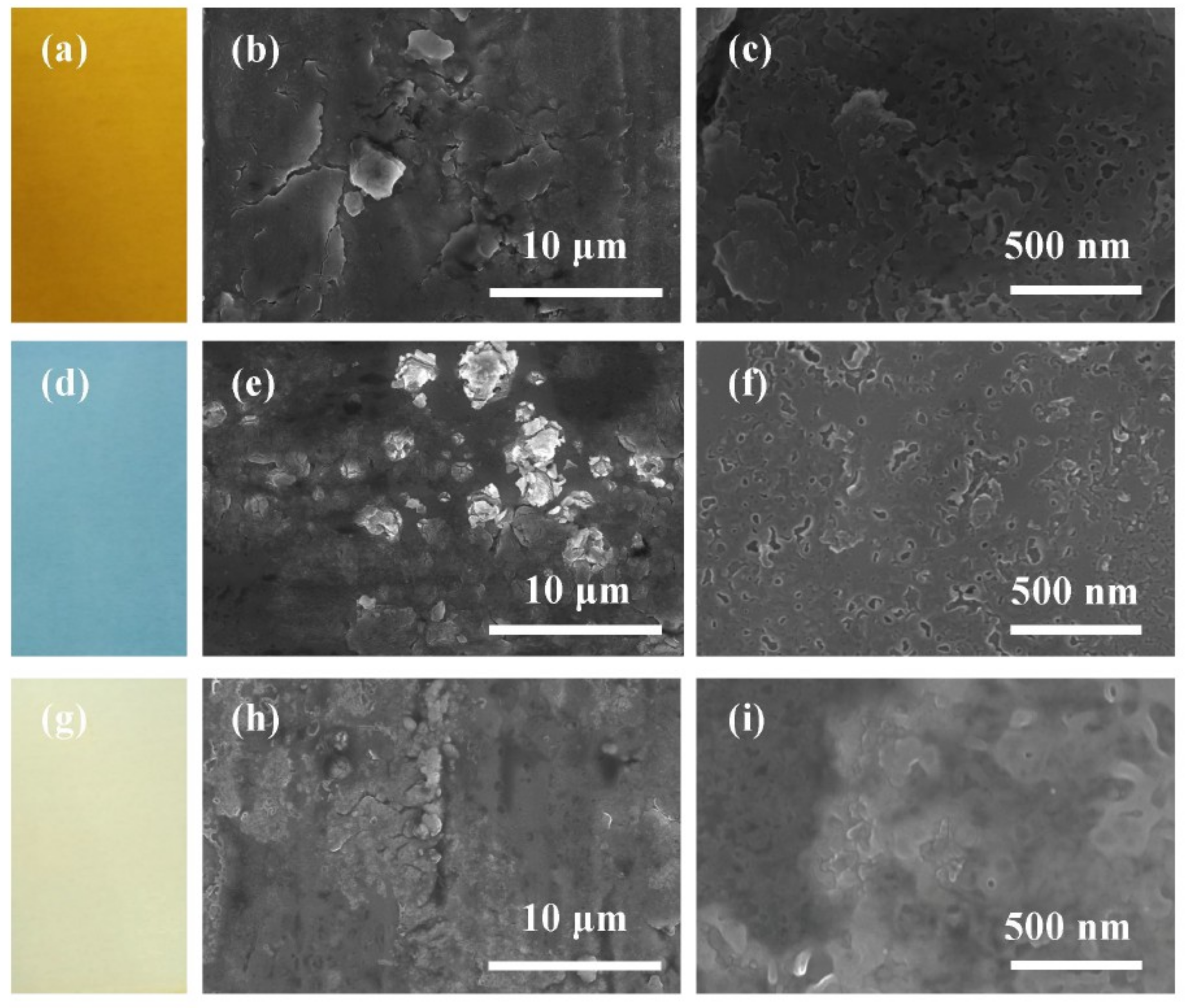
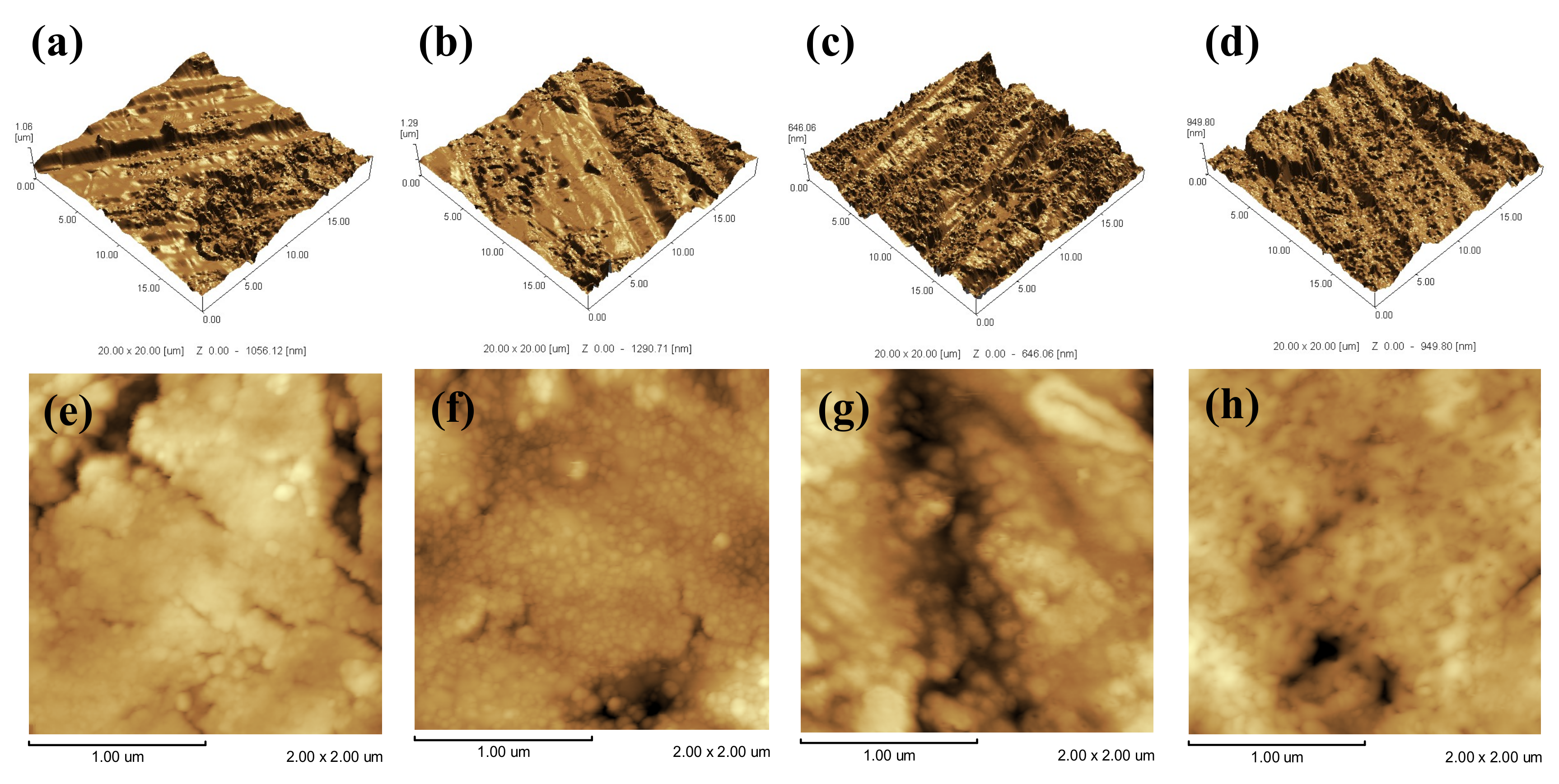
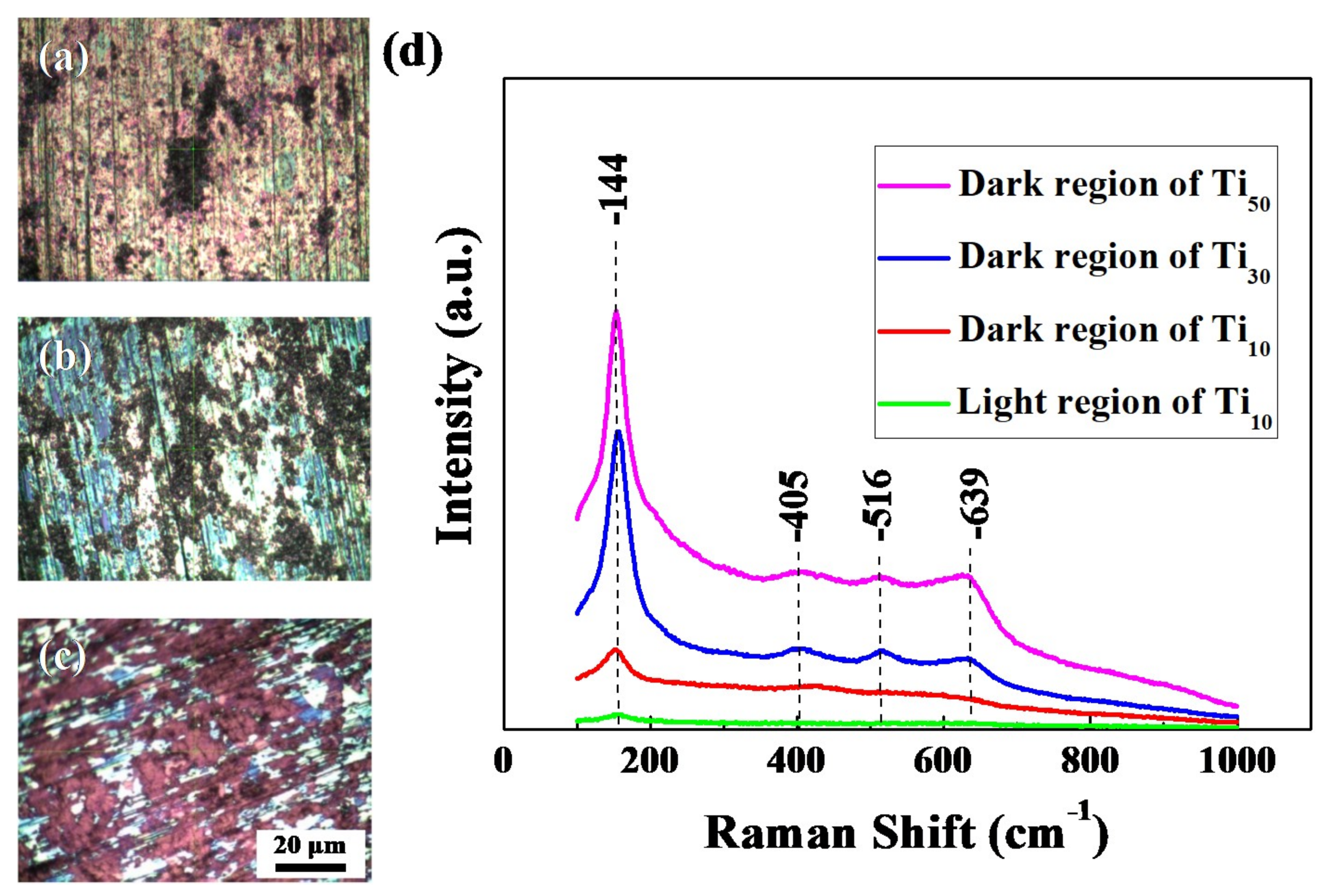
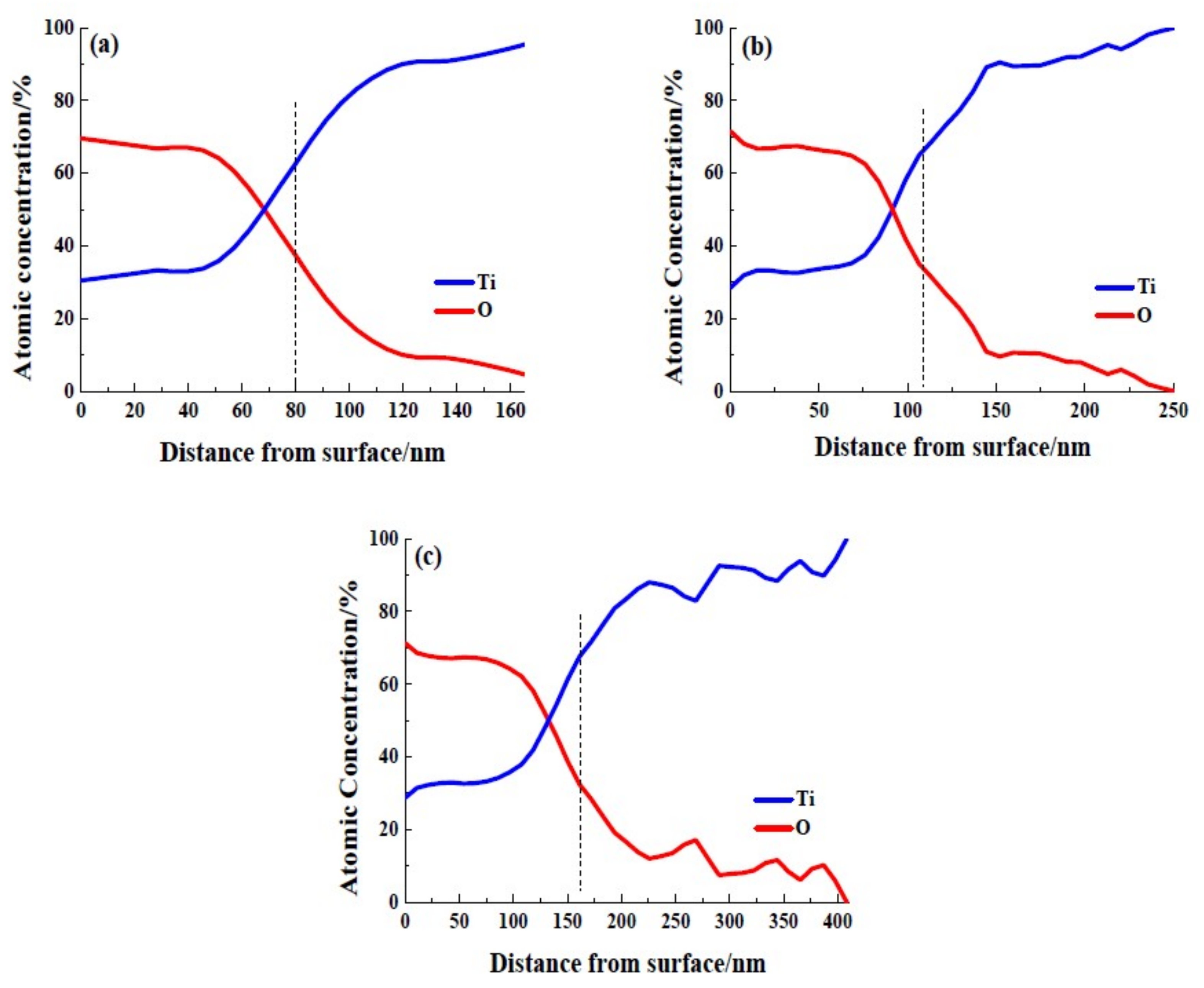
3.1. Surface Properties
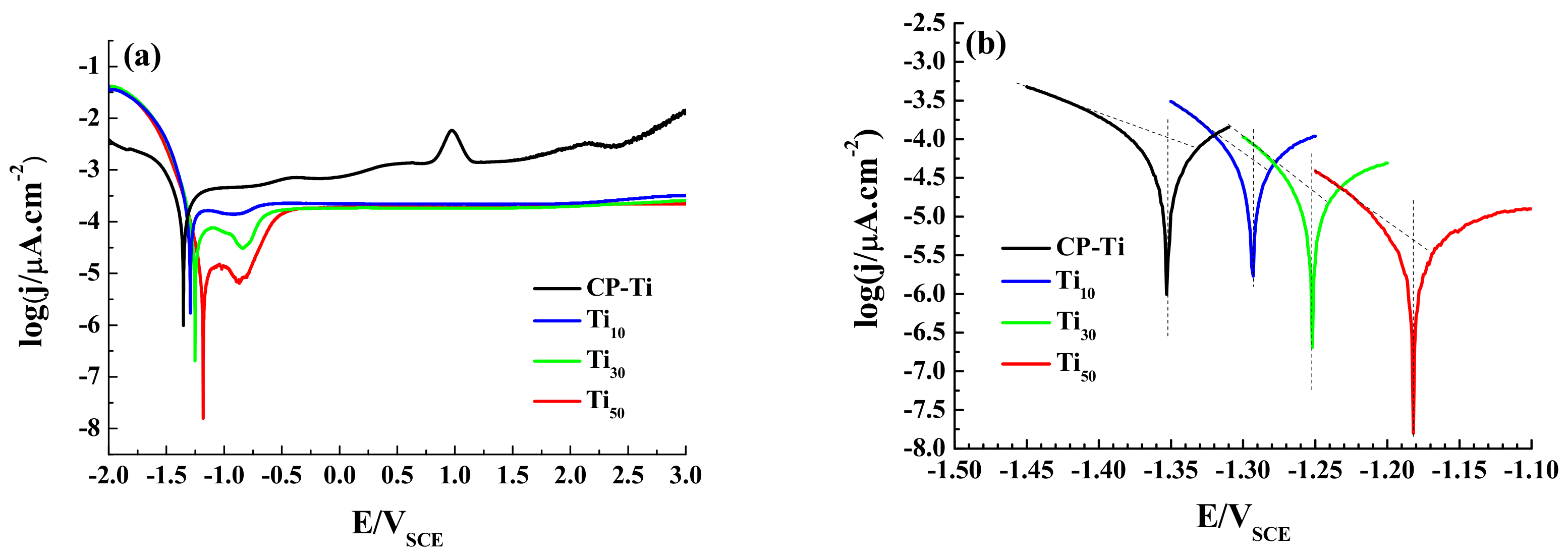
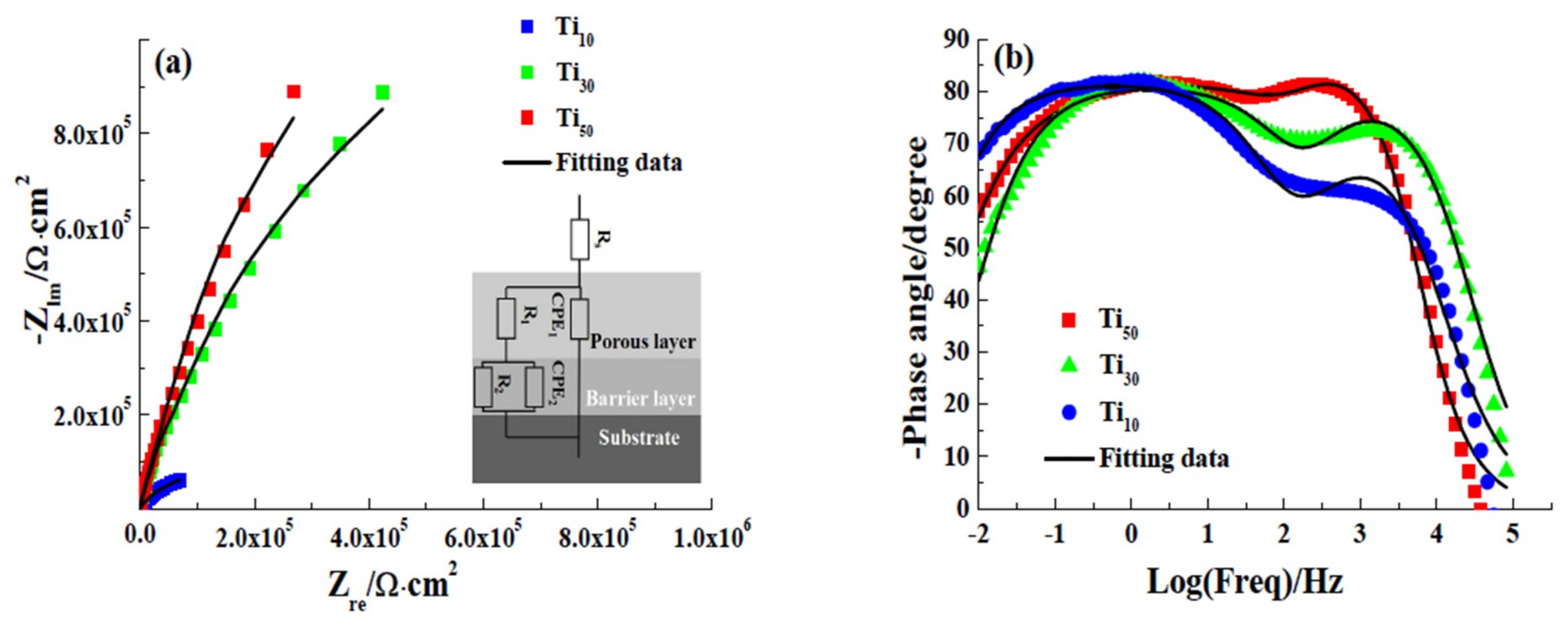
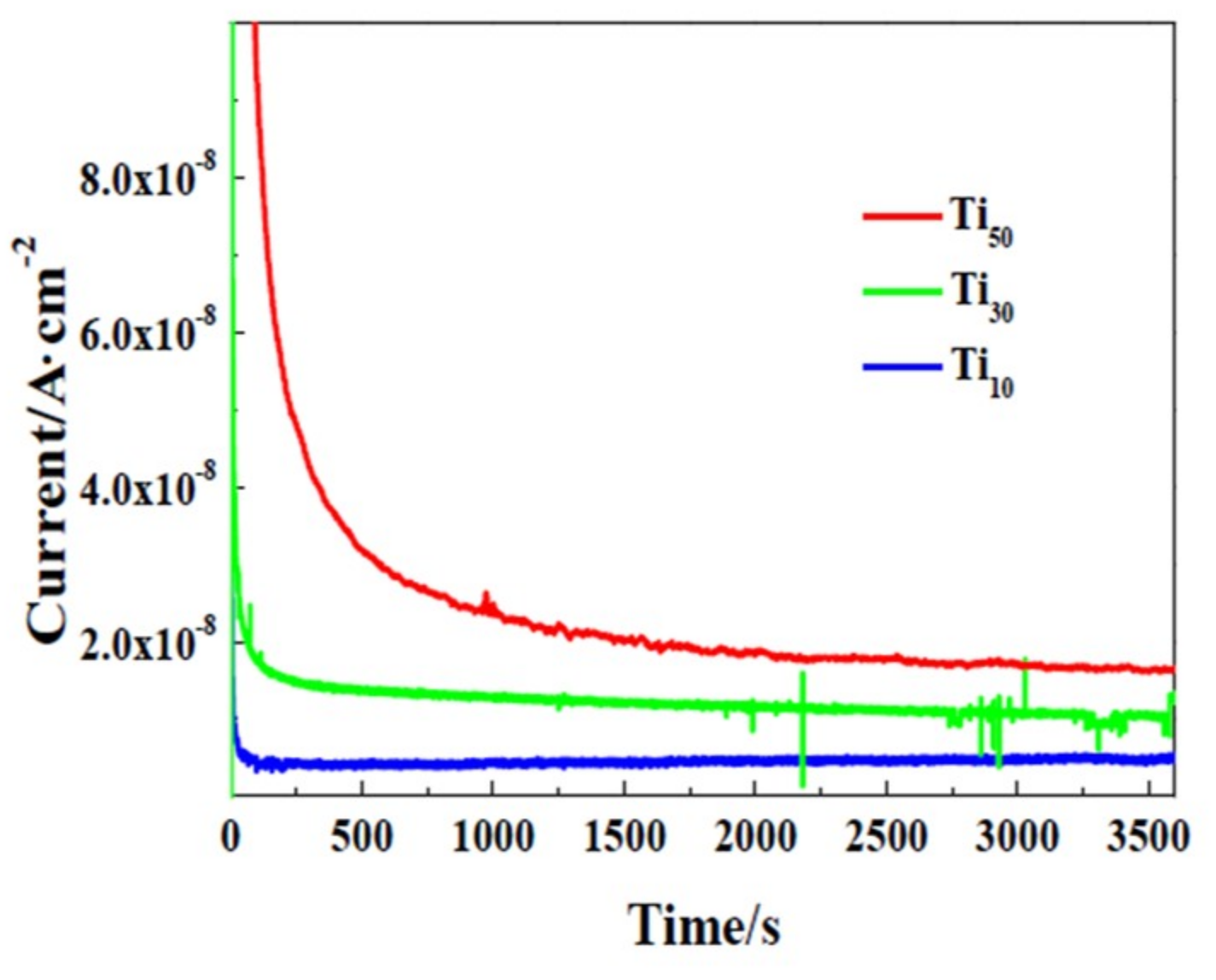
3.2. Electrochemical Tests
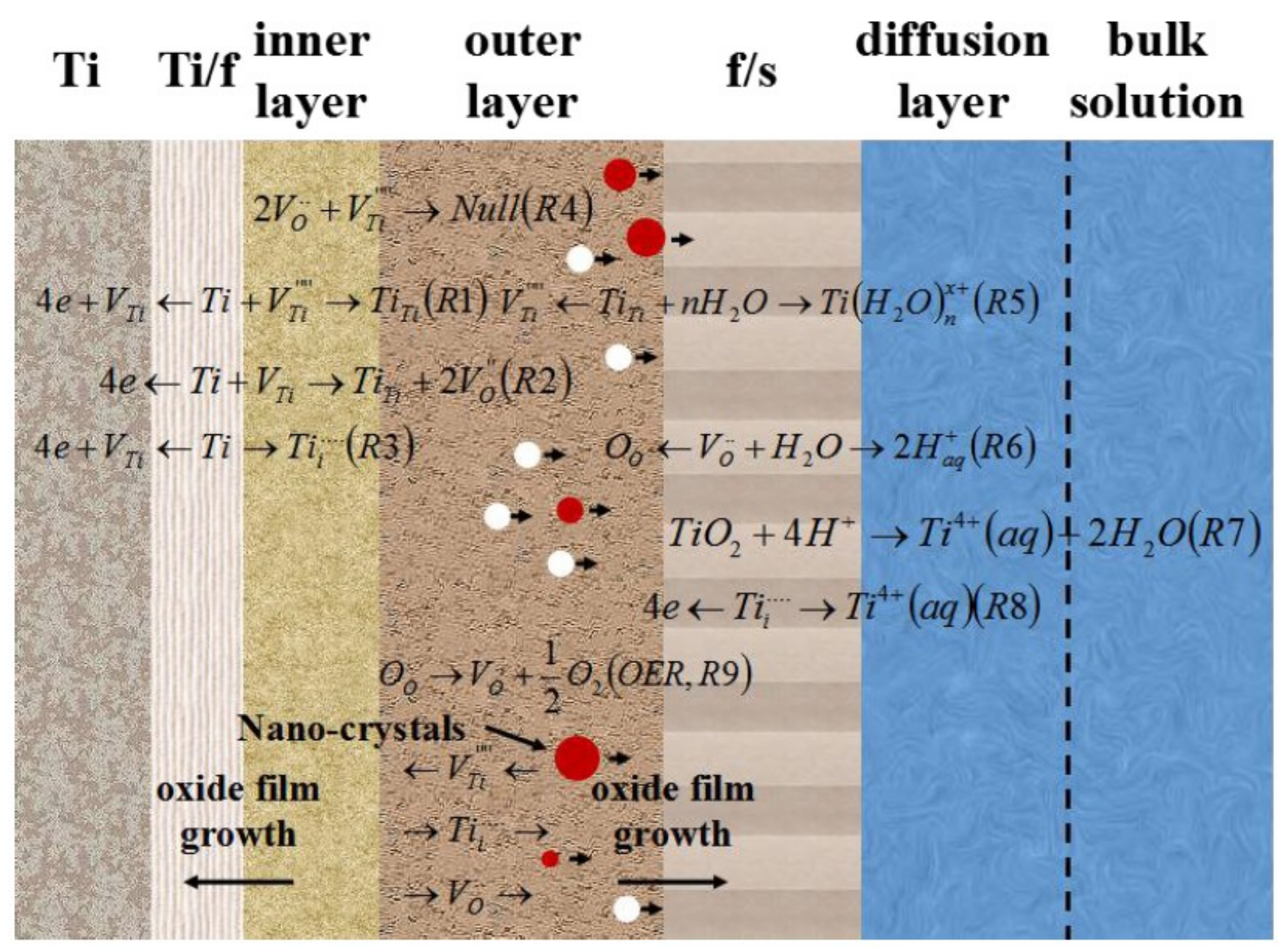
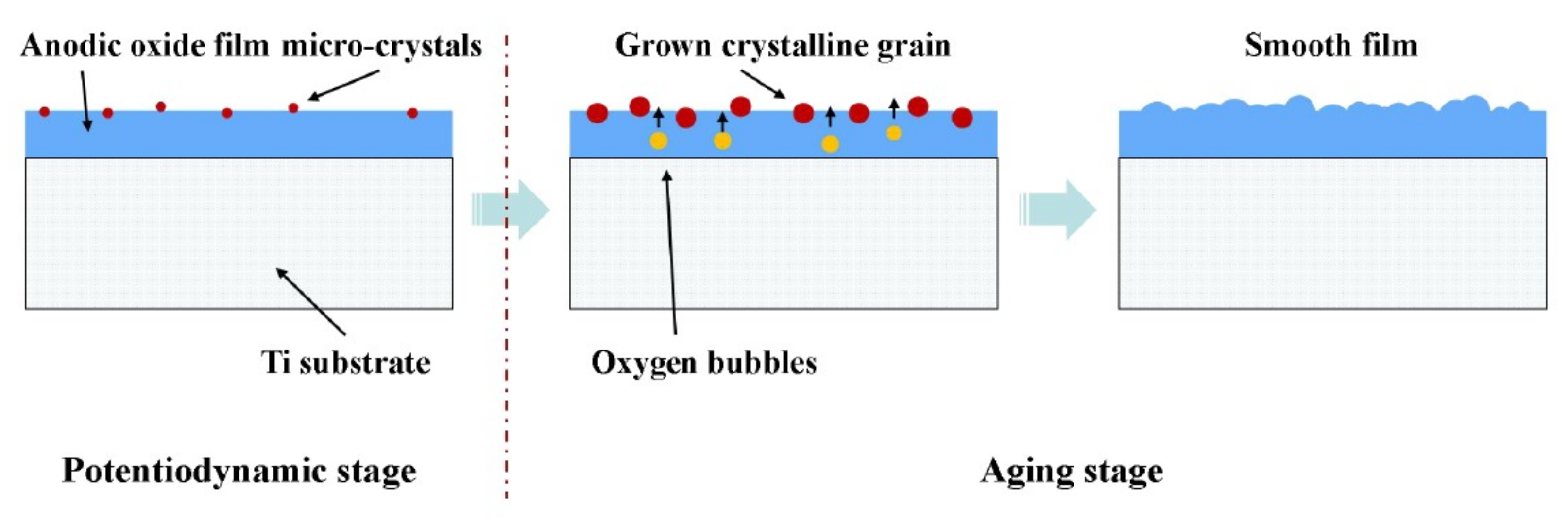
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diamanti, M.V.; Pedeferri, M.P. Effect of anodic oxidation parameters on the titanium oxides formation. Corros. Sci. 2007, 49, 939–948. [Google Scholar] [CrossRef]
- Oliveira, V.M.C.A.; Aguiar, C.; Vazquez, A.M.; Robin, A.; Barboza, M.J.R. Improving corrosion resistance of Ti–6Al–4V alloy through plasma-assisted PVD deposited nitride coatings. Corros. Sci. 2014, 88, 317–327. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Norby, T.; Middleton, H. Investigation of pitting resistance of titanium based on a modified point defect model. Corros. Sci. 2011, 53, 815–821. [Google Scholar] [CrossRef]
- Li, S.; Yao, W.; Liu, J.; Yu, M.; Wu, L.; Ma, K. Study on anodic oxidation process and property of composite film formed on Ti–10V–2Fe–3Al alloy in SiC nanoparticle suspension. Surf. Coat. Technol. 2015, 277, 234–241. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Jiang, Z.; Norby, T.; Middleton, H. Evaluation of metastable pitting on titanium by charge integration of current transients. Corros. Sci. 2010, 52, 3158–3161. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Tamborim, S.M.; Santos, C.B.D.; Müller, I.L. Tribological, electrochemical and tribo-electrochemical characterization of bare and nitrided Ti6Al4V in simulated body fluid solution. Corros. Sci. 2011, 53, 1786–1793. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Guan, J.; Zhang, D. Characterizations of anodic oxide films formed on Ti6Al4V in the silicate electrolyte with sodium polyacrylate as an additive. Surf. Coat. Technol. 2018, 338, 14–21. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K. Phosphoric acid anodization of Ti–6Al–4V—Structural and corrosion aspects. Corros. Sci. 2007, 49, 542–558. [Google Scholar] [CrossRef]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure Commercial Titanium Color Anodizing and Corrosion Resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Simka, W.; Sadkowski, A.; Warczak, M.; Iwaniak, A.; Dercz, G.; Michalska, J.; Maciej, A. Characterization of passive films formed on titanium during anodic oxidation. Electrochim. Acta 2011, 56, 8962–8968. [Google Scholar] [CrossRef]
- Xing, J.H.; Xia, Z.B.; Hui, L.I.; Wang, Y.Y.; Zhong, L. Growth and crystallization behaviors of anodic oxide films on sputter-deposited titanium at very low potentials. Trans. Nonferrous Met. Soc. China 2013, 23, 3286–3292. [Google Scholar] [CrossRef]
- Kuromoto, N.K.; Simão, R.A.; Soares, G.A. Titanium oxide films produced on commercially pure titanium by anodic oxidation with different voltages. Mater. Charact. 2007, 58, 114–121. [Google Scholar] [CrossRef]
- Nanjo, H.; Deng, H.; Oconer, I.S.; Ishikawa, I.; Suzuki, T.M. Effect of Ultraviolet Light Irradiation on Structure and Electrochemical Properties of Iron Surface. Jpn. J. Appl. Phys. 2005, 44, 539–542. [Google Scholar] [CrossRef]
- Sivakumar, B.; Pathak, L.C.; Singh, R. Role of surface roughness on corrosion and fretting corrosion behaviour of commercially pure titanium in Ringer’s solution for bio-implant application. Appl. Surf. Sci. 2017, 401, 385–398. [Google Scholar] [CrossRef]
- Xia, Z.; Nanjo, H.; Aizawa, T.; Kanakubo, M.; Fujimura, M.; Onagawa, J. Growth process of atomically flat anodic films on titanium under potentiostatical electrochemical treatment in H2SO4 solution. Surf. Sci. 2007, 601, 5133–5141. [Google Scholar] [CrossRef]
- Xing, J.H.; Xia, Z.B.; Hu, J.F.; Zhang, Y.H.; Zhong, L. Growth and Crystallization of Titanium Oxide Films at Different Anodization Modes. J. Electrochem. Soc. 2013, 160, C239–C246. [Google Scholar] [CrossRef]
- Delplancke, J.L.; Garnier, A.; Massiani, Y.; Winand, R. Influence of the anodizing procedure on the structure and the properties of titanium oxide films and its effect on copper nucleation. J. Appl. Polym. Sci. 1994, 39, 1281–1289. [Google Scholar] [CrossRef]
- Kudelka, S.; Michaelis, A.; Schultze, J.W. Effect of texture and formation rate on ionic and electronic properties of passive layers on Ti single crystals. Electrochim. Acta 1996, 41, 863–870. [Google Scholar] [CrossRef]
- Alves, A.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Pinto, A.; Rocha, L.; Fernandes, J. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Mu, S.; Li, N.; Li, D.; Zou, Z. Investigation of a transparent chromate (III) passive film on electroless Ni–P coating by XPS and electrochemical methods. Electrochim. Acta 2009, 54, 6718–6724. [Google Scholar] [CrossRef]
- Komatsu, I.; Aoki, H.; Ebisawa, M.; Kuroda, A.; Kuroda, K.; Maeda, S. Color change mechanism of niobium oxide thin film with incidental light angle and applied voltage. Thin Solid Film. 2016, 603, 180–186. [Google Scholar] [CrossRef]
- Xing, J.; Xia, Z.; Hu, J.; Zhang, Y.; Zhong, L. Time dependence of growth and crystallization of anodic titanium oxide films in potentiostatic mode. Corros. Sci. 2013, 75, 212–219. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E.; Habazaki, H. Influence of grain orientation on oxygen generation in anodic titania. Thin Solid Film. 2008, 516, 2296–2305. [Google Scholar] [CrossRef]
- Benea, L.; Danaila, E.; Ponthiaux, P. Effect of titania anodic formation and hydroxyapatite electrodeposition on electrochemical behaviour of Ti–6Al–4V alloy under fretting conditions for biomedical applications. Corros. Sci. 2015, 91, 262–271. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Guo, J.; Sato, N. ChemInform Abstract: Raman Spectra of the Anodic Oxide Film on Titanium in Acidic Sulfate and Neutral Phosphate Solutions. Cheminform 1987, 18, 2473–2476. [Google Scholar] [CrossRef]
- Si, H.Y.; Sun, Z.H.; Kang, X.; Zi, W.W.; Zhang, H.L. Voltage-dependent morphology, wettability and photocurrent response of anodic porous titanium dioxide films. Microporous Mesoporous Mater. 2009, 119, 75–81. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A. Passivity of AISI 321 stainless steel in 0.5 M H2SO4 solution studied by Mott–Schottky analysis in conjunction with the point defect model. Arab. J. Chem. 2016, 9, S1342–S1348. [Google Scholar] [CrossRef]
- Wang, C.; Pan, L.; Zhang, Y.; Xiao, S.; Chen, Y. Deoxidization mechanism of hydrogen in TiH2 dehydrogenation process. Int. J. Hydrogen Energy 2016, 41, 14836–14841. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middleton, H. Effect of silicon on corrosion resistance of Ti–Si alloys. Mater. Sci. Eng. B 2011, 176, 79–86. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Nishimura, D.; Doi, H.; Nomura, N.; Hanawa, T. Difference in surface reactions between titanium and zirconium in Hanks’ solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater. Sci. Eng. B 2009, 29, 1702–1708. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Drenik, A.; Hauptman, N.; Balat-Pichelin, M. High temperature oxidation of stainless steel AISI316L in air plasma. Appl. Surf. Sci. 2008, 255, 1759–1765. [Google Scholar] [CrossRef]
- Gui, Y.; Meng, X.B.; Zheng, Z.J.; Gao, Y. Critical temperature determination of detectable Cr diffusion enhancement by nanostructure through structural evolution analysis of the oxide films at 25–450 °C on 304 stainless steel. Appl. Surf. Sci. 2017, 419, 512–521. [Google Scholar] [CrossRef]
- Kirchheim, R. Growth kinetics of passive films. Electrochim. Acta 1987, 32, 1619–1629. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Schwarzenboeck, E.; Banas, J. Role of plastic deformation and microstructure in the micro-electrochemical behaviour of Ti–6Al–4V in sodium chloride solution. Electrochim. Acta 2013, 104, 400–406. [Google Scholar] [CrossRef]
- Mccafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Poorqasemi, E.; Abootalebi, O.; Peikari, M.; Haqdar, F. Investigating accuracy of the Tafel extrapolation method in HCl solutions. Corros. Sci. 2009, 51, 1043–1054. [Google Scholar] [CrossRef]
- Kelly, E.J. Electrochemical Behavior of Titanium; Springer: New York, NY, USA, 1982. [Google Scholar]
- Fu, T.; Zhan, Z.; Zhang, L.; Yang, Y.; Liu, Z.; Liu, J.; Li, L.; Yu, X. Effect of surface mechanical attrition treatment on corrosion resistance of commercial pure titanium. Surf. Coat. Technol. 2015, 280, 129–135. [Google Scholar] [CrossRef]
- Hernández-López, J.M.; Conde, A.; Damborenea, J.J.D.; Arenas, M.A. Electrochemical response of TiO2 anodic layers fabricated on Ti6A14V alloy with nanoporous, dual and nanotubular morphology. Corros. Sci. 2016, 112, 194–203. [Google Scholar] [CrossRef]
- Ningshen, S.; Mudali, U.K.; Ramya, S.; Raj, B. Corrosion behaviour of AISI type 304L stainless steel in nitric acid media containing oxidizing species. Corros. Sci. 2011, 53, 64–70. [Google Scholar] [CrossRef]
- Robin, A.; Meirelis, J.P. Influence of fluoride concentration and pH on corrosion behavior of Ti-6Al-4V and Ti-23Ta alloys in artificial saliva. Mater. Corros. 2015, 58, 173–180. [Google Scholar] [CrossRef]
- Ter-Ovanessian, B.; Alemany-Dumont, C.; Normand, B. Electronic and transport properties of passive films grown on different Ni-Cr binary alloys in relation to the pitting susceptibility. Electrochim. Acta 2014, 133, 373–381. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Li, Z.; Munroe, P.; Xie, Z.H. Niobium addition enhancing the corrosion resistance of nanocrystalline Ti5Si3 coating in H2SO4 solution. Acta Mater. 2014, 63, 245–260. [Google Scholar] [CrossRef]
- Jurczakowski, R.; Hitz, C.; Lasia, A. Impedance of porous Au based electrodes. J. Electroanal. Chem. 2004, 572, 355–366. [Google Scholar] [CrossRef]
- Leiva-García, R.; Fernandes, J.C.S.; Muñoz-Portero, M.J.; García-Antón, J. Study of the sensitisation process of a duplex stainless steel (UNS 1.4462) by means of confocal microscopy and localised electrochemical techniques. Corros. Sci. 2015, 94, 327–341. [Google Scholar] [Green Version]
- Orazem, M.E.; PéBèRe, N.; Tribollet, B. Enhanced Graphical Representation of Electrochemical Impedance Data. J. Electrochem. Soc. 2006, 153, B129–B36. [Google Scholar] [CrossRef]
- Mantzila, A.G.; Prodromidis, M.I. Development and study of anodic Ti/TiO electrodes and their potential use as impedimetric immunosensors. Electrochim. Acta 2006, 51, 3537–3542. [Google Scholar] [CrossRef]
- Busani, T.; Devine, R.A.B. Dielectric and infrared properties of TiO2 films containing anatase and rutile. Semicond. Sci. Technol. 2005, 20, 870. [Google Scholar] [CrossRef]
- Habazaki, H.; Uozumi, M.; Konno, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E. Crystallization of anodic titania on titanium and its alloys. Corros. Sci. 2003, 45, 2063–2073. [Google Scholar] [CrossRef]
- Hwang, B.J.; Hwang, J.R. Kinetic model of anodic oxidation of titanium in sulphuric acid. J. Appl. Electrochem. 1993, 23, 1056–1062. [Google Scholar] [CrossRef]
- Fu, T.; Wang, X.; Liu, J.; Li, L.; Yu, X.; Zhan, Z. Characteristics and Corrosion Behavior of Pure Titanium Subjected to Surface Mechanical Attrition. JOM 2017, 69, 1–4. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middleton, H. Investigation on passivity of titanium under steady-state conditions in acidic solutions. Mater. Chem. Phys. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Kong, D.-S.; Lu, W.-H.; Yu, Z.-Y.; Wu, J.-X.; Fan, W.-J.; Liu, H.-Y.; Feng, Y.-Y. Studying on the Point-Defect-Conductive Property of the Semiconducting Anodic Oxide Films on Titanium. J. Electrochem. Soc. 2009, 156, C39–C44. [Google Scholar] [CrossRef]
- Veluchamy, A.; Sherwood, D.; Emmanuel, B.; Cole, I.S. Critical review on the passive film formation and breakdown on iron electrode and the models for the mechanisms underlying passivity. J. Electroanal. Chem. 2017, 785, 196–215. [Google Scholar] [CrossRef]
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Xia, D.-H.X.; Song, S.; Zhu, R.; Behnamian, Y.; Shen, C.; Wang, J.; Luo, J.; Lu, Y.; Klimas, S. A mechanistic study on thiosulfate-enhanced passivity degradation of Alloy 800 in chloride solutions. Electrochim. Acta 2013, 111, 510–525. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; García-Antón, J. Passive and transpassive behaviour of Alloy 31 in a heavy brine LiBr solution. Electrochim. Acta 2013, 95, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vanhumbeeck, J.F.; Ryelandt, L.; Proost, J. On the relationship between local voltage maxima and efficiency changes during galvanostatic Ti anodising. Electrochim. Acta 2009, 54, 3330–3338. [Google Scholar] [CrossRef]
- Leach, J.S.L.; Pearson, B.R. ChemInform Abstract: Crystallization in Anodic Oxide Films. Cheminform 1988. [Google Scholar] [CrossRef]
- Dyer, C.K.; Leach, J.S.L. ChemInform Abstract: Breakdown and Efficiency of Anodic Oxide Growth on Titanium. Chem. Inf. 1978, 9, 1032–1038. [Google Scholar] [CrossRef]

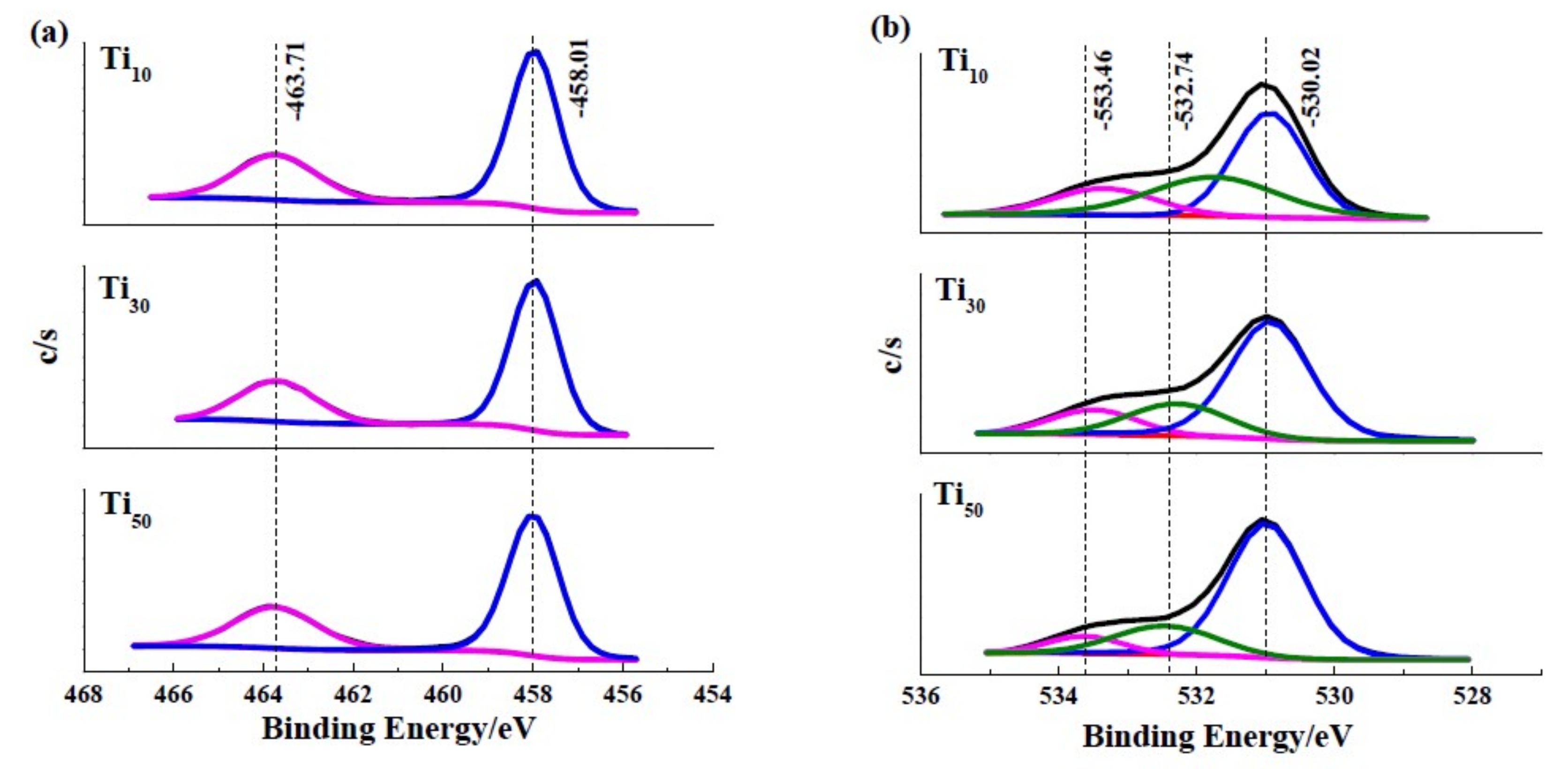

| Ti 2p3/2 (eV) | O 1s (eV) | |||||
|---|---|---|---|---|---|---|
| Ti | Ti2+ | Ti3+ | Ti4+ | O2− | OH− | H2O |
| 453.6 | 455.9 | 457.3 | 458.7 | 530.2 | 532.5 | 533.4 |
| Samples | The Contribution of O 1s Components (%) | ||
|---|---|---|---|
| O2− | OH− | H2O | |
| Ti10 | 73.65 | 21.88 | 4.47 |
| Ti30 | 78.03 | 17.59 | 4.38 |
| Ti50 | 84.09 | 12.99 | 2.92 |
| Sample | Ecorr/VSCE | jcorr (μA cm−2) |
|---|---|---|
| CP-Ti | −1.352 | 1.258 × 10−4 |
| Ti10 | −1.297 | 5.623 × 10−5 |
| Ti30 | −1.252 | 1.995 × 10−5 |
| Ti50 | −1.181 | 5.623 × 10−6 |
| Samples | Rs (kΩ·cm2) | Q1/10−5 (Ω−1·cm−2sα) | α1 | Q2/10−5 (Ω−1·cm−2sα) | α2 | R1 (kΩ·cm2) | R2 (kΩ·cm2) | χ2 (10−4) |
|---|---|---|---|---|---|---|---|---|
| Ti10 | 1.02 | 8.5501 | 0.92 | 2.5680 | 0.67 | 1.79 | 489.53 | 3.47 |
| Ti30 | 1.12 | 2.5727 | 0.89 | 1.7535 | 0.64 | 43.78 | 502.56 | 2.87 |
| Ti50 | 1.08 | 1.9826 | 0.93 | 1.3068 | 0.59 | 89.45 | 512.48 | 2.67 |
| Samples | C1 (μF·cm−2) | C2 (μF·cm−2) | L1 (nm) | L2 (nm) |
|---|---|---|---|---|
| Ti10 | 0.08121 | 0.08935 | 52.33 | 32.68 |
| Ti30 | 0.02611 | 0.05964 | 162.75 | 49.86 |
| Ti50 | 0.02078 | 0.04896 | 204.48 | 59.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Duan, Y.; Gao, R.; Yang, J.; Wei, K.; Tang, D.; Fu, T. The Effect of Potential on Surface Characteristic and Corrosion Resistance of Anodic Oxide Film Formed on Commercial Pure Titanium at the Potentiodynamic-Aging Mode. Materials 2019, 12, 370. https://doi.org/10.3390/ma12030370
Zhang L, Duan Y, Gao R, Yang J, Wei K, Tang D, Fu T. The Effect of Potential on Surface Characteristic and Corrosion Resistance of Anodic Oxide Film Formed on Commercial Pure Titanium at the Potentiodynamic-Aging Mode. Materials. 2019; 12(3):370. https://doi.org/10.3390/ma12030370
Chicago/Turabian StyleZhang, Ling, Yanqing Duan, Rui Gao, Jianyun Yang, Keyi Wei, Danyu Tang, and Tianlin Fu. 2019. "The Effect of Potential on Surface Characteristic and Corrosion Resistance of Anodic Oxide Film Formed on Commercial Pure Titanium at the Potentiodynamic-Aging Mode" Materials 12, no. 3: 370. https://doi.org/10.3390/ma12030370




