Anodic Polarization Behavior of X80 Steel in Na2SO4 Solution under High Potential and Current Density Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solution
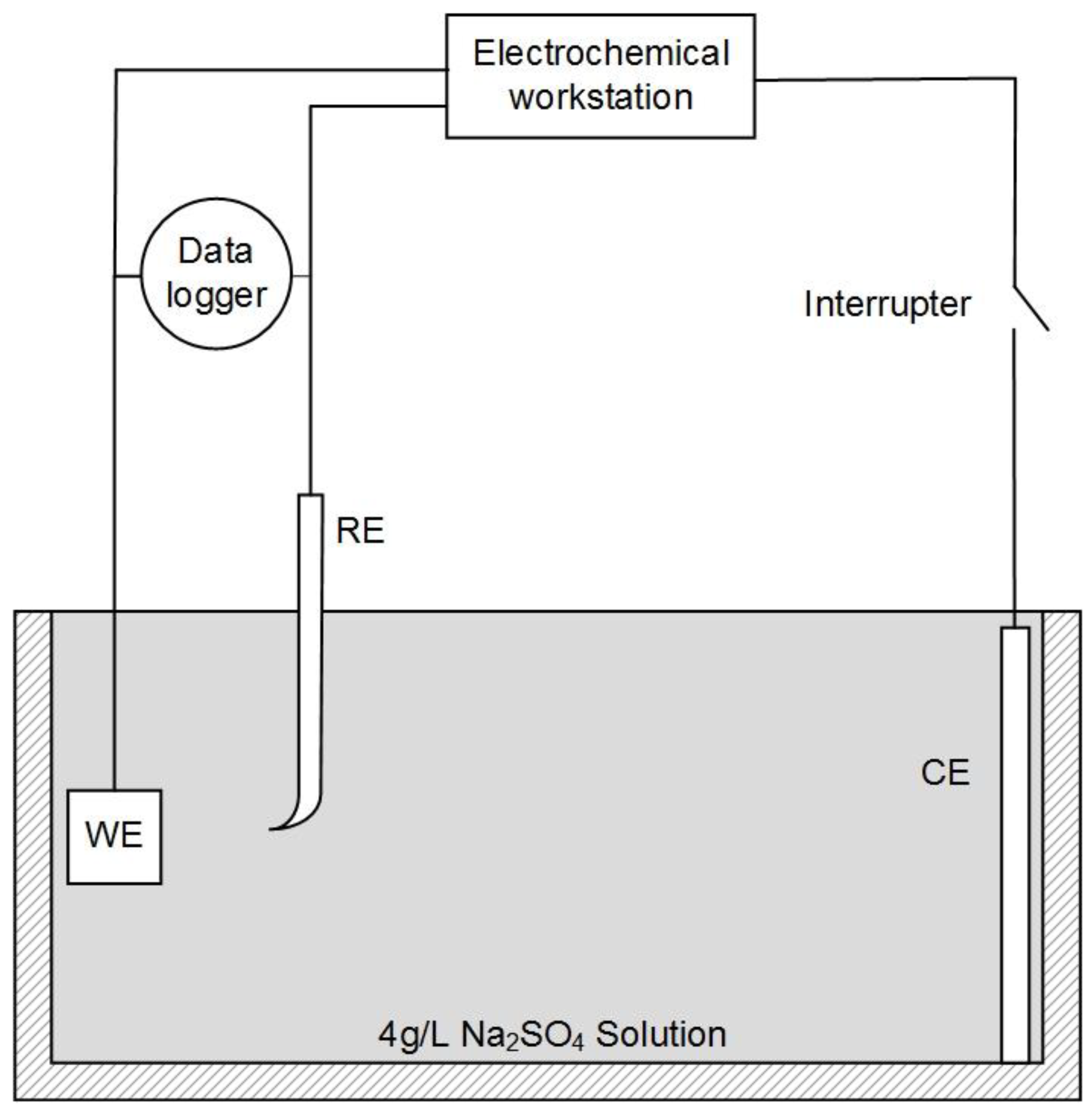
2.2. Potentiodynamic Polarization Measurement and IR Drop Elimination
2.3. Corrosion Rate and Product Characterization
3. Results
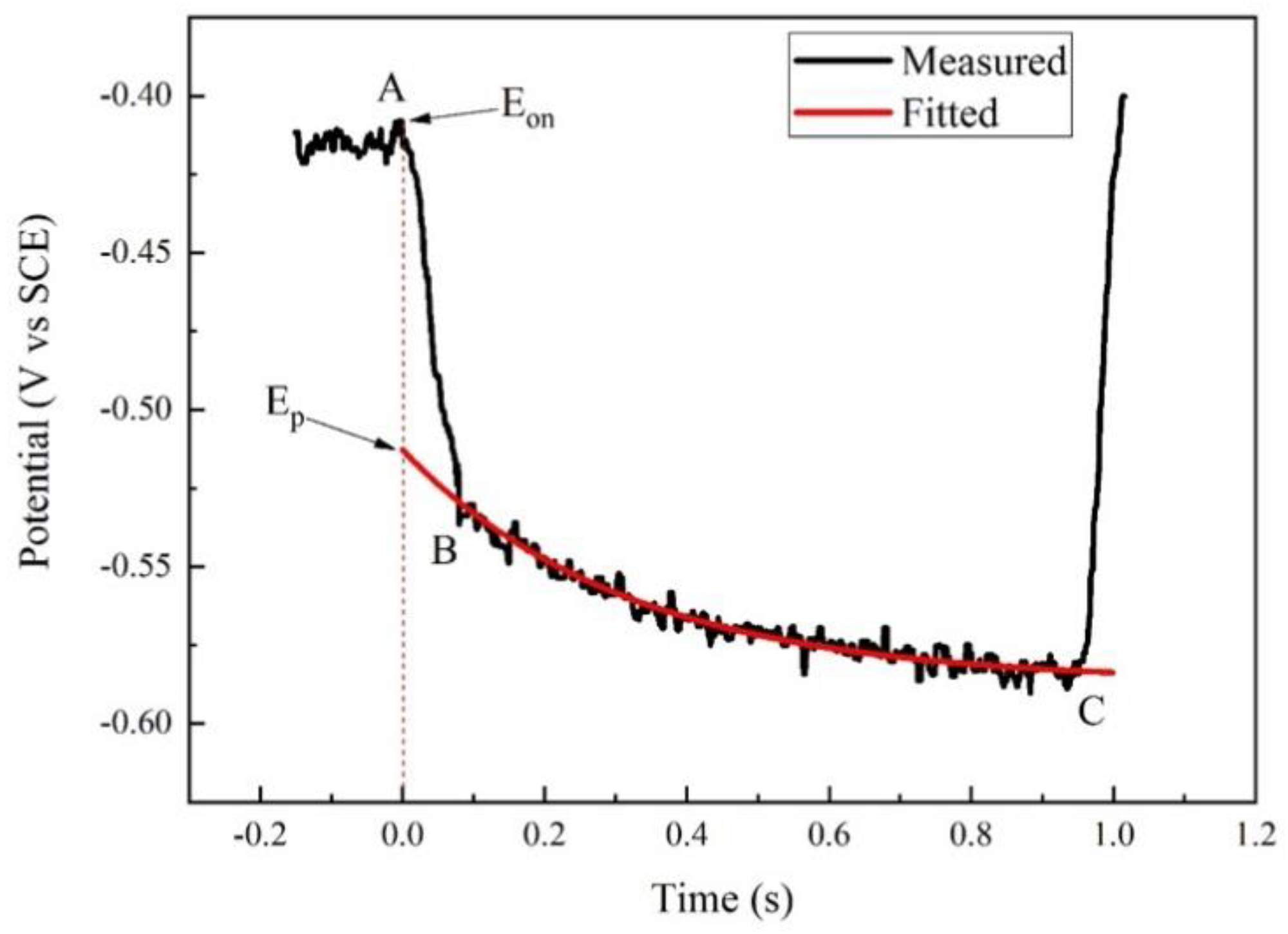
3.1. IR Drop Elimination
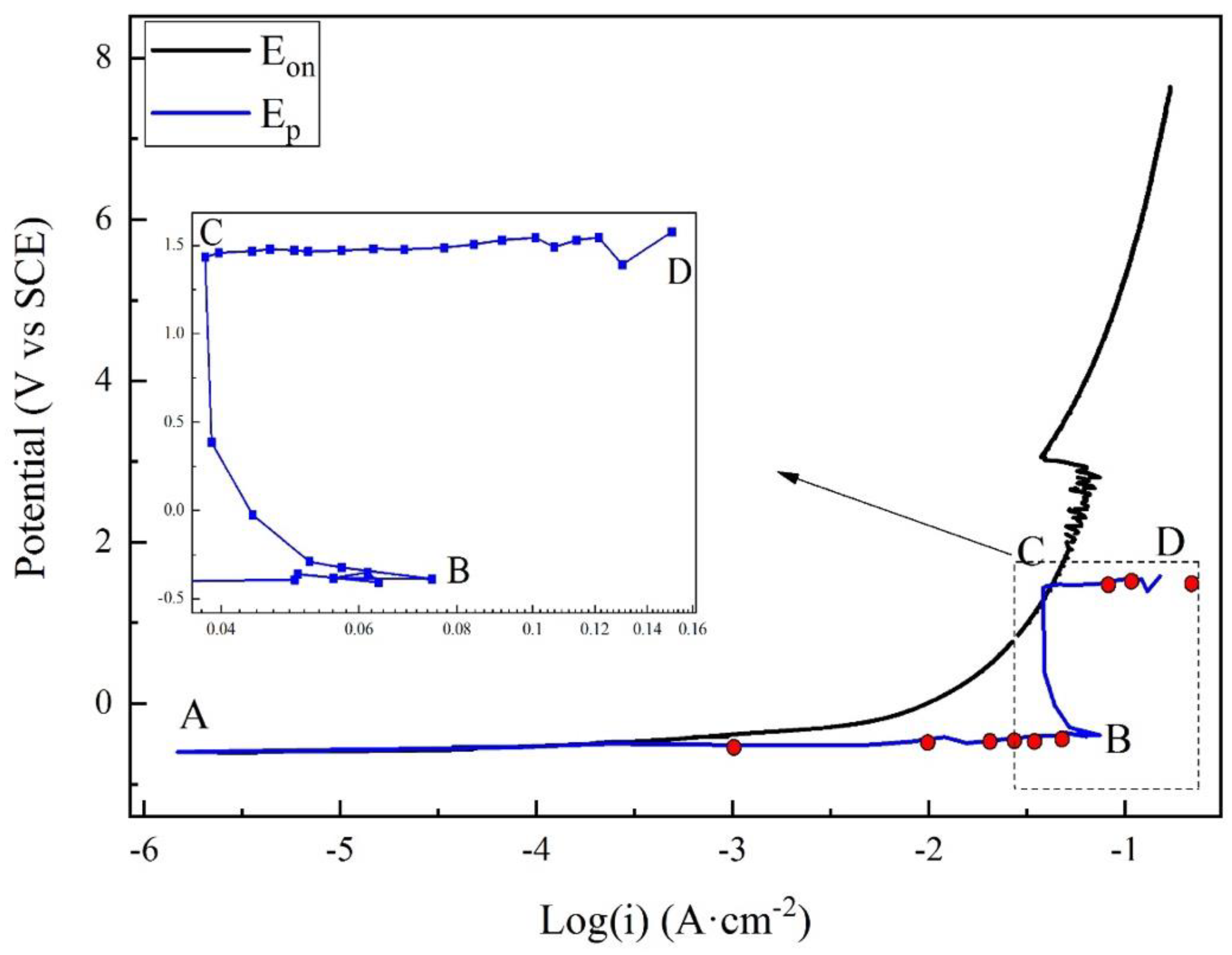
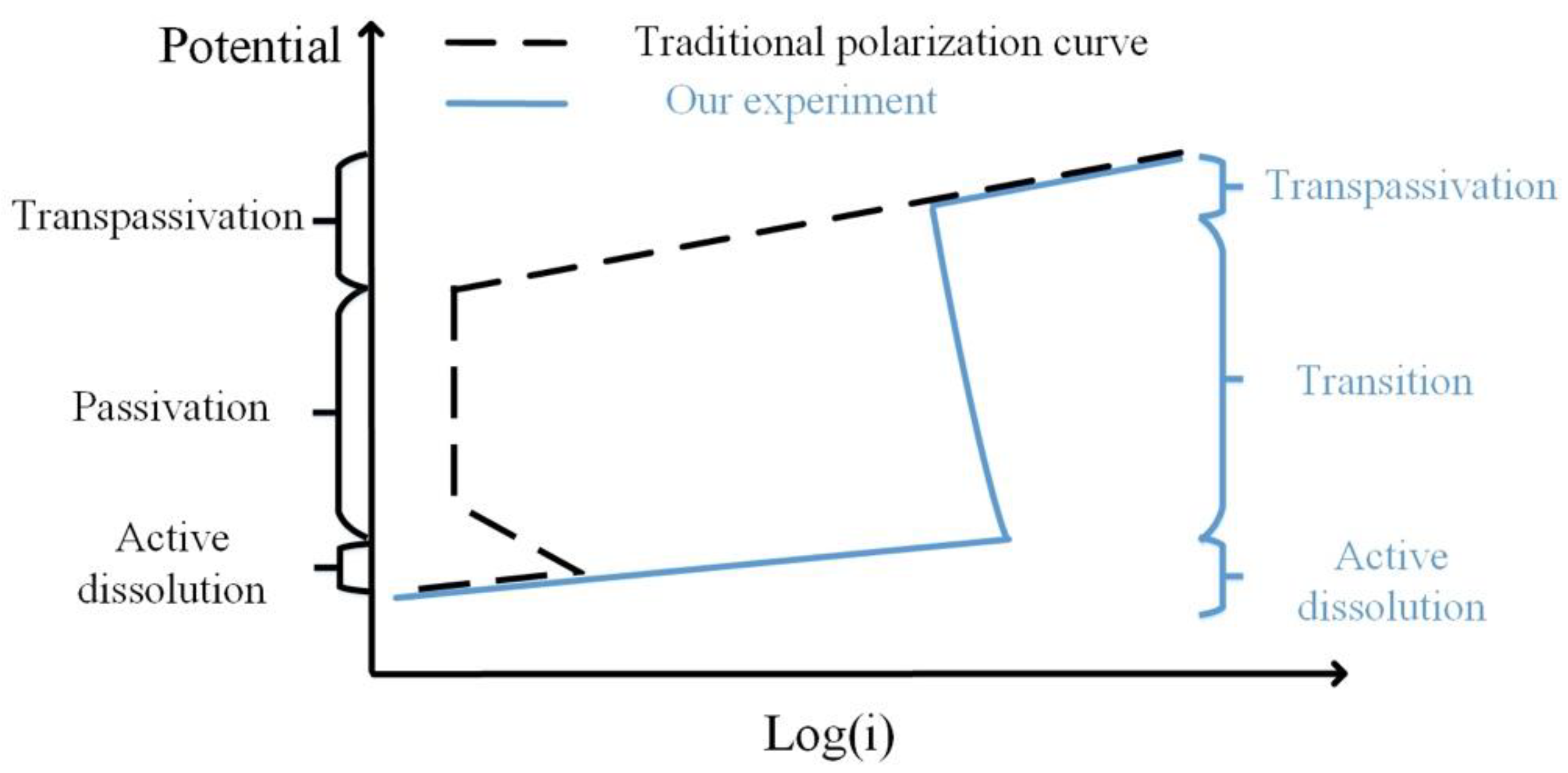
3.2. Potentiodynamic Polarization Results
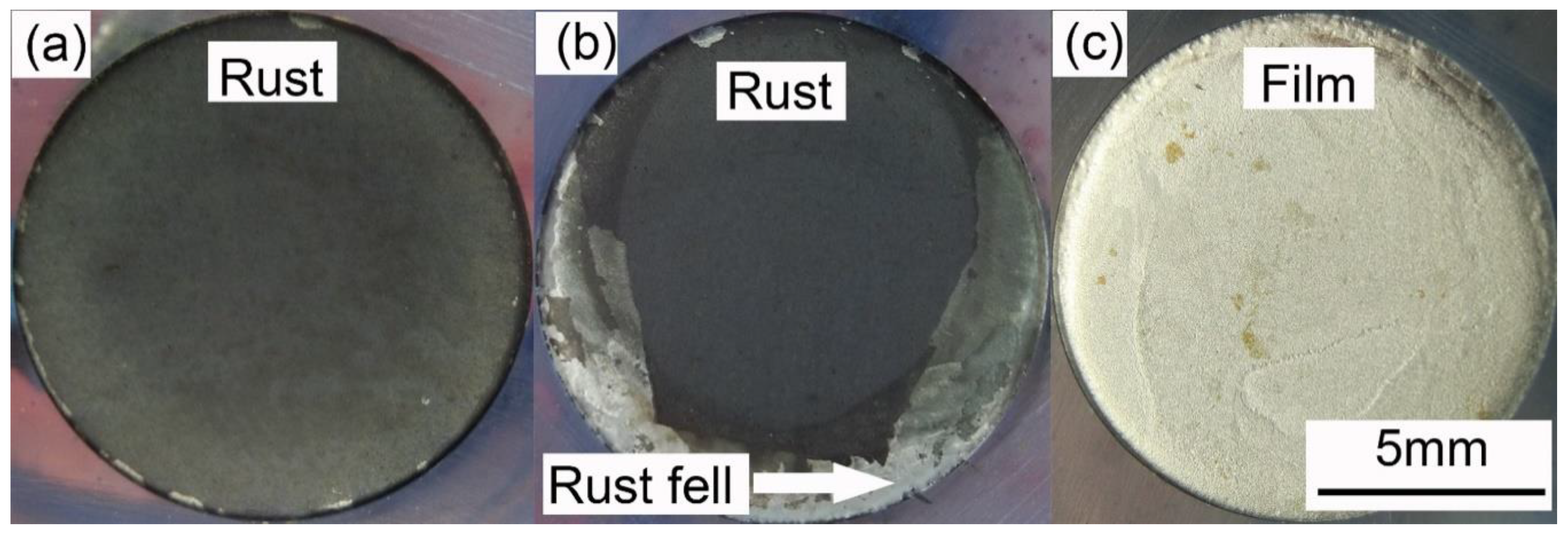
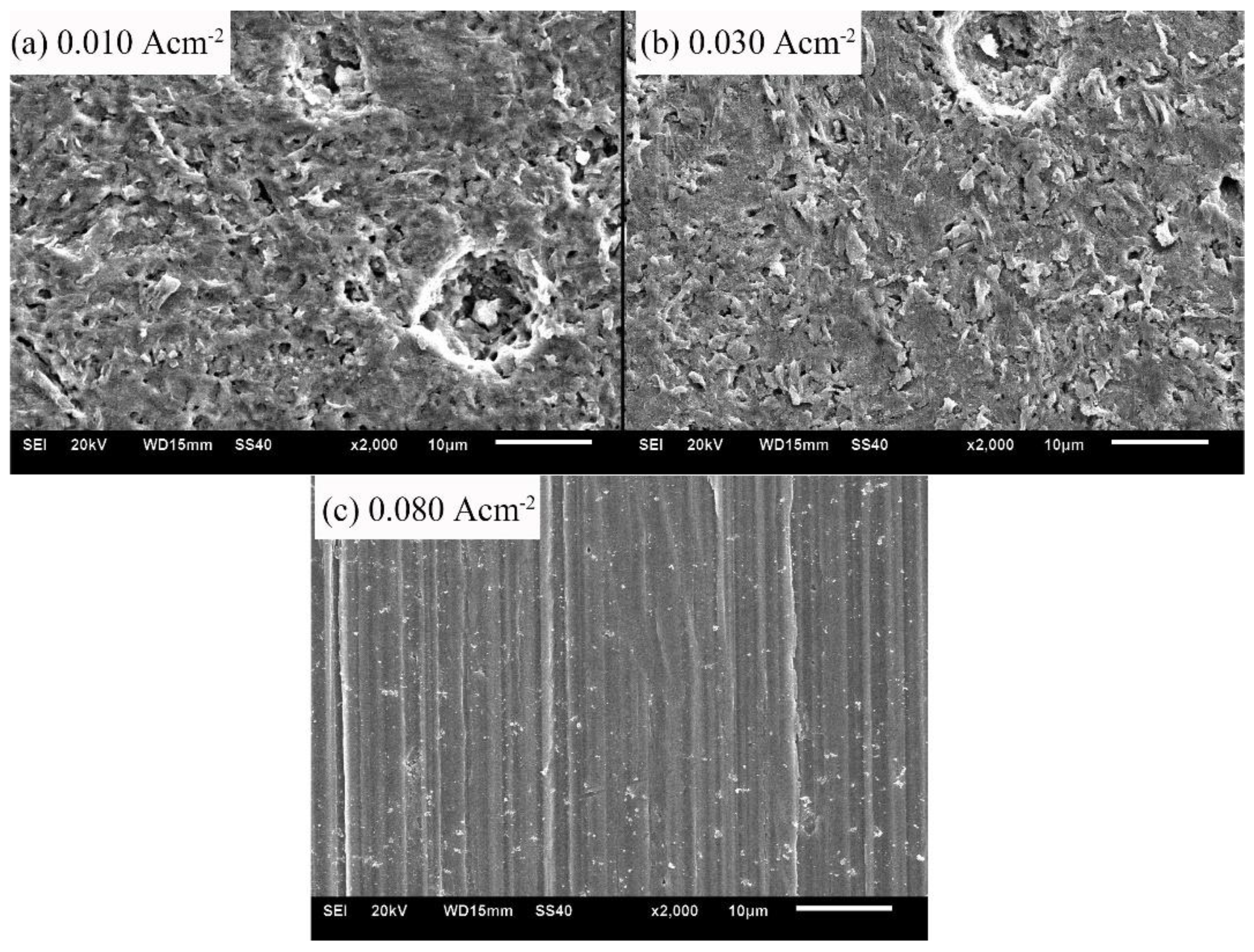
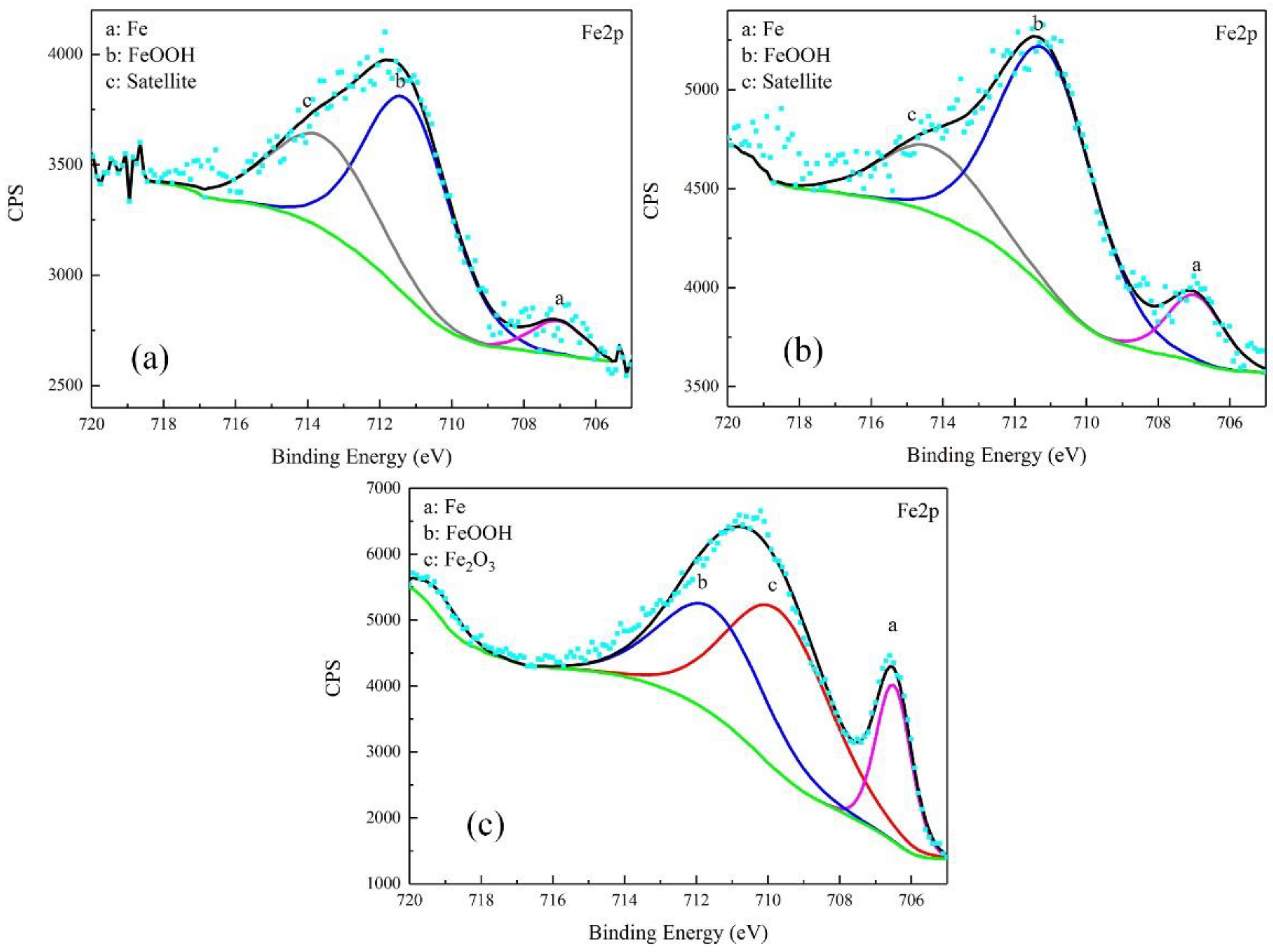
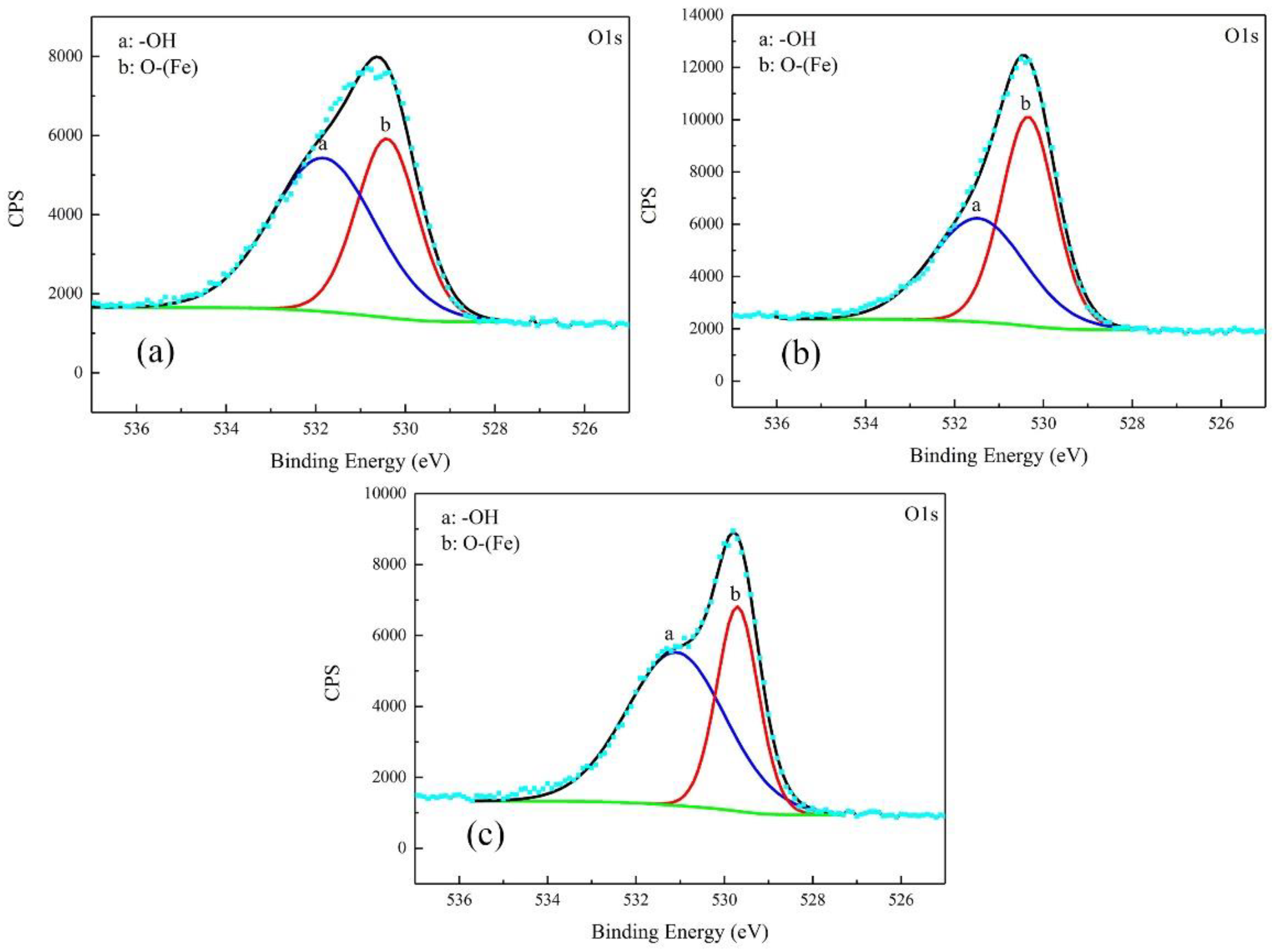
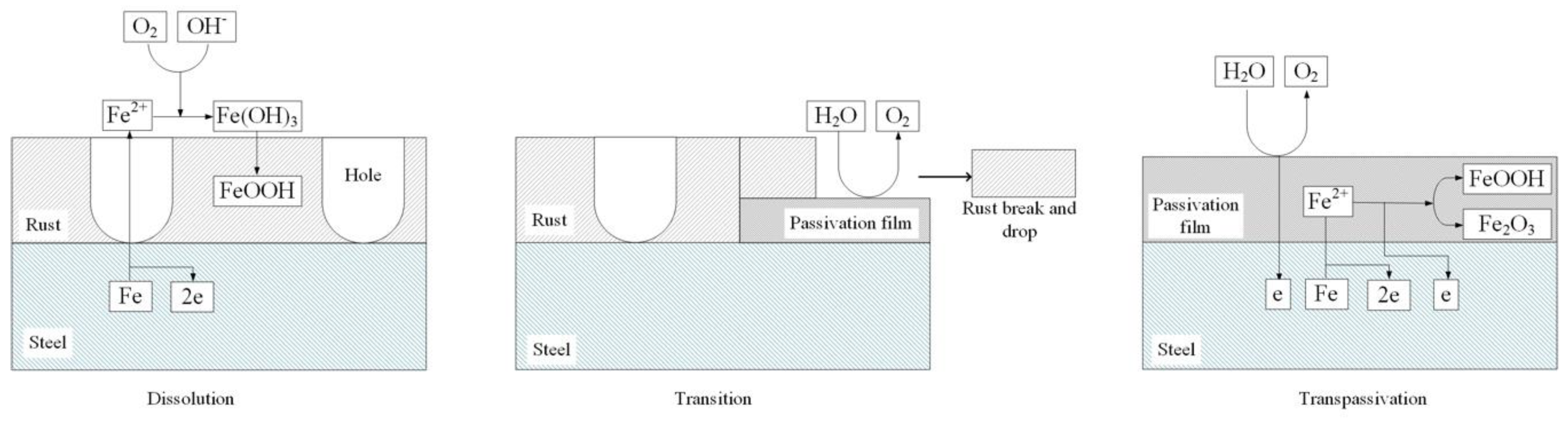
3.3. Corrosion Product Characterization
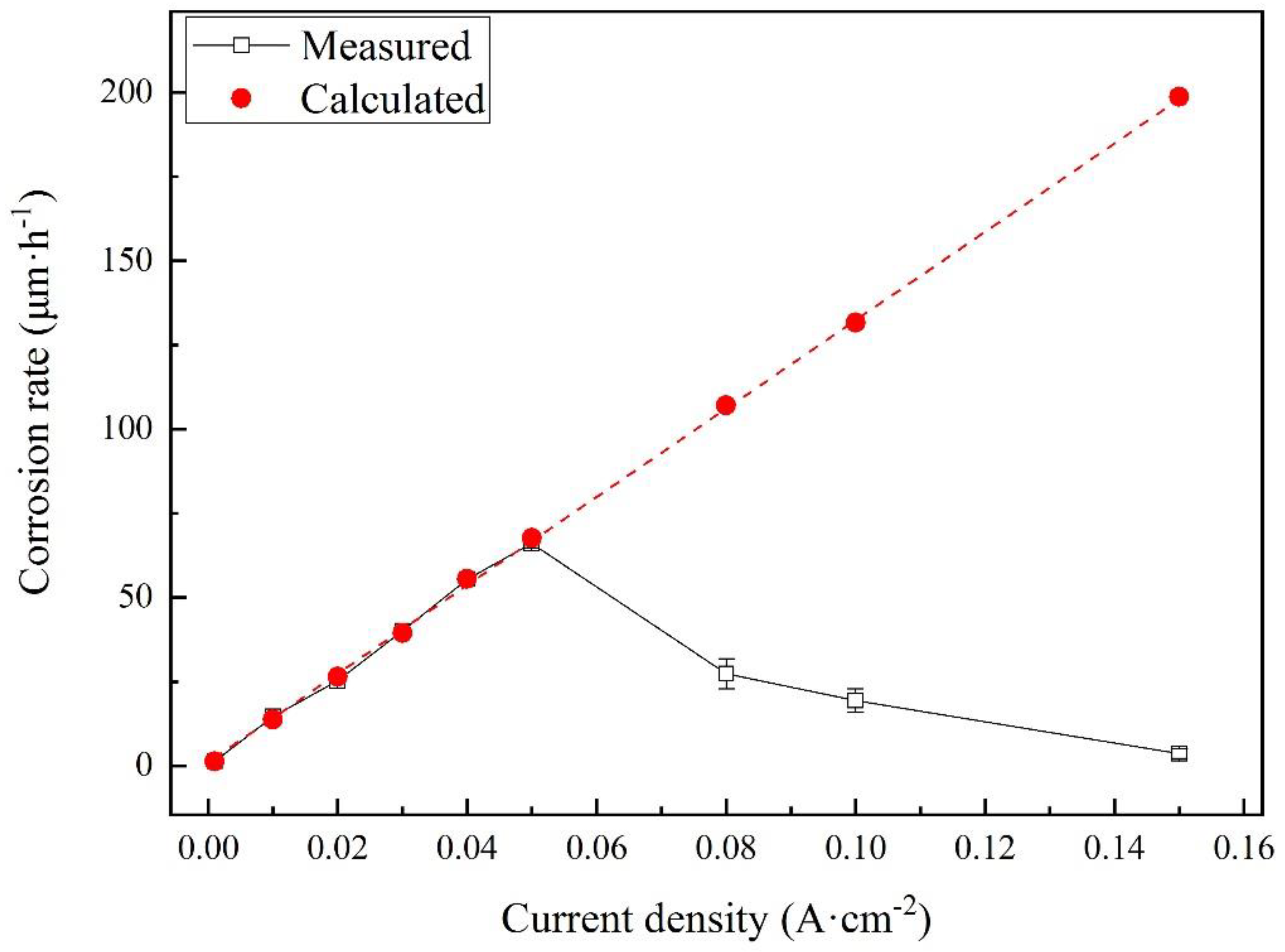
3.4. Corrosion Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gong, Y.; Xue, C.; Yuan, Z.; Li, Y.; Dawalibi, F.P. Advanced analysis of HVDC electrodes interference on neighboring pipelines. J. Power Energy Eng. 2015, 3, 332–341. [Google Scholar] [CrossRef]
- Qin, R.Z.; Du, Y.X.; Peng, G.Z.; Lu, M.X.; Jiang, Z.T. High Voltage Direct Current Interference on Buried Pipelines: Case Study and Mitigation Design. In Proceedings of the Corrosion Conference 2017, New Orleans, LA, USA, 28–31 March 2017. [Google Scholar]
- Jiang, Z.T.; Du, Y.X.; Lu, M.X.; Zhang, Y.; Tang, D.; Dong, L. New findings on the factors accelerating AC corrosion of buried pipeline. Corros. Sci. 2014, 81, 1–10. [Google Scholar] [CrossRef]
- Smith, P.; Roy, S.; Swailes, D.; Maxwell, S.; Page, D.; Lawson, J. A model for the corrosion of steel subjected to synthetic produced water containing sulfate, chloride and hydrogen sulfide. Chem. Eng. Sci. 2011, 66, 5775–5790. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion—Part 1: Effects on overpotentials of anodic and cathodic processes. Corros. Sci. 2010, 52, 491–497. [Google Scholar] [CrossRef]
- Gil, P.M.; Julien, B.; Palomar-Pardavé, M.; de Oca-Yemha, M.G.M.; Ramírez-Silva, M.T.; Romero-Romo, M. Electrochemical behaviour of API 5L X52 steel samples immersed in sulphate aqueous solutions with different pH. Int. J. Electrochem. Sci. 2018, 13, 3297–3308. [Google Scholar]
- Bockris, J.O.M.; Drazic, D.; Despic, A.R. The electrode kinetics of the deposition and dissolution of iron. Electrochim. Acta 1961, 4, 325–361. [Google Scholar] [CrossRef]
- Lorentz, W.J.; Heusler, K.E. Corrosion Mechanisms; Marcel Dekker: New York, NY, USA, 1987. [Google Scholar]
- Gonçalves, R.S.; Mello, L.D. Electrochemical investigation of ascorbic acid adsorption on low-carbon steel in 0.50 M Na2SO4 solutions. Corros. Sci. 2001, 43, 457–470. [Google Scholar] [CrossRef]
- Barbalat, M.; Lanarde, L.; Caron, D.; Meyer, M.; Vittonato, J.; Castillon, F.; Fontaine, S.; Refait, P. Electrochemical study of the corrosion rate of carbon steel in soil: Evolution with time and determination of residual corrosion rates under cathodic protection. Corros. Sci. 2012, 55, 246–253. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Wang, Y.; Liu, M.; Tang, K.; Si, S.; Li, H. Effects of SO42− on pitting corrosion behavior of X100 pipeline steel in alkaline solution. J. Chin. Univ. Pet. Nat. Sci. Ed. 2016, 40, 128. [Google Scholar]
- Zhang, X.; Shi, Z.; Wang, Y.; Liu, M.; Yang, S. Corrosion behavior of X100 pipeline steel in simulated solution of alkaline soil. J. Chin. Soc. Corros. Prot. 2015, 35, 33–37. [Google Scholar]
- Xie, F.; Wu, M.; Chen, X.; Wang, D.; Hu, Z.; Liu, Y. Effects of SO42− on corrosion behavior of X80 pipeline steel in simulated Ku’erle soil solution. J. Cent. South Univ. 2013, 44, 424–430. [Google Scholar]
- Myland, J.C.; Oldham, K.B. Uncompensated Resistance. 1. The Effect of Cell Geometry. Anal. Chem. 2000, 72, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Oelßner, W.; Berthold, F.; Guth, U. The iR drop-well-known but often underestimated in electrochemical polarization measurements and corrosion testing. Mater. Corros. 2006, 57, 455–466. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Hiveš, J.; Roušar, I. Measurement of anode potentials at high current densities in NaNO3 and NaClO3 media by the current interruption method for metals used in aviation technology. J. Appl. Electrochem. 1994, 24, 798–802. [Google Scholar] [CrossRef]
- Mao, K.W. Anodic Polarization Study of Mild Steel in NaCl Solution During Electrochemical Machining. J. Electrochem. Soc. 1973, 120, 1056–1060. [Google Scholar] [CrossRef]
- Britz, D.; Brocke, W.A.J. Elimination of iR-drop in electrochemical cells by the use of a current-interruption potentiostat. Electroanal. Chem. Interfacial Electrochem. 1975, 58, 301. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion rate of steel in concrete—Measurements beyond the Tafel law. Corros. Sci. 2005, 47, 3019–3033. [Google Scholar] [CrossRef]
- ISO 8407:2009. Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens; International Organization for Standardizatino: Geneva, Switzerland, 2009. [Google Scholar]
- Ghods, P.; Isgor, O.B.; Brown, J.R.; Bensebaa, F.; Kingston, D. XPS depth profiling study on the passive oxide film of carbon steel in saturated calcium hydroxide solution and the effect of chloride on the film properties. Appl. Surf. Sci. 2011, 257, 4669–4677. [Google Scholar] [CrossRef]
- Ejaz, A.; Lu, Z.; Chen, J.; Xiao, Q.; Ru, X.; Han, G.; Shoji, T. The effects of hydrogen on anodic dissolution and passivation of iron in alkaline solutions. Corros. Sci. 2015, 101, 165–181. [Google Scholar] [CrossRef]
- Mourya, P.; Singh, P.; Tewari, A.K.; Rastogi, R.B.; Singh, M.M. Relationship between structure and inhibition behaviour of quinolinium salts for mild steel corrosion: Experimental and theoretical approach. Corros. Sci. 2015, 95, 71–87. [Google Scholar] [CrossRef]
- Xu, W.; Daub, K.; Zhang, X.; Noel, J.J.; Shoesmith, D.W.; Wren, J.C. Oxide formation and conversion on carbon steel in mildly basic solutions. Electrochim. Acta 2009, 54, 5727–5738. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Deen, K.M.; Cano, L.; Haider, W. The effects of parametric changes in electropolishing process on surface properties of 316L stainless steel. Appl. Surf. Sci. 2017, 410, 432–444. [Google Scholar] [CrossRef]
- López, D.A.; Schreiner, W.H.; de Sánchez, S.R.; Simison, S.N. The influence of carbon steel microstructure on corrosion layers. Appl. Surf. Sci. 2003, 207, 69–85. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. The effect of β-FeOOH on the corrosion behavior of low carbon steel exposed in tropic marine environment. Mater. Chem. Phys. 2008, 112, 844–852. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley: New York, NY, USA, 2003. [Google Scholar]
- de La Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Castaño, J.G.; Botero, C.A.; Restrepo, A.H.; Agudelo, E.A.; Correa, E.; Echeverría, F. Atmospheric corrosion of carbon steel in Colombia. Corros. Sci. 2010, 52, 216–223. [Google Scholar] [CrossRef]
- Marcus, P. Corrosion Mechanisms in Theory and Practice; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Freire, L.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Larramona, G.; Gutiérrez, C. The Passive Film on Iron at pH 1–14 A Potential-Modulated Reflectance Study. J. Electrochem. Soc. 1989, 136, 2171–2178. [Google Scholar] [CrossRef]
- Meisel, W. Corrosion processes and their inhibition as studied by Mössbauer conversion and other electron spectroscopies. Hyperfine Interact. 1989, 45, 73–90. [Google Scholar] [CrossRef]
- Gui, J.; Devine, T.M. The influence of sulfate ions on the surface enhanced raman spectra of passive films formed on iron. Corros. Sci. 1994, 36, 441–462. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, C.; Jia, Z.; Li, X. Pitting Corrosion of X70 Pipeline Steel in the Simulated Wet Storage Environment. Acta Metall. Sin. 2011, 47, 1009–1016. [Google Scholar]
- El-Naggar, M.M. Effects of Cl−, NO3− and SO42− anions on the anodic behavior of carbon steel in deaerated 0.50M NaHCO3 solutions. Appl. Surf. Sci. 2006, 252, 6179–6194. [Google Scholar] [CrossRef]
- Yang, J.; Dong, J.; Ke, W. Effects of SO42− and Cl− on active/passive corrosion behaviors of low carbon steel in deaerated bicarbonate solution. Acta Metall. Sin. 2011, 47, 1321–1326. [Google Scholar]
- Simard, S.; Odziemkowski, M.; Irish, D.E.; Brossard, L.; Menard, H. In situ micro-Raman spectroscopy to investigate pitting corrosion product of 1024 mild steel in phosphate and bicarbonate solutions containing chloride and sulfate ions. J. Appl. Electrochem. 2001, 31, 913–920. [Google Scholar] [CrossRef]
- Gadala, I.M.; Alfantazi, A. Electrochemical behavior of API-X100 pipeline steel in NS4, near-neutral, and mildly alkaline pH simulated soil solutions. Corros. Sci. 2014, 82, 45–57. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Ni, Z.; Huang, R. Corrosion behavior of steel submitted to chloride and sulphate ions in simulated concrete pore solution. Const. Build. Mater. 2016, 115, 1–5. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Du, Y.; Xu, Z.; Lu, M. Anodic Polarization Behavior of X80 Steel in Na2SO4 Solution under High Potential and Current Density Conditions. Materials 2019, 12, 394. https://doi.org/10.3390/ma12030394
Qin R, Du Y, Xu Z, Lu M. Anodic Polarization Behavior of X80 Steel in Na2SO4 Solution under High Potential and Current Density Conditions. Materials. 2019; 12(3):394. https://doi.org/10.3390/ma12030394
Chicago/Turabian StyleQin, Runzhi, Yanxia Du, Zhenchang Xu, and Minxu Lu. 2019. "Anodic Polarization Behavior of X80 Steel in Na2SO4 Solution under High Potential and Current Density Conditions" Materials 12, no. 3: 394. https://doi.org/10.3390/ma12030394
APA StyleQin, R., Du, Y., Xu, Z., & Lu, M. (2019). Anodic Polarization Behavior of X80 Steel in Na2SO4 Solution under High Potential and Current Density Conditions. Materials, 12(3), 394. https://doi.org/10.3390/ma12030394




