Solid State Phase Equilibria of an Al–Sn–Y Ternary System
Abstract
:1. Introduction
2. Materials and Methods
3. Results
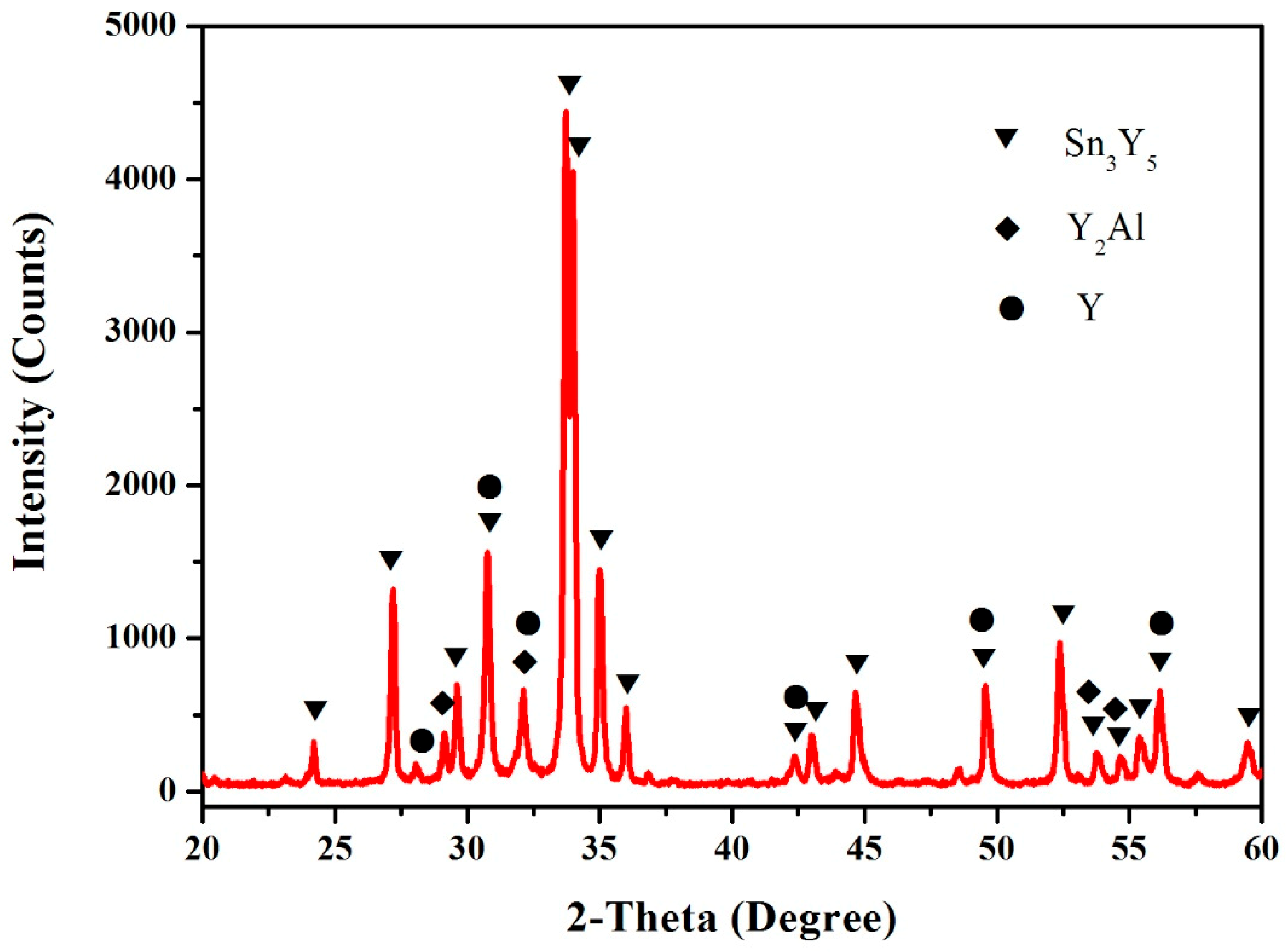
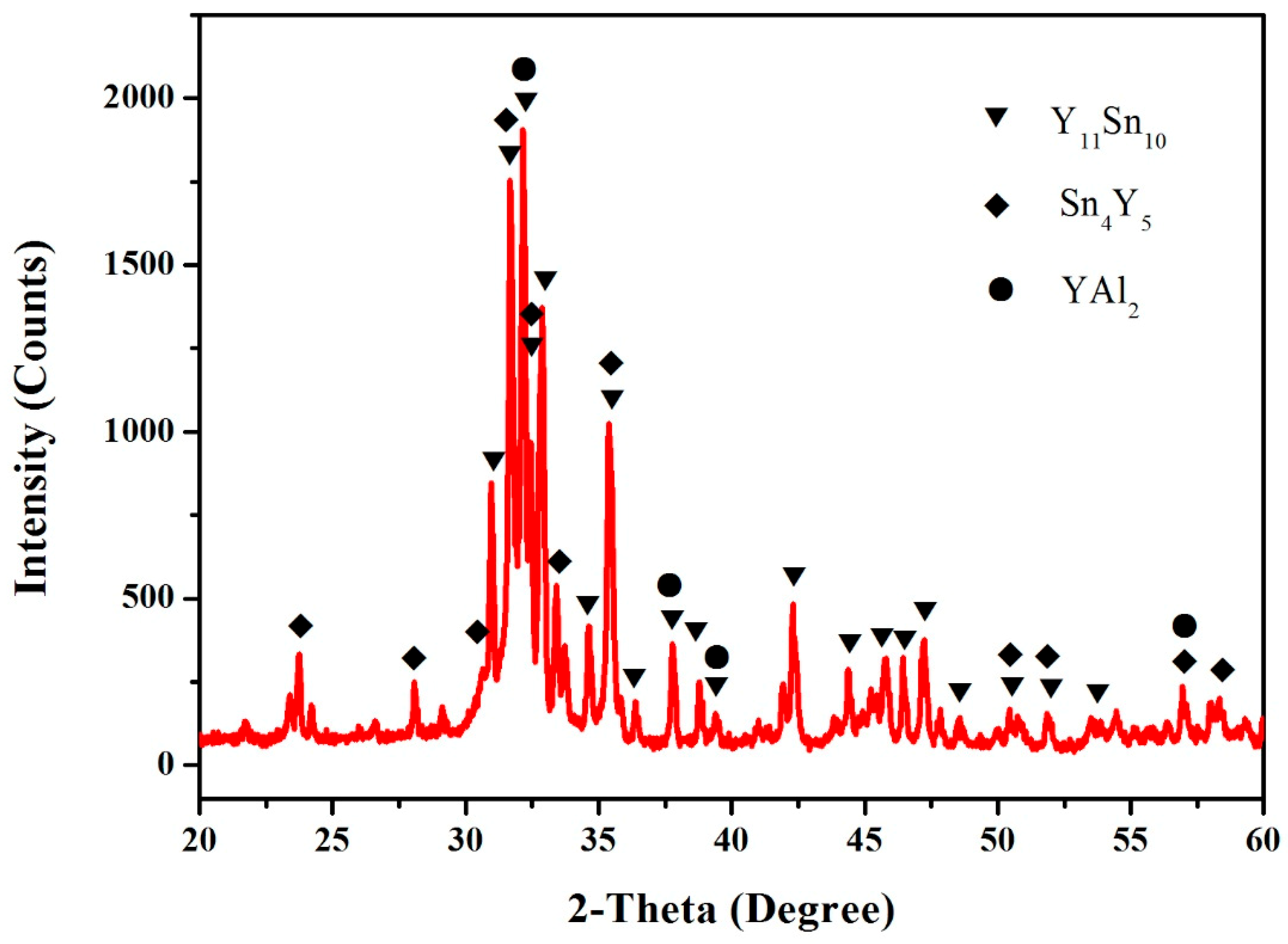
3.1. Sn–Y Binary System
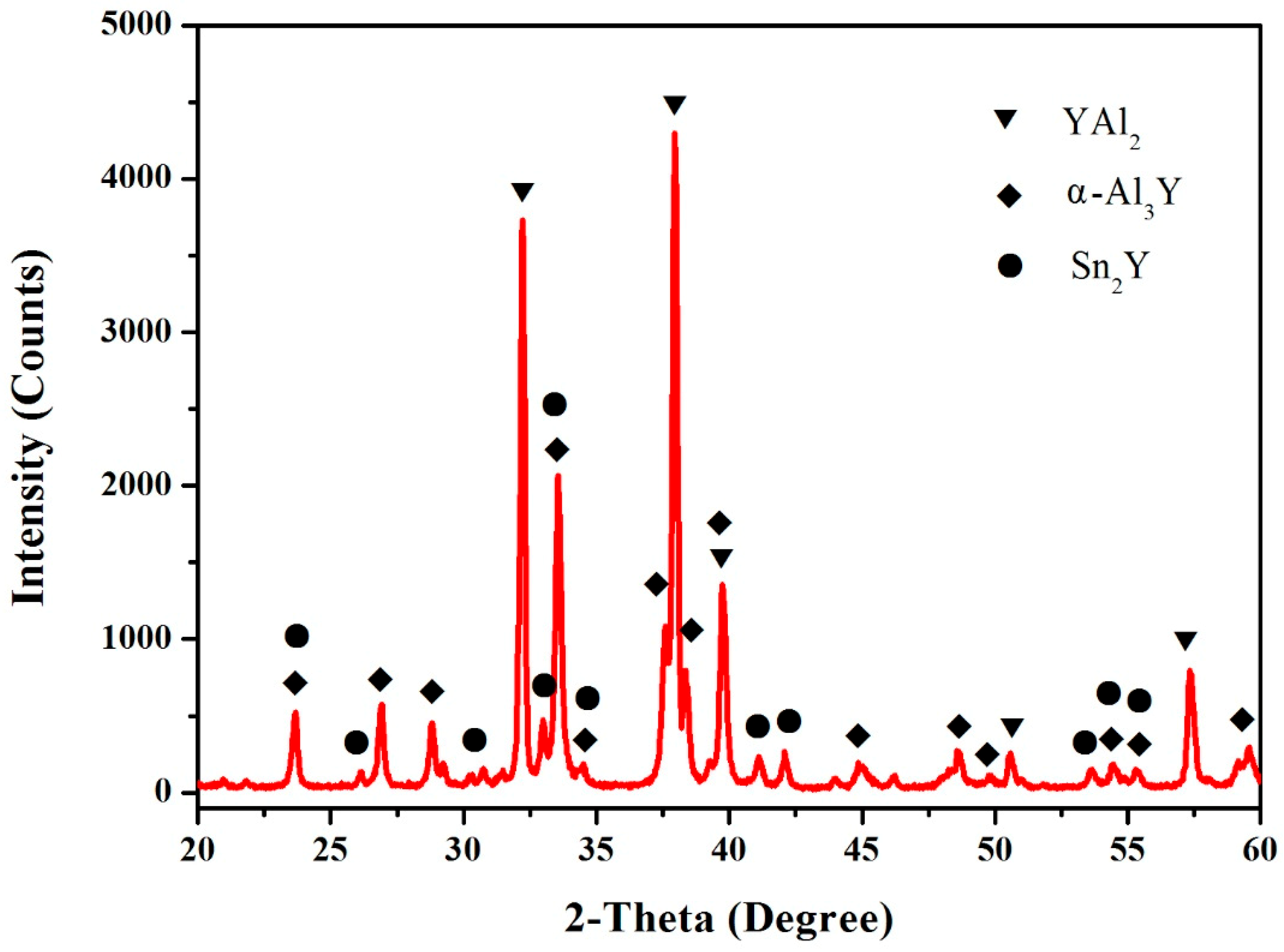
3.2. Al–Y Binary System
3.3. Sn–Al Binary System
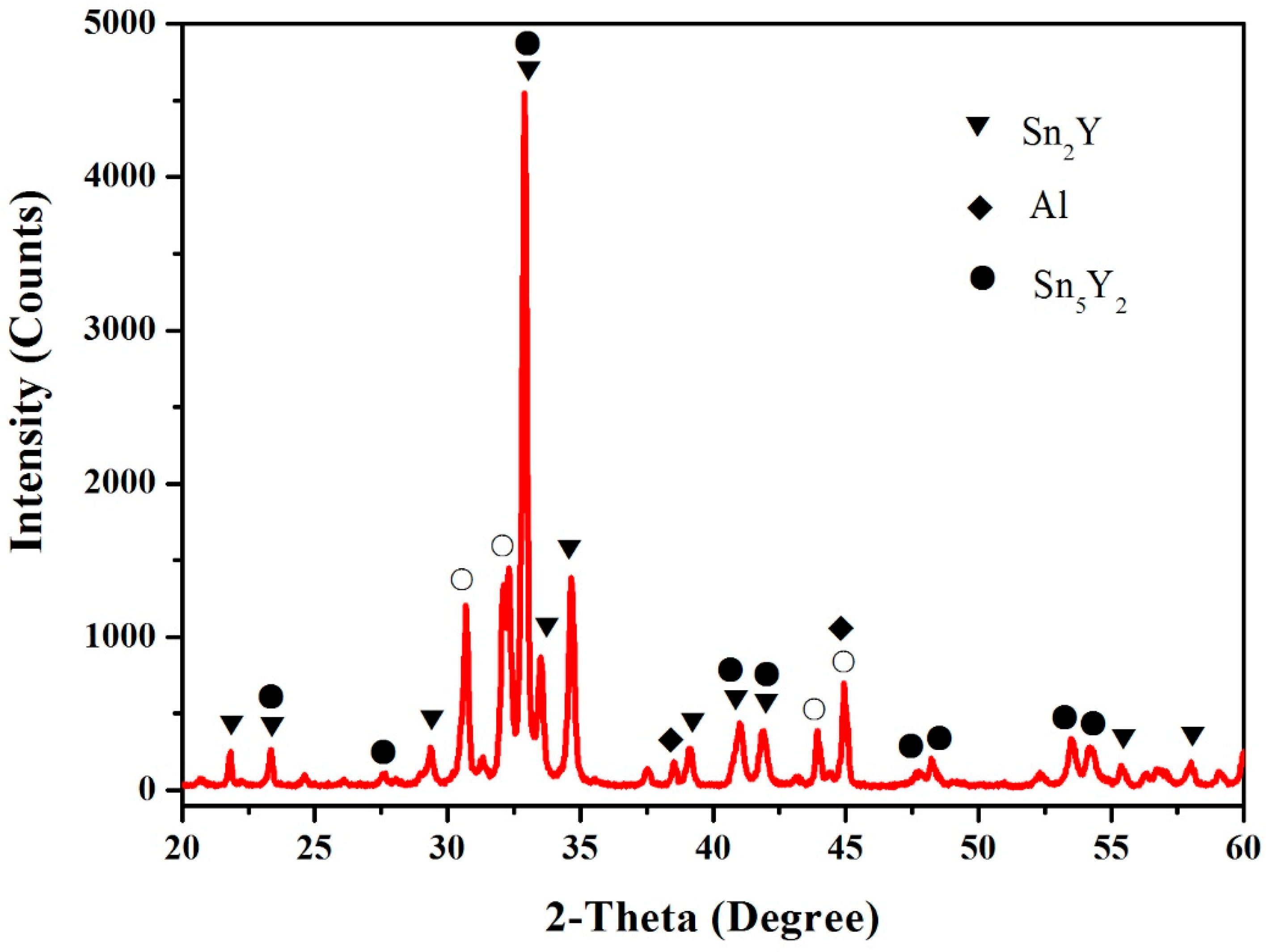
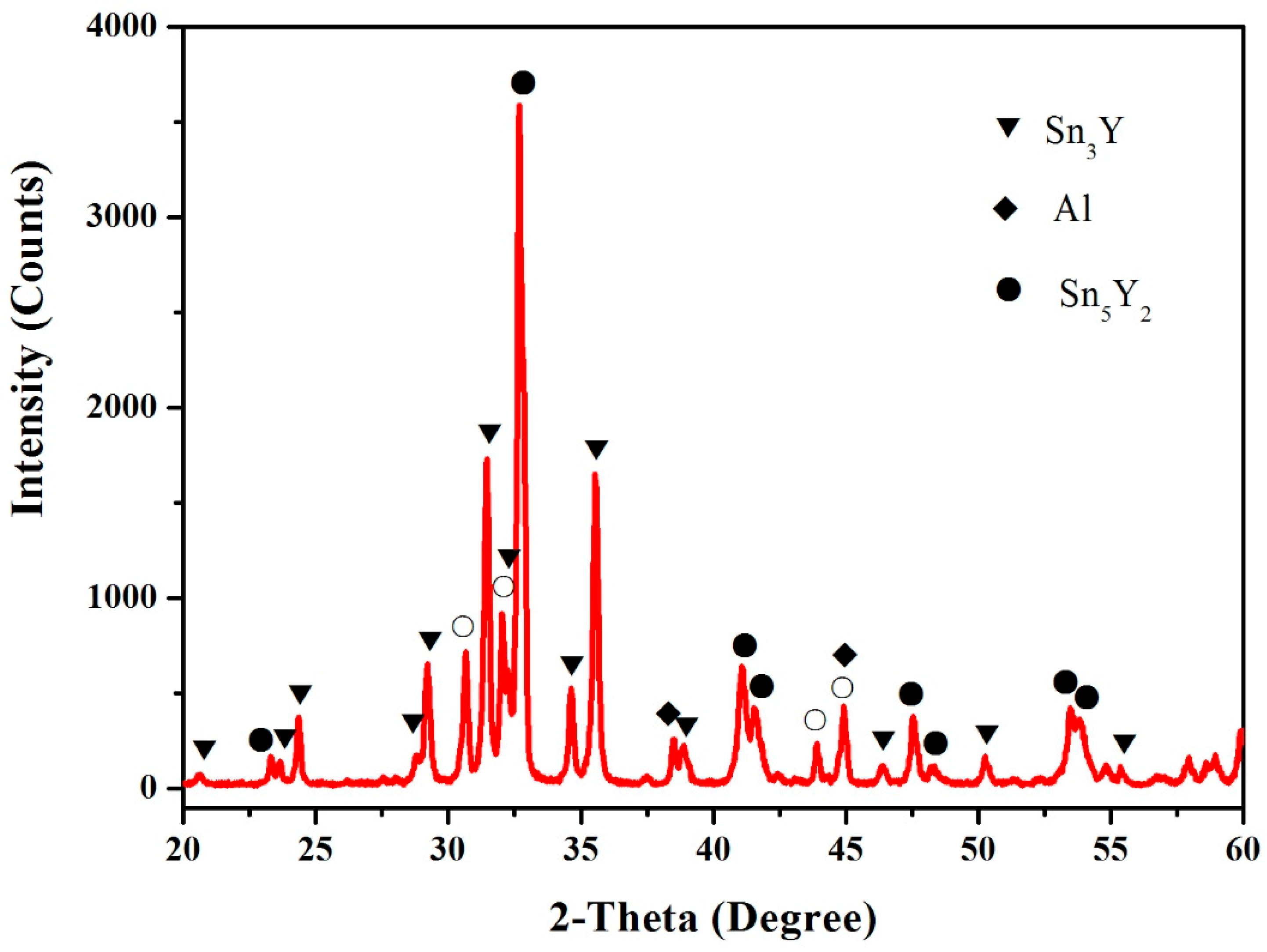
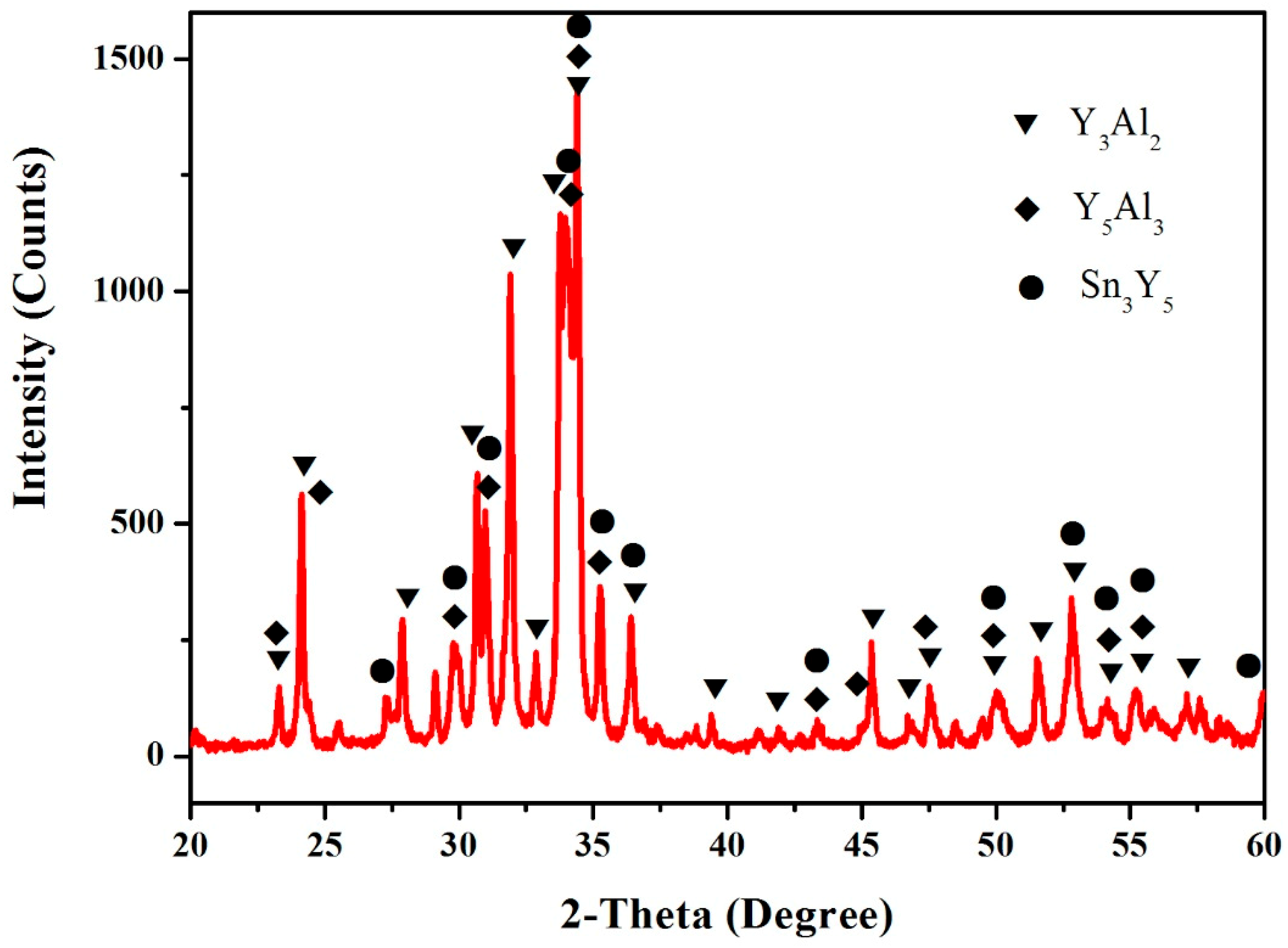
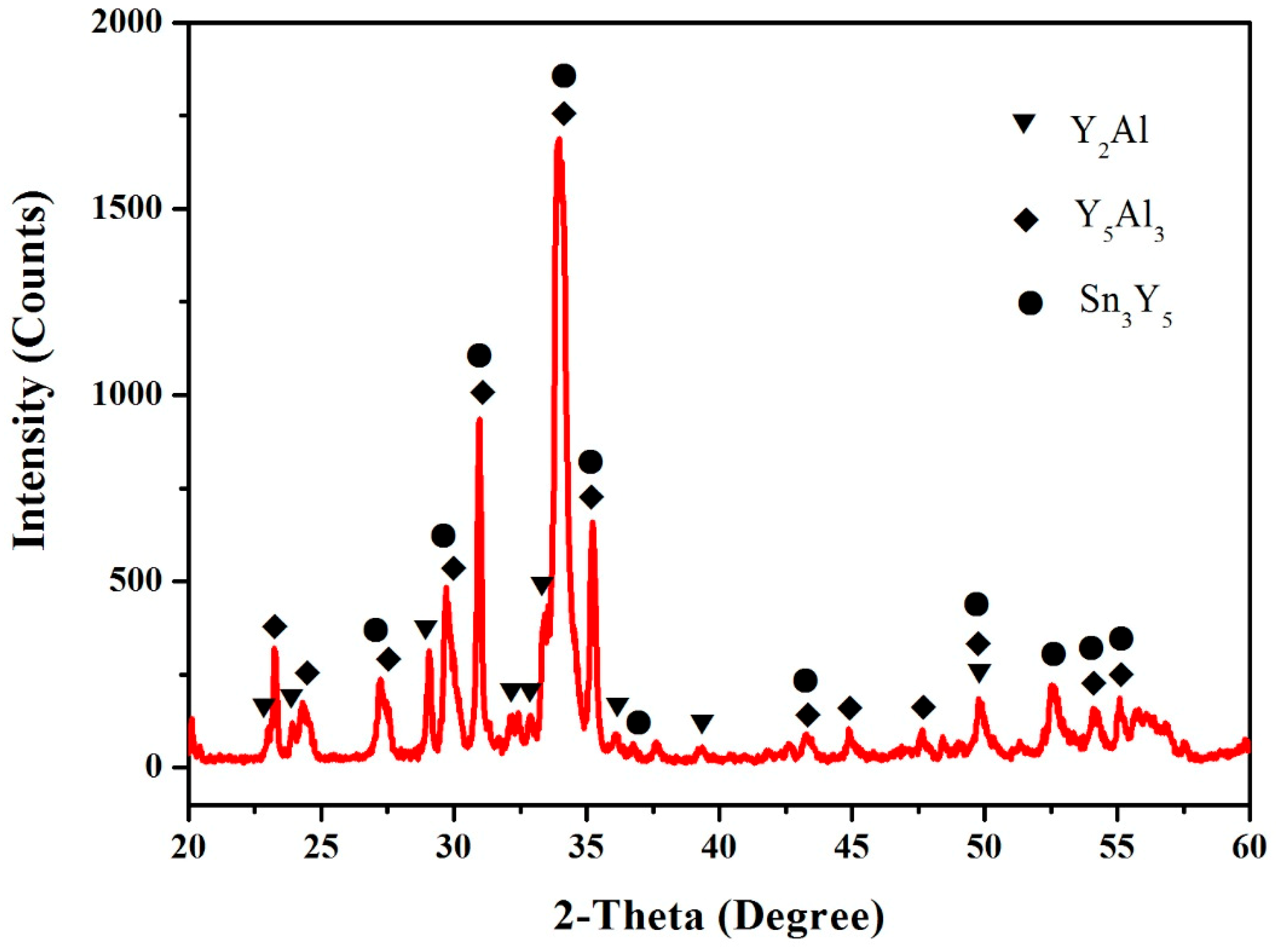
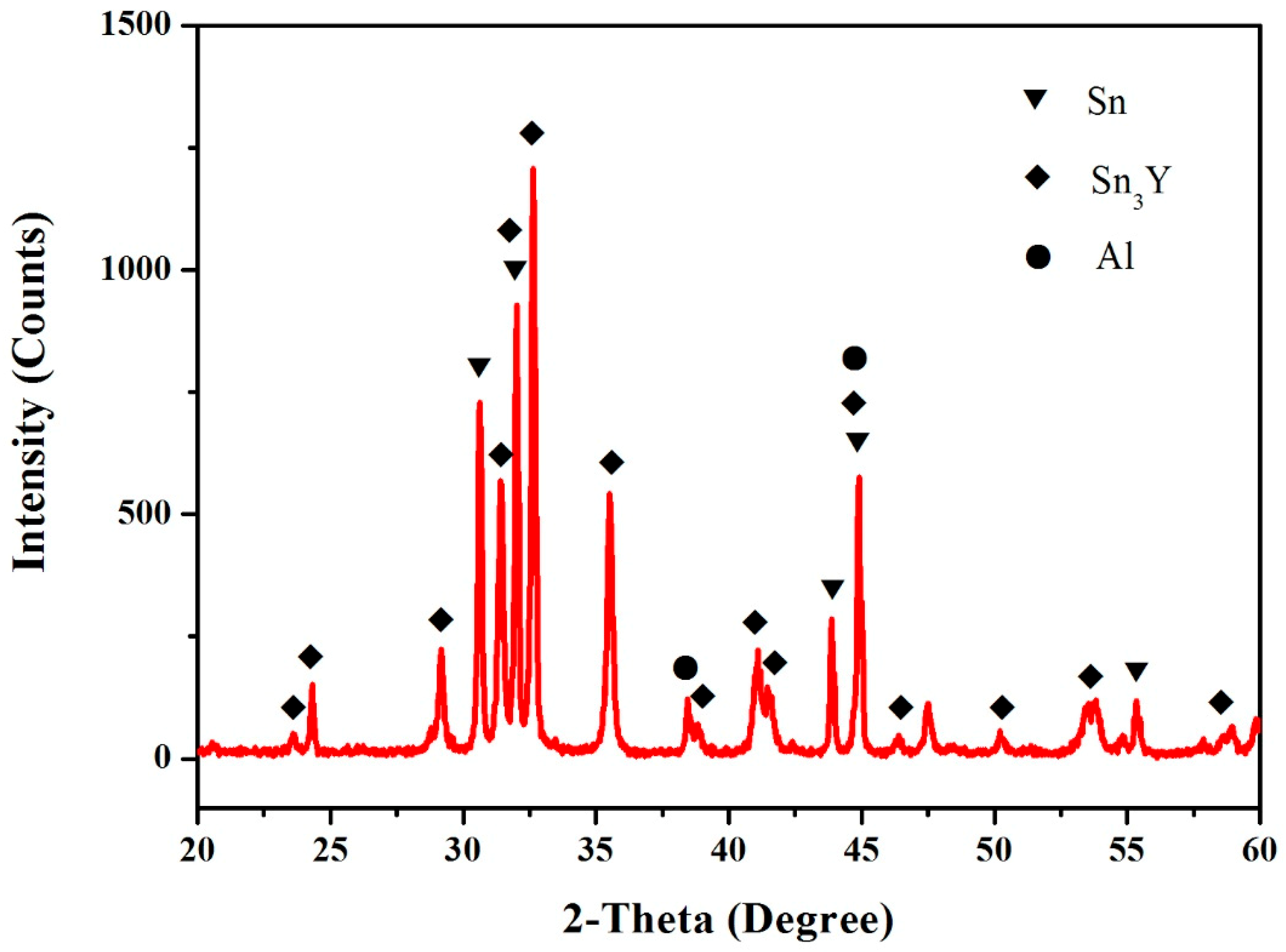
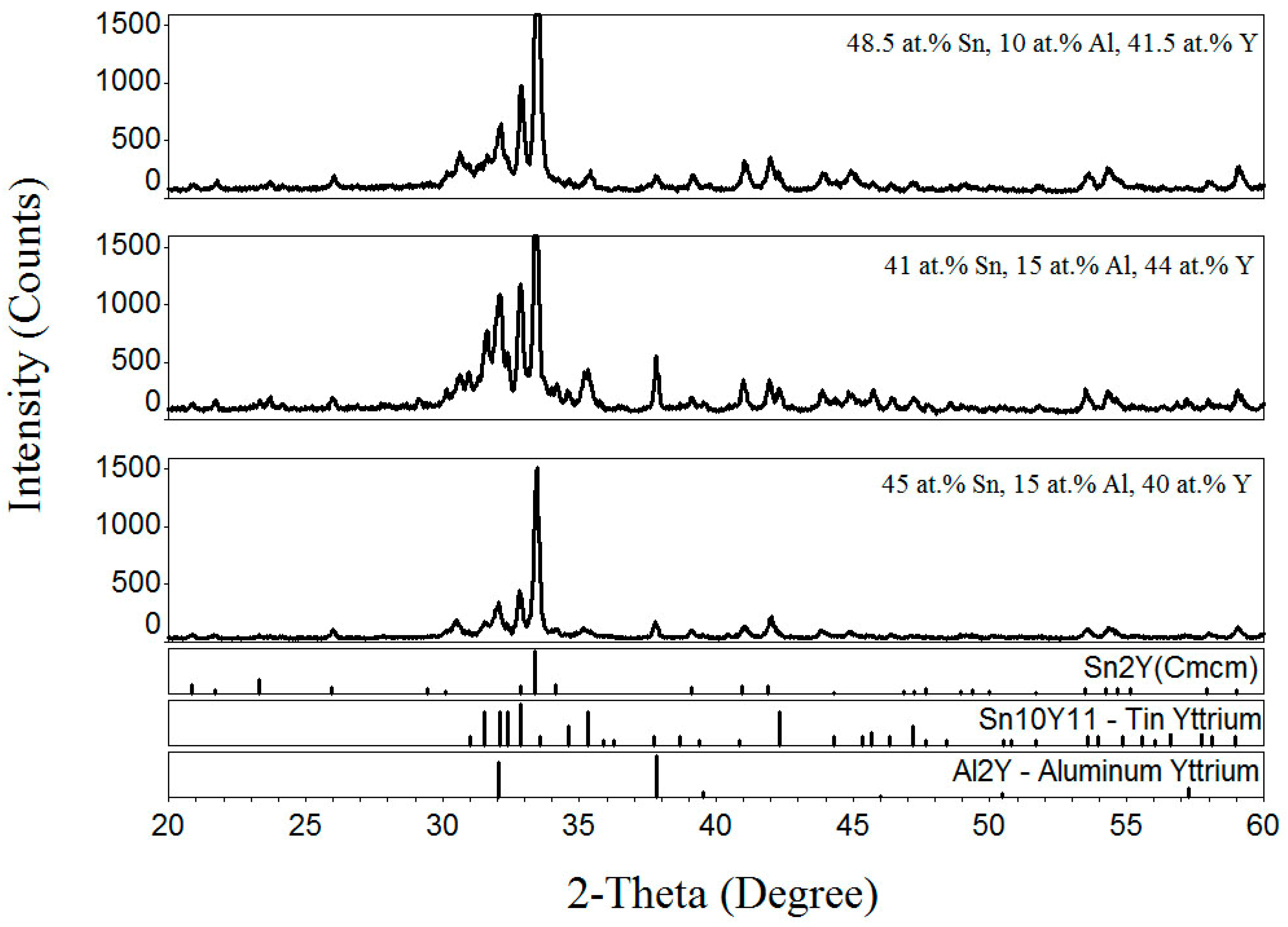
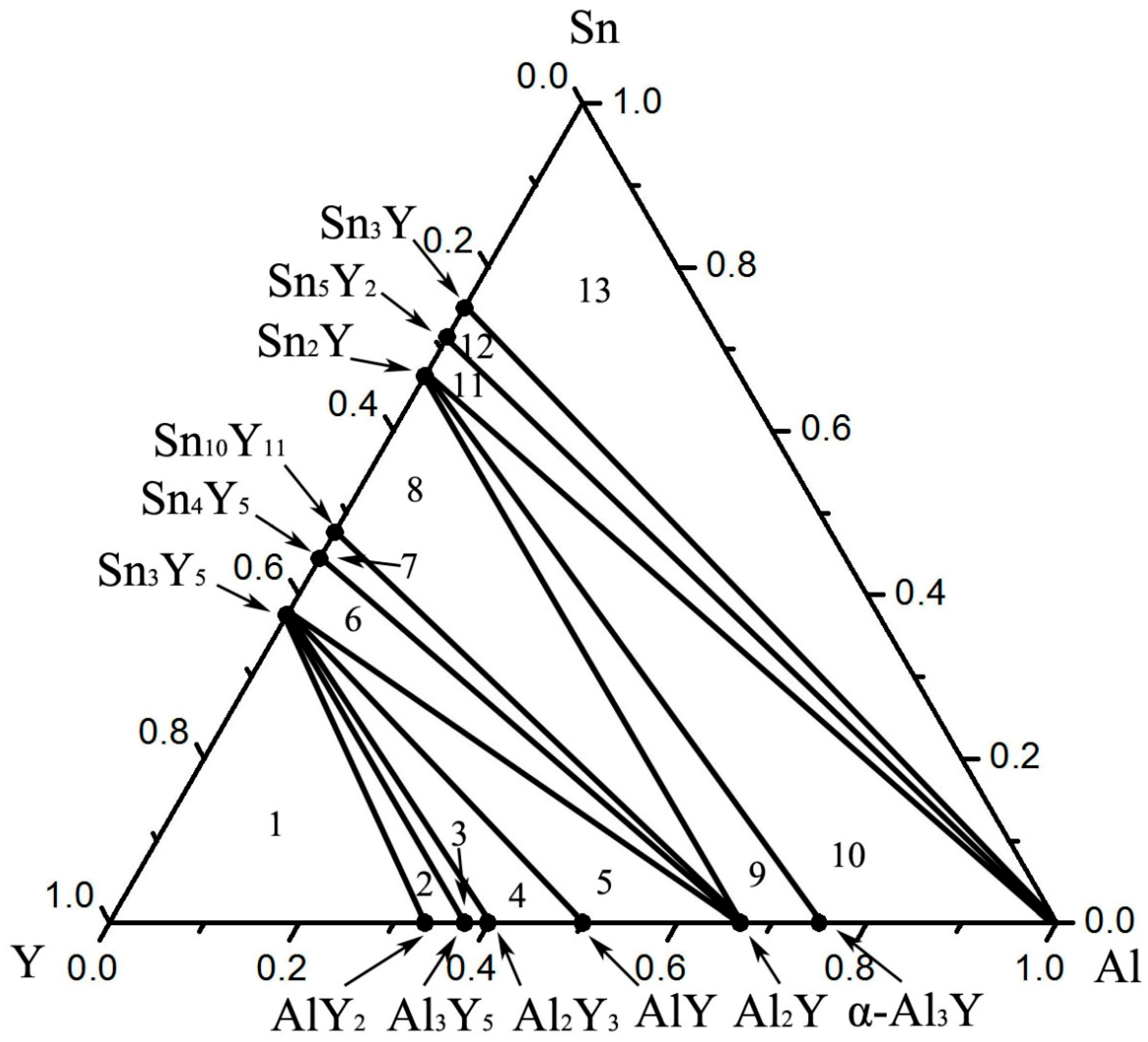
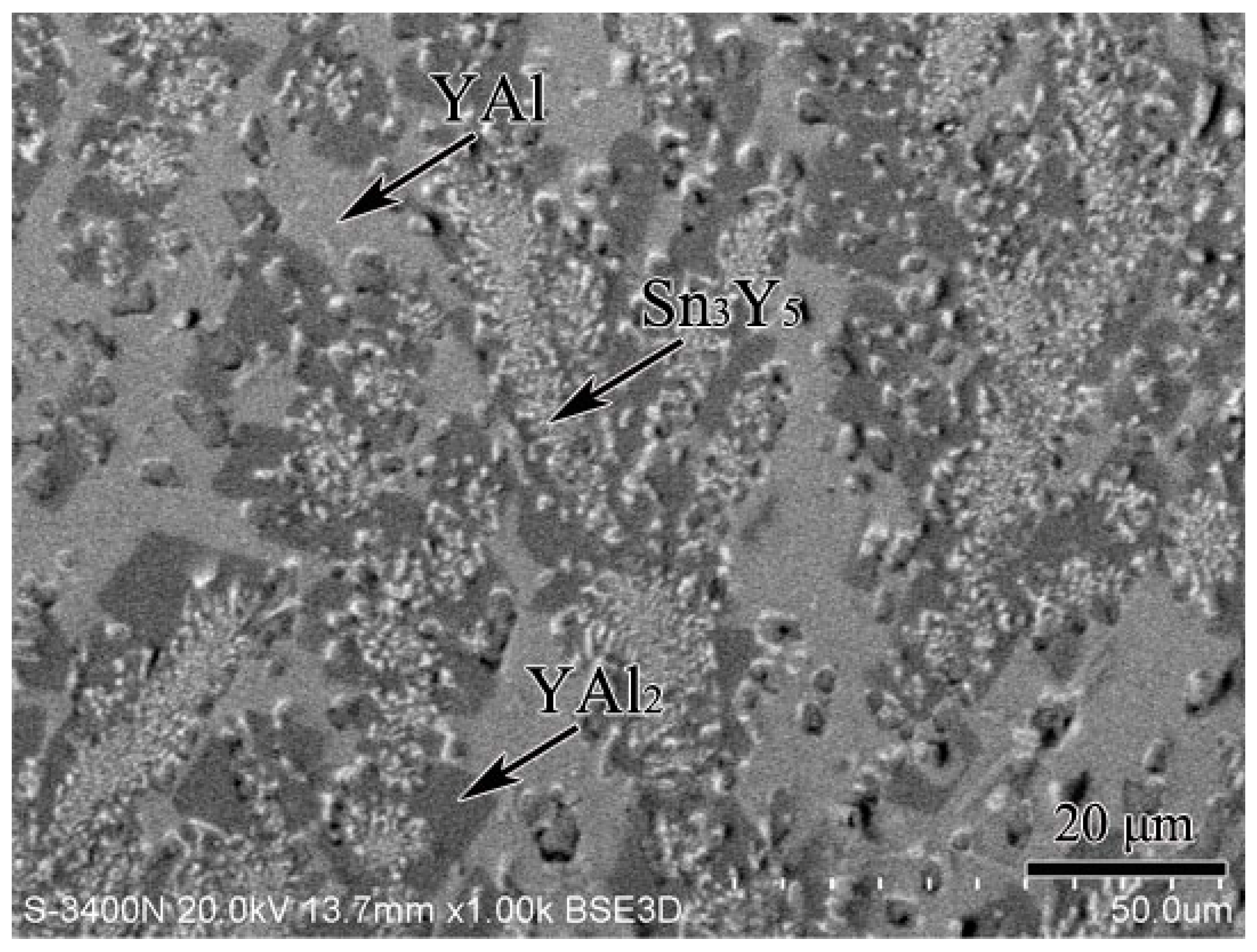
3.4. Al–Sn–Y Ternary System
3.5. Isothermal Section
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Zeng, M.Q.; Ma, Y.; Zhu, M. Wear behavior of Al–Sn alloys with different distribution of Sn dispersoids manipulated by mechanical alloying and sintering. Wear 2008, 265, 1857–1863. [Google Scholar] [CrossRef]
- Ning, X.J.; Jang, J.H.; Kim, H.J.; Li, C.J.; Lee, C. Cold spraying of Al–Sn binary alloy: Coating characteristics and particle bonding features. Surf. Coat. Technol. 2008, 202, 1681–1687. [Google Scholar] [CrossRef]
- Ueda, M.; Inaba, R.; Ohtsuka, T. Composition and structure of Al–Sn alloys formed by constant potential electrolysis in an AlCl3–NaCl–KCl–SnCl2 molten salt. Electrochim. Acta 2013, 100, 281–284. [Google Scholar] [CrossRef]
- Lu, Z.C.; Gao, Y.; Zeng, M.Q.; Zhu, M. Improving wear performance of dual-scale Al–Sn alloys: The role of Mg addition in enhancing Sn distribution and tribolayer stability. Wear 2014, 309, 216–225. [Google Scholar] [CrossRef]
- Kong, C.J.; Brown, P.D.; Harris, S.J.; McCartney, D.G. The microstructures of a thermally sprayed and heat treated Al–20 wt.%Sn–3 wt.%Si alloy. Mater. Sci. Eng. A 2005, 403, 205–214. [Google Scholar] [CrossRef]
- Hou, D.; Li, D.; Han, L.; Ji, L. Effect of lanthanum addition on microstructure and corrosion behavior of Al-Sn-Bi anodes. J. Rare Earths 2011, 29, 129–132. [Google Scholar] [CrossRef]
- Mao, Z.G.; Seidman, D.N.; Wolverton, C. First-principles phase stability, magnetic properties and solubility in aluminum–rare-earth (Al–RE) alloys and compounds. Acta Mater. 2011, 59, 3659–3666. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Yang, R.; Peng, J.; Zhao, C.; She, J.; Gao, Z.; Tang, A. Thermal and electrical conductivity of binary magnesium alloys. J. Mater. Sci. 2014, 49, 3107–3124. [Google Scholar] [CrossRef]
- Zuo, X.R.; Jing, Y.W. Investigation of the age hardening behaviour of 6063 aluminium alloys refined with Ti, RE and B. J. Mater. Process. Technol. 2009, 209, 360–366. [Google Scholar] [CrossRef]
- Chen, R.Z.; Wei, X.Z.; Liu, J.Q. The isothermal section of the phase diagram of the ternary system Al-Sn-Y at room temperature. J. Alloy. Compd. 1995, 218, 221–223. [Google Scholar]
- Okamoto, H. Comment on Sn-Y (Tin-Yttrium). J. Phase Equilibria 1995, 16, 104. [Google Scholar] [CrossRef]
- Tang, C.; Hu, B.; Du, Y.; Zhao, D.; Zhou, P.; Zheng, F.; Gao, Q.; Wang, J. Thermodynamic modeling of the Hf–Sn and Sn–Y systems. Calphad 2012, 39, 91–96. [Google Scholar] [CrossRef]
- Mudryk, Y.; Romaka, L.; Stadnyk, Y.; Bodak, O.; Fruchart, D. X-ray investigation of the R–Fe–Sn ternary systems (R–Y, Gd). J. Alloy. Compd. 2004, 383, 162–165. [Google Scholar] [CrossRef]
- Zhan, Y.; He, L.; Ma, J.; Sun, Z.; Zhang, G.; Zhuang, Y. Experimental study of Ti–Sn–Y system phase equilibria at 473 K. J. Alloy. Compd. 2009, 470, 173–175. [Google Scholar] [CrossRef]
- Romaka, L.; Dovgalyuk, Y.; Romaka, V.V.; Lototska, I.; Stadnyk, Y. Interaction of the components in Y–Ni–Sn ternary system at 770 K and 670 K. Intermetallics 2012, 29, 116–122. [Google Scholar] [CrossRef]
- Hao, H.; Dong, W.X.; Shi, Y.W. Mechanism of Tin Whisker Morphology. Chin. J. Nonferrous Met. 2009, 2, 021. [Google Scholar]
- Hao, H.; Shi, Y.W. Tin whisker growth accelerated by the oxidization of RE-phase on lead-free solder surface. Electron. Compon. Mater. 2009, 8, 011. [Google Scholar]
- Bailey, D.M. The Structures of Two Polymorphic Froms of YAl3. Acta Crystallogr. 1967, 23, 729–733. [Google Scholar] [CrossRef]
- Lukas, H.L. System Al-Y, Cost 507. Thermochem. Database Light Met. Alloy. 1998, 99–102. [Google Scholar]
- Richter, R.; Altounian, Z.; Strom-Olsen, J.O.; Köster, U.; Blank-Bewersdorff, M. Y5Al3: A new Y-Al compound. J. Mater. Sci. 1987, 22, 2983–2986. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Xu, H.; He, C.; Schuster, J.C. Experimental investigation of the Al–Y phase diagram. J. Alloy. Compd. 2006, 414, 60–65. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.; Chen, H.; Liang, J.; Tao, X.; Ouyang, Y.; Du, Y. Experimental Investigation on Phase Equilibria of Al-Fe-Y System at 773 K. J. Phase Equilibria Diffus. 2014, 35, 256–261. [Google Scholar] [CrossRef]
- She, J.; Zhan, Y.; Hu, Z.; Li, C.; Hu, J.; Du, Y.; Xu, H. Experimental study of Al–Zr–Y system phase equilibria at 773 K. J. Alloy. Compd. 2010, 497, 118–120. [Google Scholar] [CrossRef]
- Zeng, L.M.; Wang, S.Y. The 800 K isothermal section of the Y–Al–Sb phase diagram. J. Alloy. Compd. 2003, 351, 176–179. [Google Scholar] [CrossRef]
- Liu, X.J.; Wen, M.Z.; Wang, C.P.; Pan, F.S. Thermodynamic assessment of the Zn–Y and Al–Zn–Y systems. J. Alloy. Compd. 2008, 452, 283–290. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Calderwood, F.W. The Al-Y (Aluminum-Yttrium) System. Bull. Alloy Phase Digrams 1989, 10, 44–47. [Google Scholar] [CrossRef]




| Phase | Pearson’s Symbol | Crystal Structure | Space Group | Lattice Parameters (nm) | Refs. | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Sn3Y | oC16 | Gd4Sn11 | Amm2 | 0.4345 | 0.4391 | 2.1937 | [12] |
| Sn5Y2 | oP14 | Ge5Er2 | Pmmm | 0.4322 | 0.4409 | 1.9089 | [12] |
| Sn2Y | oC12 | Si2Zr | Cmcm | 0.4398 | 1.632 | 0.4304 | [12] |
| Sn10Y11 | tI84 | Ge10Ho11 | I4/mmm | 1.154 | – | 1.692 | [12] |
| Sn4Y5 | oP36 | Ge4Sm5 | Pnma | 0.805 | 1.529 | 0.805 | [12] |
| Sn3Y5 | hP16 | Si3Mn5 | P63/mcm | 0.8902 | – | 0.6536 | [12] |
| αYAl3 | hP8 | Ni3Sn | P63/mmc | 0.6276 | – | 0.4582 | [26] |
| YAl2 | cF24 | Cu2Mg | 0.78611 | – | – | [26] | |
| YAl | oC8 | CrB | Cmcm | 0.3884 | 1.1522 | 0.4385 | [26] |
| Y3Al2 | tP20 | Al2Zr3 | P42/mnm | 0.8239 | – | 0.7648 | [26] |
| Y2Al | oP12 | Co2Si | Pnma | 0.6642 | 0.5084 | 0.9469 | [26] |
| Y5Al3 | hP16 | Mn5Si3 | P63/mcm | 0.8787 | – | 0.6435 | [20] |
| Phase Regions | Alloy Composition (at.%) | Phase Composition | ||
|---|---|---|---|---|
| Sn | Al | Y | ||
| 1 | 20 | 9 | 71 | Y + Sn3Y5 + Y2Al |
| 2 | 5 | 30 | 65 | Y2Al + Sn3Y5 + Y5Al3 |
| 3 | 4 | 35 | 61 | Y5Al3 + Sn3Y5 + Y3Al2 |
| 4 | 6.3 | 37 | 56.7 | Y3Al2 + Sn3Y5 + AlY |
| 5 | 6.5 | 47.8 | 45.7 | AlY + Sn3Y5 + Al2Y |
| 6 | 33.5 | 12.5 | 54 | Al2Y + Sn3Y5 + Sn4Y5 |
| 7 | 39 | 10 | 51 | Sn4Y5 + Al2Y + Sn10Y11 |
| 8 | 45 | 15 | 40 | Sn10Y11 + Al2Y + Sn2Y |
| 9 | 6.5 | 64 | 29.5 | Sn2Y + Al2Y + α–Al3Y |
| 10 | 20 | 59 | 21 | α–Al3Y + Sn2Y + Al |
| 11 | 60.5 | 12.5 | 27 | Al + Sn2Y + Sn5Y2 |
| 12 | 64.5 | 12.5 | 23 | Sn5Y2 + Al + Sn3Y |
| 13 | 69.8 | 14.9 | 15.3 | Sn3Y + Al + Sn |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Liu, M.; Feng, J.; Wu, J.; Mao, J.; Du, Z.; Ke, X.; Zhang, X.; Zhan, Y. Solid State Phase Equilibria of an Al–Sn–Y Ternary System. Materials 2019, 12, 444. https://doi.org/10.3390/ma12030444
Yang W, Liu M, Feng J, Wu J, Mao J, Du Z, Ke X, Zhang X, Zhan Y. Solid State Phase Equilibria of an Al–Sn–Y Ternary System. Materials. 2019; 12(3):444. https://doi.org/10.3390/ma12030444
Chicago/Turabian StyleYang, Wenchao, Moumiao Liu, Junli Feng, Jingwu Wu, Jun Mao, Zaixiang Du, Xiaojun Ke, Xinjiang Zhang, and Yongzhong Zhan. 2019. "Solid State Phase Equilibria of an Al–Sn–Y Ternary System" Materials 12, no. 3: 444. https://doi.org/10.3390/ma12030444




