Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System
Abstract
:1. Introduction
2. Methods
- Iron oxide AND magnetic AND nanoparticles AND synthesis AND central nervous system
- OR
- Iron oxide AND nanoparticles AND magnetization AND properties AND central nervous system
- OR
- Iron oxide AND nanoparticles AND functionalization AND central nervous system
- OR
- Iron oxide AND nanoparticles AND toxicity AND central nervous system.
3. Results
4. Discussion
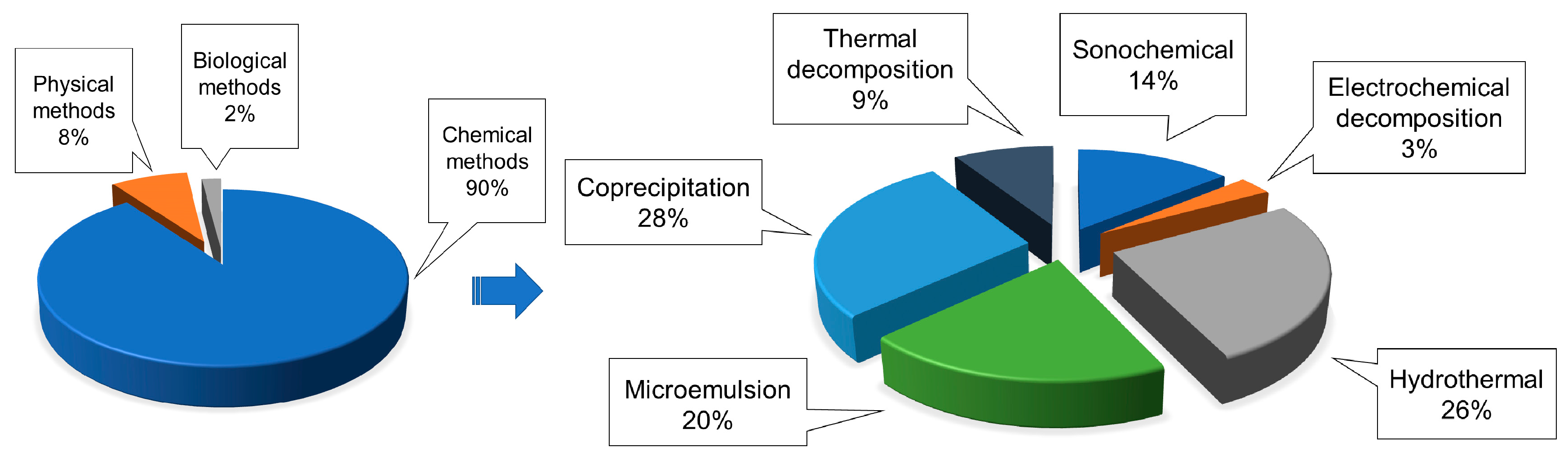
4.1. Synthesis of IONPs
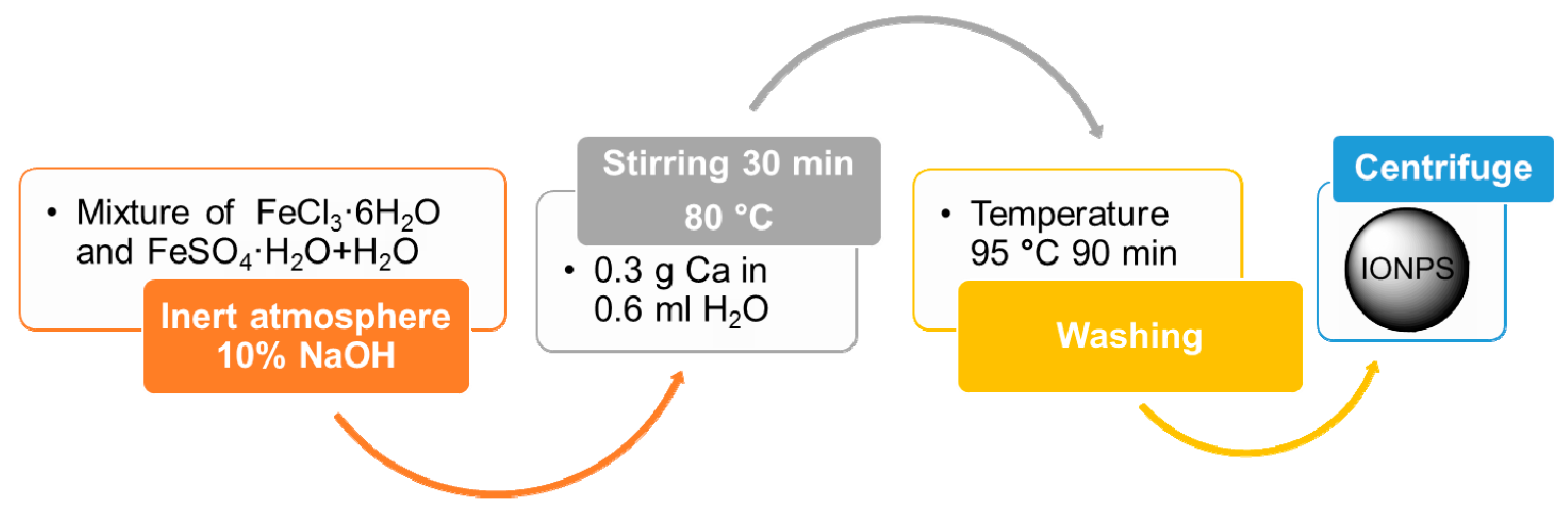
4.1.1. Co-Precipitation Method
4.1.2. Thermal Decomposition Method
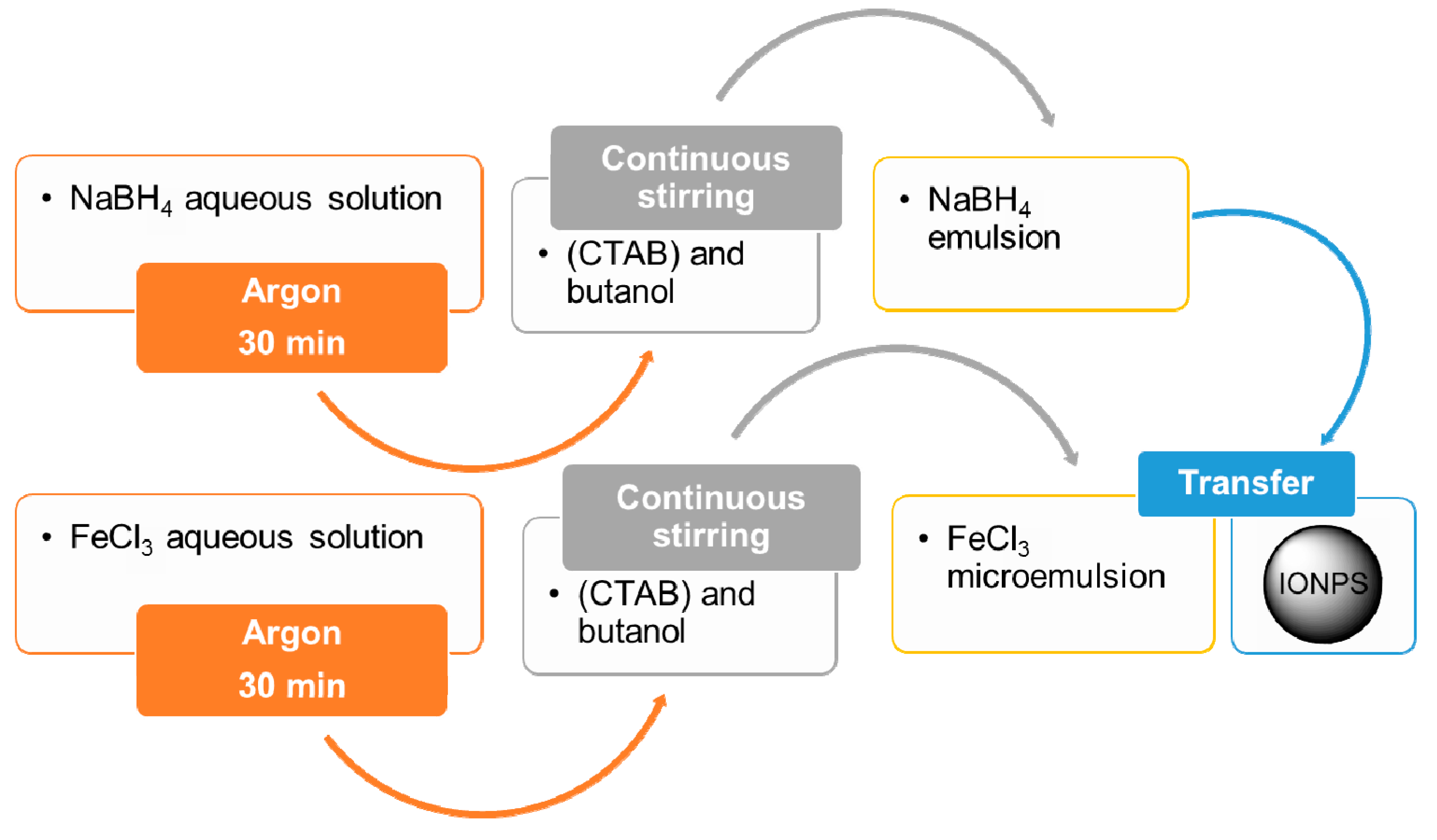
4.1.3. Microemulsion Method
4.1.4. Hydrothermal Method
4.1.5. Polyol Method
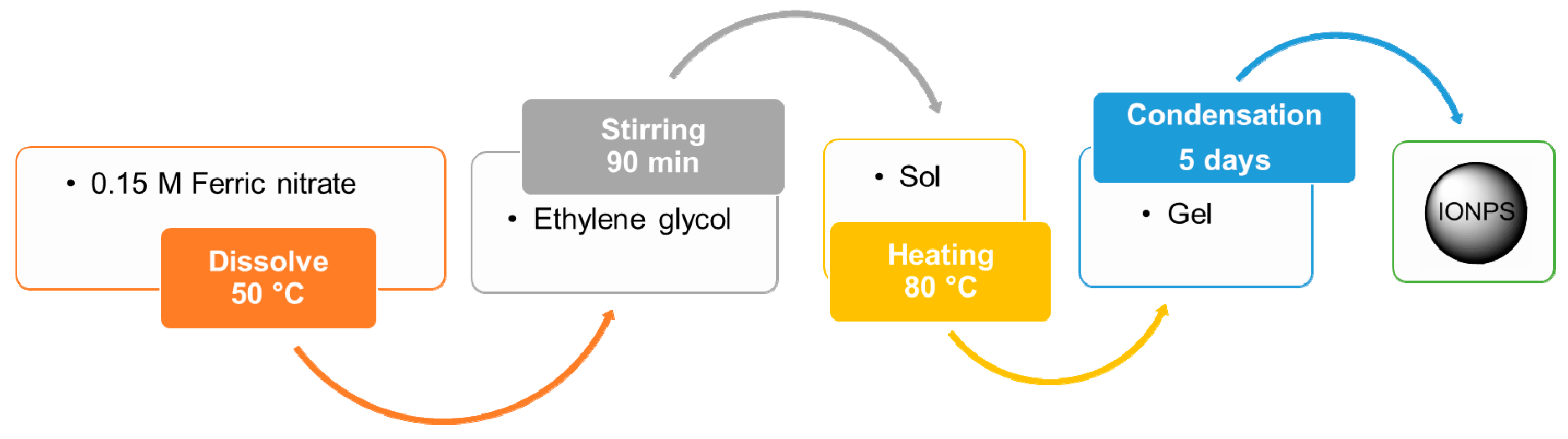
4.1.6. Sol–Gel Method
4.1.7. Biomineralization
4.1.8. Sputter Deposition
4.1.9. Application to CNS
4.2. Characterization of IONPs
4.2.1. Microscopic Techniques
4.2.2. Spectroscopic Techniques
4.2.3. Magnetometric Techniques
4.2.4. Magnetic Properties of IONPs
4.2.5. Application to CNS
4.3. Functionalization of IONPs
- To improve or modify dispersion in tissues;
- To improve the surface activity;
- To enhance physicochemical and mechanical properties;
- To improve biocompatibility.
4.3.1. Ligand Addition
4.3.2. Ligand Exchange
4.3.3. Silica Coating
4.3.4. Aminosilane Coating
4.3.5. Polymer Coating
4.3.6. Application to CNS
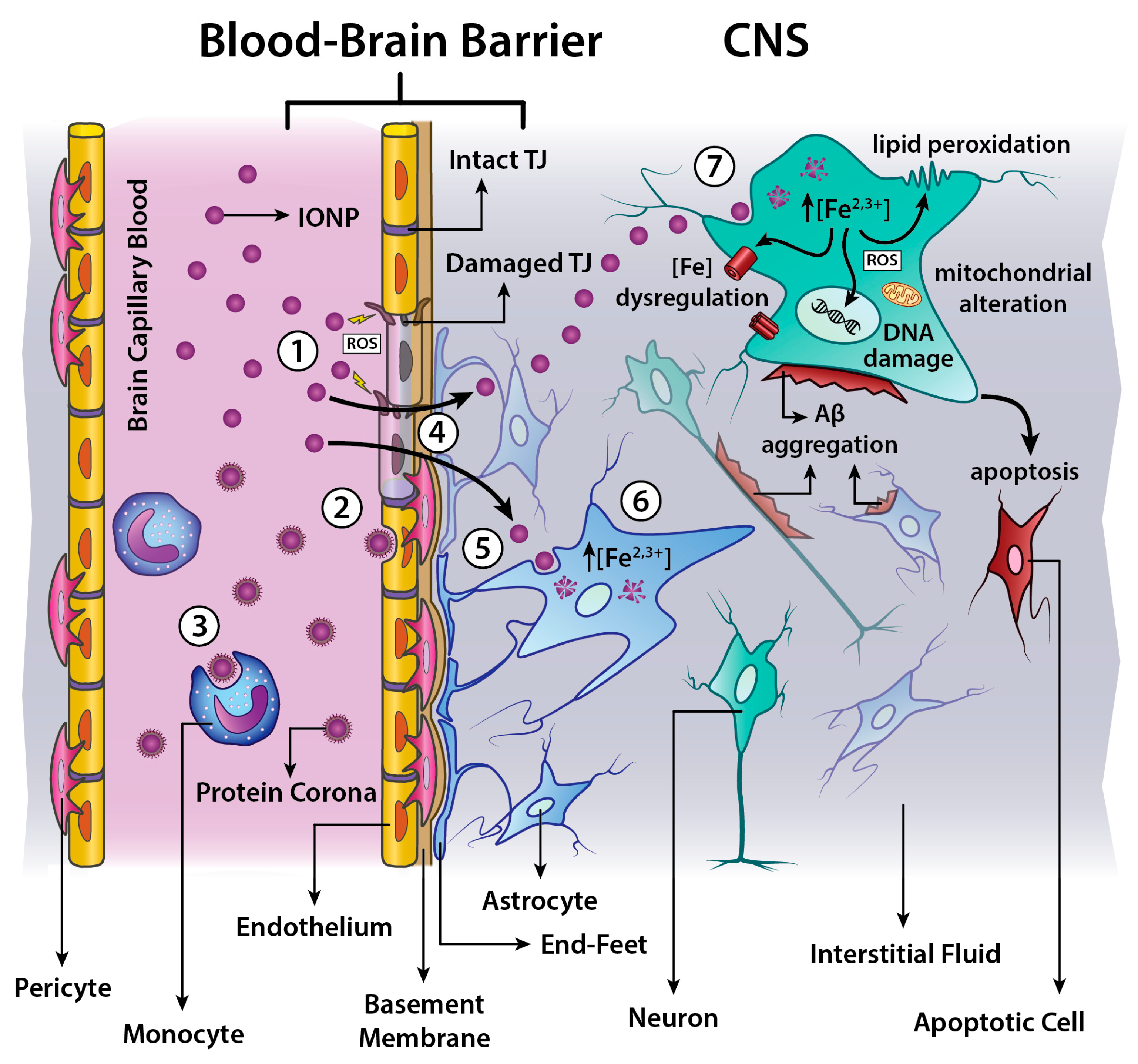
4.4. Toxicity in CNS
- dose and time-dependent cell viability and/or proliferation;
- production of Reactive Oxygen Species (ROS);
- cell membrane disruptions;
- alteration of mitochondrial activity;
- genotoxicity induction.
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | 2-Dimension |
| 3D | 3-Dimension |
| AmS | AminoSilane |
| Aβ | Amyloid-beta |
| BBB | Blood-Brain-Barrier |
| CNS | Central Nervous System |
| CTAB | cetyltrimethylammonium bromide |
| DLS | Dynamic Light Scattering |
| DNA | DeoxyriboNucleic Acid |
| FTIR | Fourier-Transform Infrared |
| IONP | Iron Oxides Nanoparticle |
| IR | Infrared |
| MNP | Magnetic Nanoparticle |
| MR | Magnetic Resonance |
| MRI | Magnetic Resonance Imaging |
| NMR | Nuclear Magnetic Resonance |
| NP | Nanoparticles |
| PET | Positron Emission Tomography |
| PIONP | Polymer-coated Iron Oxides Nanoparticle |
| ROS | Reactive Oxygen Species |
| SEM | Scanning Electron Microscopy |
| SPIONs | Superparamagnetic Iron Oxides Nanoparticles |
| SQUID | Superconducting Quantum Interference Device |
| TEM | Transmission Electron Microscopy |
| USPION | Ultra-Small Iron Oxides Nanoparticle |
| VSM | Vibrating Sample Magnetometer |
| W/O | Water-in-Oil |
| XRD | X-Ray Diffraction |
References
- McNamara, K.; Tofail, S.A.M. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Khanna, L.; Verma, N.K.; Tripathi, S.K. Burgeoning tool of biomedical applications—Superparamagnetic nanoparticles. J. Alloys Compd. 2018, 752, 332–353. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Neurological Disorders: A Public Health Approach; World Health Organization: Geneva, Switzerland, 2006; ISBN 9241563362. [Google Scholar]
- D’Agata, F.; Ruffinatti, F.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules 2017, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C.; Velasco-Aguirre, C.; Gallardo-Toledo, E.; Araya, E.; Kogan, M.J. Metal Nanoparticles as Targeted Carriers Circumventing the Blood-Brain Barrier. Int. Rev. Neurobiol. 2016, 130, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Menon, P.K.; Lafuente, J.V.; Aguilar, Z.P.; Wang, Y.A.; Muresanu, D.F.; Mössler, H.; Patnaik, R.; Sharma, A. The role of functionalized magnetic iron oxide nanoparticles in the central nervous system injury and repair: New potentials for neuroprotection with Cerebrolysin therapy. J. Nanosci. Nanotechnol. 2014, 14, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health Concerns of Various Nanoparticles: A Review of Their in Vitro and in Vivo Toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Khan, F.A.; Almohazey, D.; Alomari, M.; Almofty, S.A. Impact of nanoparticles on neuron biology: Current research trends. Int. J. Nanomed. 2018, 13, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Meera, V. Synthesis of Iron Oxide Nanoparticles Coated Sand by Biological Method and Chemical Method. Procedia Technol. 2016, 24, 210–216. [Google Scholar] [CrossRef]
- Osial, M.; Rybicka, P.; Pękała, M.; Cichowicz, G.; Cyrański, M.; Krysiński, P. Easy Synthesis and Characterization of Holmium-Doped SPIONs. Nanomaterials 2018, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single step synthesis, characterization and applications of curcumin functionalized iron oxide magnetic nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Biehl, P.; von der Lühe, M.; Dutz, S.; Schacher, F. Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef]
- Fatima, H.; Kim, K.S. Iron-based magnetic nanoparticles for magnetic resonance imaging. Adv. Powder Technol. 2018, 29, 2678–2685. [Google Scholar] [CrossRef]
- Gruskiene, R.; Krivorotova, T.; Staneviciene, R.; Ratautas, D.; Serviene, E.; Sereikaite, J. Preparation and characterization of iron oxide magnetic nanoparticles functionalized by nisin. Colloids Surf. B Biointerfaces 2018, 169, 126–134. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Belaïd, S.; Stanicki, D.; Vander Elst, L.; Muller, R.N.; Laurent, S. Influence of experimental parameters on iron oxide nanoparticle properties synthesized by thermal decomposition: Size and nuclear magnetic resonance studies. Nanotechnology 2018, 29, 165603. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous synthesis of iron oxide (Fe3O4) nanoparticles via thermal decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Effenberger, F.B.; Couto, R.A.; Kiyohara, P.K.; Machado, G.; Masunaga, S.H.; Jardim, R.F.; Rossi, L.M. Economically attractive route for the preparation of high quality magnetic nanoparticles by the thermal decomposition of iron(III) acetylacetonate. Nanotechnology 2017, 28, 115603. [Google Scholar] [CrossRef] [PubMed]
- Jović Orsini, N.; Babić-Stojić, B.; Spasojević, V.; Calatayud, M.P.; Cvjetićanin, N.; Goya, G.F. Magnetic and power absorption measurements on iron oxide nanoparticles synthesized by thermal decomposition of Fe(acac)3. J. Magn. Magn. Mater. 2018, 449, 286–296. [Google Scholar] [CrossRef]
- Grüttner, C.; Müller, K.; Teller, J.; Westphal, F. Synthesis and functionalisation of magnetic nanoparticles for hyperthermia applications. Int. J. Hyperthermia 2013, 29, 777–789. [Google Scholar] [CrossRef]
- Kekalo, K.; Koo, K.; Zeitchick, E.; Baker, I. Microemulsion synthesis of iron core/iron oxide shell magnetic nanoparticles and their physicochemical properties. Mater. Res. Soc. Symp. Proc. 2012, 1416, 61–66. [Google Scholar] [CrossRef]
- López, R.G.; Pineda, M.G.; Hurtado, G.; de León, R.D.; Fernández, S.; Saade, H.; Bueno, D. Chitosan-coated magnetic nanoparticles prepared in one step by reverse microemulsion precipitation. Int. J. Mol. Sci. 2013, 14, 19636–19650. [Google Scholar] [CrossRef]
- Ban, I.; Stergar, J.; Drofenik, M.; Ferk, G.; Makovec, D. Synthesis of chromium-Nickel nanoparticles prepared by a microemulsion method and mechanical milling. Acta Chim. Slov. 2013, 60, 750–755. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.X.; Yan, X.P. Hydrothermal and biomineralization synthesis of a dual-modal nanoprobe for targeted near-infrared persistent luminescence and magnetic resonance imaging. Nanoscale 2017, 9, 9049–9055. [Google Scholar] [CrossRef] [PubMed]
- Nejati, K.; Zabihi, R. Preparation and magnetic properties of nano size nickel ferrite particles using hydrothermal method. Chem. Cent. J. 2012, 6, 394. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A. Synthesis, photoluminescence and Magnetic properties of iron oxide (α-Fe2O3) nanoparticles through precipitation or hydrothermal methods. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 101, 212–219. [Google Scholar] [CrossRef]
- Gyergyek, S.; Makovec, D.; Jagodič, M.; Drofenik, M.; Schenk, K.; Jordan, O.; Kovač, J.; Dražič, G.; Hofmann, H. Hydrothermal growth of iron oxide NPs with a uniform size distribution for magnetically induced hyperthermia: Structural, colloidal and magnetic properties. J. Alloys Compd. 2017, 694, 261–271. [Google Scholar] [CrossRef]
- Wegmann, M.; Scharr, M. Chapter 8—Synthesis of Magnetic Iron Oxide Nanoparticles; Elsevier Inc.: New York, NY, USA, 2018; ISBN 978-0-12-805364-5. [Google Scholar]
- Park, J.-H.; Shin, S.-H.; Kim, S.-H.; Park, J.-K.; Lee, J.-W.; Shin, J.-H.; Park, J.-H.; Kim, S.-W.; Choi, H.-J.; Lee, K.-S.; et al. Effect of Synthesis Time and Composition on Magnetic Properties of FeCo Nanoparticles by Polyol Method. J. Nanosci. Nanotechnol. 2018, 18, 7115–7119. [Google Scholar] [CrossRef] [PubMed]
- Hemery, G.; Keyes, A.C.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; Plazaola, F.; Garanger, E.; Sandre, O. Tuning Sizes, Morphologies, and Magnetic Properties of Monocore Versus Multicore Iron Oxide Nanoparticles through the Controlled Addition of Water in the Polyol Synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef] [PubMed]
- Hasany, S.F.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic Review of the Preparation Techniques of Iron Oxide Magnetic Nanoparticles. Nanosci. Nanotechnol. 2013, 2, 148–158. [Google Scholar] [CrossRef]
- Riaz, S.; Shah, S.Z.H.; Kayani, Z.N.; Naseem, S. Magnetic and Structural Phase Transition in Iron Oxide Nanostructures; Elsevier Ltd.: New York, NY, USA, 2015; Volume 2. [Google Scholar]
- Raja, K.; Mary Jaculine, M.; Jose, M.; Verma, S.; Prince, A.A.M.; Ilangovan, K.; Sethusankar, K.; Jerome Das, S. Sol-gel synthesis and characterization of α-Fe2O3 nanoparticles. Superlattices Microstruct. 2015, 86, 306–312. [Google Scholar] [CrossRef]
- Mathevula, L.E.; Noto, L.L.; Mothudi, B.M.; Chithambo, M.; Dhlamini, M.S. Structural and optical properties of sol-gel derived α-Fe2O3 nanoparticles. J. Lumin. 2017, 192, 879–887. [Google Scholar] [CrossRef]
- Kotelnikova, P.A.; Shipunova, V.O.; Aghayeva, U.F.; Stremovskiy, O.A.; Nikitin, M.P.; Novikov, I.A.; Schulga, A.A.; Deyev, S.M.; Petrov, R.V. Synthesis of Magnetic Nanoparticles Stabilized by Magnetite-Binding Protein for Targeted Delivery to Cancer Cells. Dokl. Biochem. Biophys. 2018, 481, 198–200. [Google Scholar] [CrossRef]
- Gorobets, O.; Gorobets, S.; Koralewski, M. Physiological origin of biogenic magnetic nanoparticles in health and disease: From bacteria to humans. Int. J. Nanomed. 2017, 12, 4371–4395. [Google Scholar] [CrossRef] [PubMed]
- Khanal, L.R.; Williams, T.; Qiang, Y. High-temperature investigation on morphology, phase and size of iron/iron-oxide core–shell nanoclusters for radiation nanodetector. J. Phys. D Appl. Phys. 2018, 51, 255302. [Google Scholar] [CrossRef]
- Xing, L.; Ten Brink, G.H.; Chen, B.; Schmidt, F.P.; Haberfehlner, G.; Hofer, F.; Kooi, B.J.; Palasantzas, G. Synthesis and morphology of iron-iron oxide core-shell nanoparticles produced by high pressure gas condensation. Nanotechnology 2016, 27, 215703. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ten Brink, G.H.; Kooi, B.J.; Palasantzas, G. Preparation of tunable-sized iron nanoparticles based on magnetic manipulation in inert gas condensation (IGC). J. Appl. Phys. 2017, 121, 24305. [Google Scholar] [CrossRef]
- Sundararajan, J.A.; Kaur, M.; Jiang, W.; McCloy, J.S.; Qiang, Y. Oxide shell reduction and magnetic property changes in core-shell Fe nanoclusters under ion irradiation. J. Appl. Phys. 2014, 115, 17B507. [Google Scholar] [CrossRef]
- Vernieres, J.; Steinhauer, S.; Zhao, J.; Chapelle, A.; Menini, P.; Dufour, N.; Diaz, R.E.; Nordlund, K.; Djurabekova, F.; Grammatikopoulos, P.; et al. Gas Phase Synthesis of Multifunctional Fe-Based Nanocubes. Adv. Funct. Mater. 2017, 27, 1605328. [Google Scholar] [CrossRef]
- Jiang, W.; McCloy, J.S.; Lea, A.S.; Sundararajan, J.A.; Yao, Q.; Qiang, Y. Magnetization and susceptibility of ion-irradiated granular magnetite films. Phys. Rev. B 2011, 83, 134435. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef]
- Jiang, F.; Li, X.; Zhu, Y.; Tang, Z. Synthesis and magnetic characterizations of uniform iron oxide nanoparticles. Phys. B Condens. Matter 2014, 443, 1–5. [Google Scholar] [CrossRef]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Järås, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and functionalization study of microemulsion-prepared magnetic iron oxide nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef]
- Zhou, J.-P.; Lv, L.; Liu, Q.; Zhang, Y.-X.; Liu, P. Hydrothermal synthesis and properties of NiFe2O4@BaTiO3 composites with well-matched interface. Sci. Technol. Adv. Mater. 2012, 13, 45001. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Jasso-Terán, R.A.; Zugasti-Cruz, A. Bioactive magnetic nanoparticles of Fe–Ga synthesized by sol–gel for their potential use in hyperthermia treatment. J. Mater. Sci. Mater. Med. 2014, 25, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Majid, F.; Mirza, S.T.; Riaz, S.; Naseem, S. Sol-Gel Synthesis of BiFeO3Nanoparticles. Mater. Today Proc. 2015, 2, 5293–5297. [Google Scholar] [CrossRef]
- Hurley, K.R.; Ring, H.L.; Kang, H.; Klein, N.D.; Haynes, C.L. Characterization of Magnetic Nanoparticles in Biological Matrices. Anal. Chem. 2015, 87, 11611–11619. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.V.; Mini, R.; Jothy, V.B.; Joe, I.H. Synthesis and Characterization of Iron Oxide Nanoparticles using DMSO as a Stabilizer. Mater. Today Proc. 2015, 2, 1051–1055. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Bagheri, Z.; Rashidzadeh, H.; Davaran, S.; Danafar, H. Preparation, characterization, and evaluation of amino acid modified magnetic nanoparticles: Drug delivery and MRI contrast agent applications. Pharm. Dev. Technol. 2018, 1–12. [Google Scholar] [CrossRef]
- Babić-Stojić, B.; Jokanović, V.; Milivojević, D.; Požek, M.; Jagličić, Z.; Makovec, D.; Orsini, N.J.; Marković, M.; Arsikin, K.; Paunović, V. Ultrasmall iron oxide nanoparticles: Magnetic and NMR relaxometric properties. Curr. Appl. Phys. 2018, 18, 141–149. [Google Scholar] [CrossRef]
- Das, B.; Kusz, J.; Reddy, V.R.; Zubko, M.; Bhattacharjee, A. Solventless synthesis, morphology, structure and magnetic properties of iron oxide nanoparticles. Solid State Sci. 2017, 74, 62–69. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Brollo, M.E.F.; Veintemillas-Verdaguer, S.; del Puerto Morales, M. Design Strategies for Shape-Controlled Magnetic Iron Oxide Nanoparticles. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface charge and dosage dependent potential developmental toxicity and biodistribution of iron oxide nanoparticles in pregnant CD-1 mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tarantash, M.; Nosrati, H.; Kheiri Manjili, H.; Baradar Khoshfetrat, A. Preparation, characterization and in vitro anticancer activity of paclitaxel conjugated magnetic nanoparticles. Drug Dev. Ind. Pharm. 2018, 44, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.R.; Kashefi, M.; Shams, S.F.; Jaafari, M.R. Perspective of Fe3O4 Nanoparticles Role in Biomedical Applications. Biochem. Res. Int. 2016, 2016, 7840161. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y.P.; Jin, Y.; et al. Appropriate Size of Magnetic Nanoparticles for Various Bioapplications in Cancer Diagnostics and Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, D.; Cai, C.; Chen, X.; Zhou, Y.; Wu, L.; Sun, Y.; Dai, H.; Kong, X.; Liu, P. Size-dependent cytotoxicity of Fe3O4 nanoparticles induced by biphasic regulation of oxidative stress in different human hepatoma cells. Int. J. Nanomed. 2016, 11, 3557–3570. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Sun, X.; Sun, S. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Han, S.; Li, C.; Lei, B.; Lu, W.; Fang, J.; Zhou, C. Single crystalline magnetite nanotubes. J. Am. Chem. Soc. 2005, 127, 6–7. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, T.; Wu, T.; Li, Y.; Tong, G. Excellent microwave-absorbing properties of elliptical Fe3O4 nanorings made by a rapid microwave-assisted hydrothermal approach. Nanotechnology 2016, 27, 165707. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rizvi, S.M.D.; Ahmad, V.; Baig, M.H.; Kamal, M.A.; Ahmad, S.; Rai, M.; Zafar Iqbal, A.N.M.; Mushtaq, G.; Khan, M.S. Magnetic Nanoparticles: Properties, Synthesis and Biomedical Applications. Curr. Drug Metab. 2015, 16, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Hubert, A.; Schäfer, R. Magnetic Domains; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-64108-7. [Google Scholar]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Diaz-Cano, A.I.; Guillen-Cervantes, A.; Santoyo-Salazar, J. Magnetic domain interactions of Fe3O4 nanoparticles embedded in a SiO2 matrix. Sci. Rep. 2018, 8, 5096. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Nikolaev, B.; Marchenko, Y.; Yakovleva, L.; Skvortsov, N.; Mazur, A.; Tolstoy, P.; Ryzhov, V.; Multhoff, G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs). Int. J. Nanomed. 2018, 13, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Kandibanda, S.R.; Gundeboina, N.; Das, S.; Sunkara, V.M. Synthesis, characterisation, cellular uptake and cytotoxicity of functionalised magnetic ruthenium (II) polypyridine complex core-shell nanocomposite. J. Photochem. Photobiol. B 2018, 178, 270–276. [Google Scholar] [CrossRef]
- Toropova, Y.G.; Golovkin, A.S.; Malashicheva, A.B.; Korolev, D.V.; Gorshkov, A.N.; Gareev, K.G.; Afonin, M.V.; Galagudza, M.M. In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int. J. Nanomedicine 2017, 12, 593–603. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef]
- Sharma, K.S.; Ningthoujam, R.S.; Dubey, A.K.; Chattopadhyay, A.; Phapale, S.; Juluri, R.R.; Mukherjee, S.; Tewari, R.; Shetake, N.G.; Pandey, B.N.; et al. Synthesis and characterization of monodispersed water dispersible Fe3O4 nanoparticles and in vitro studies on human breast carcinoma cell line under hyperthermia condition. Sci. Rep. 2018, 8, 14766. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xu, K.; Gu, J.; Huang, L.; Zhang, L.; Liu, N.; Kong, J.; Xing, M.; Zhang, L.; et al. Characterization of superparamagnetic iron oxide nanoparticle-induced apoptosis in PC12 cells and mouse hippocampus and striatum. Toxicol. Lett. 2018, 292, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Park, I.K.; Cho, C.S. Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol. Adv. 2013, 31, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Zullino, S.; Soster, M.; Khadjavi, A.; Gabriele, D.; Ciprian, R.; Albertini, F.; Cavalli, R.; Guiot, C. Characterization of superparamagnetic, oxygen loaded NanoBubbles for hyperthermia and radiotherapy. Radiother. Oncol. 2015, 115, S576. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Davis, K.; Vidmar, M.; Khasanov, A.; Cole, B.; Ghelardini, M.; Mayer, J.; Kitchens, C.; Nath, A.; Powell, B.A.; Mefford, O.T. The effect of post-synthesis aging on the ligand exchange activity of iron oxide nanoparticles. J. Colloid Interface Sci. 2018, 511, 374–382. [Google Scholar] [CrossRef]
- Wierzbinski, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 3682. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Li, Q.; Tang, G.; Xue, S.; He, X.; Miao, P.; Li, Y.; Wang, J.; Xiong, L.; Wang, Y.; Zhang, C.; et al. Silica-coated superparamagnetic iron oxide nanoparticles targeting of EPCs in ischemic brain injury. Biomaterials 2013, 34, 4982–4992. [Google Scholar] [CrossRef]
- Sun, S.; Wei, C.; Zhu, Z.; Hou, Y.; Venkatraman, S.S.; Xu, Z. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 37503. [Google Scholar] [CrossRef]
- Alwi, R.; Telenkov, S.; Mandelis, A.; Leshuk, T.; Gu, F.; Oladepo, S.; Michaelian, K. Silica-coated super paramagnetic iron oxide nanoparticles (SPION) as biocompatible contrast agent in biomedical photoacoustics. Biomed. Opt. Express 2012, 3, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Rivet, C.J.; Yuan, Y.; Borca-Tasciuc, D.-A.; Gilbert, R.J. Altering iron oxide nanoparticle surface properties induce cortical neuron cytotoxicity. Chem. Res. Toxicol. 2012, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yathindranath, V.; Worden, M.; Thliveris, J.A.; Chu, S.; Parkinson, F.E.; Hegmann, T.; Miller, D.W. Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int. J. Nanomed. 2013, 8, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Corem-Salkmon, E.; Perlstein, B.; Margel, S. Design of near-infrared fluorescent bioactive conjugated functional iron oxide nanoparticles for optical detection of colon cancer. Int. J. Nanomed. 2012, 7, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kievit, F.M.; Veiseh, O.; Arami, H.; Stephen, Z.R.; Fang, C.; Liu, Y.; Ellenbogen, R.G.; Zhang, M. Targeted cell uptake of a noninternalizing antibody through conjugation to iron oxide nanoparticles in primary central nervous system lymphoma. World Neurosurg. 2013, 80, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Skoczeń, A.; Matusiak, K.; Setkowicz, Z.; Kubala-Kukuś, A.; Stabrawa, I.; Ciarach, M.; Janeczko, K.; Chwiej, J. Low Doses of Polyethylene Glycol Coated Iron Oxide Nanoparticles Cause Significant Elemental Changes within Main Organs. Chem. Res. Toxicol. 2018, 31, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Mi, G.; Bhattacharya, S.; Nayar, S.; Webster, T. Optimizing superparamagnetic iron oxide nanoparticles as drug carriers using an in vitro blood—Brain barrier model. Int. J. Nanomed. 2016, 11, 5371–5379. [Google Scholar] [CrossRef]
- Ivask, A.; Pilkington, E.H.; Blin, T.; Käkinen, A.; Vija, H.; Visnapuu, M.; Quinn, J.F.; Whittaker, M.R.; Qiao, R.; Davis, T.P.; et al. Uptake and transcytosis of functionalized superparamagnetic iron oxide nanoparticles in an in vitro blood brain barrier model. Biomater. Sci. 2018, 6, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Fernández-Bertólez, N.; Kiliç, G.; Costa, C.; Costa, S.; Fraga, S.; Bessa, M.J.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016, 38, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef]
- Imam, S.Z.; Lantz-McPeak, S.M.; Cuevas, E.; Rosas-Hernandez, H.; Liachenko, S.; Zhang, Y.; Sarkar, S.; Ramu, J.; Robinson, B.L.; Jones, Y.; et al. Iron Oxide Nanoparticles Induce Dopaminergic Damage: In vitro Pathways and In Vivo Imaging Reveals Mechanism of Neuronal Damage. Mol. Neurobiol. 2015, 52, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E.; et al. In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells. Evaluation of nanoparticle interference with viability tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and Metabolism of Iron Oxide Nanoparticles in Brain Cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef]
- Coccini, T.; Caloni, F.; Ramírez Cando, L.J.; De Simone, U. Cytotoxicity and proliferative capacity impairment induced on human brain cell cultures after short- and long-term exposure to magnetite nanoparticles. J. Appl. Toxicol. 2017, 37, 361–373. [Google Scholar] [CrossRef]
- Khalid, M.K.; Asad, M.; Henrich-Noack, P.; Sokolov, M.; Hintz, W.; Grigartzik, L.; Zhang, E.; Dityatev, A.; van Wachem, B.; Sabel, B.A. Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2613. [Google Scholar] [CrossRef]
- De Simone, U.; Roccio, M.; Gribaldo, L.; Spinillo, A.; Caloni, F.; Coccini, T. Human 3D Cultures as Models for Evaluating Magnetic Nanoparticle CNS Cytotoxicity after Short- and Repeated Long-Term Exposure. Int. J. Mol. Sci. 2018, 19, 1993. [Google Scholar] [CrossRef]
- Theumer, A.; Gräfe, C.; Bähring, F.; Bergemann, C.; Hochhaus, A.; Clement, J.H. Superparamagnetic iron oxide nanoparticles exert different cytotoxic effects on cells grown in monolayer cell culture versus as multicellular spheroids. J. Magn. Magn. Mater. 2015, 380, 27–33. [Google Scholar] [CrossRef]
- Pellen-Mussi, P.; Tricot-Doleux, S.; Neaime, C.; Nerambourg, N.; Cabello-Hurtado, F.; Cordier, S.; Grasset, F.; Jeanne, S. Evaluation of Functional SiO2 Nanoparticles Toxicity by a 3D Culture Model. J. Nanosci. Nanotechnol. 2018, 18, 3148–3157. [Google Scholar] [CrossRef] [PubMed]

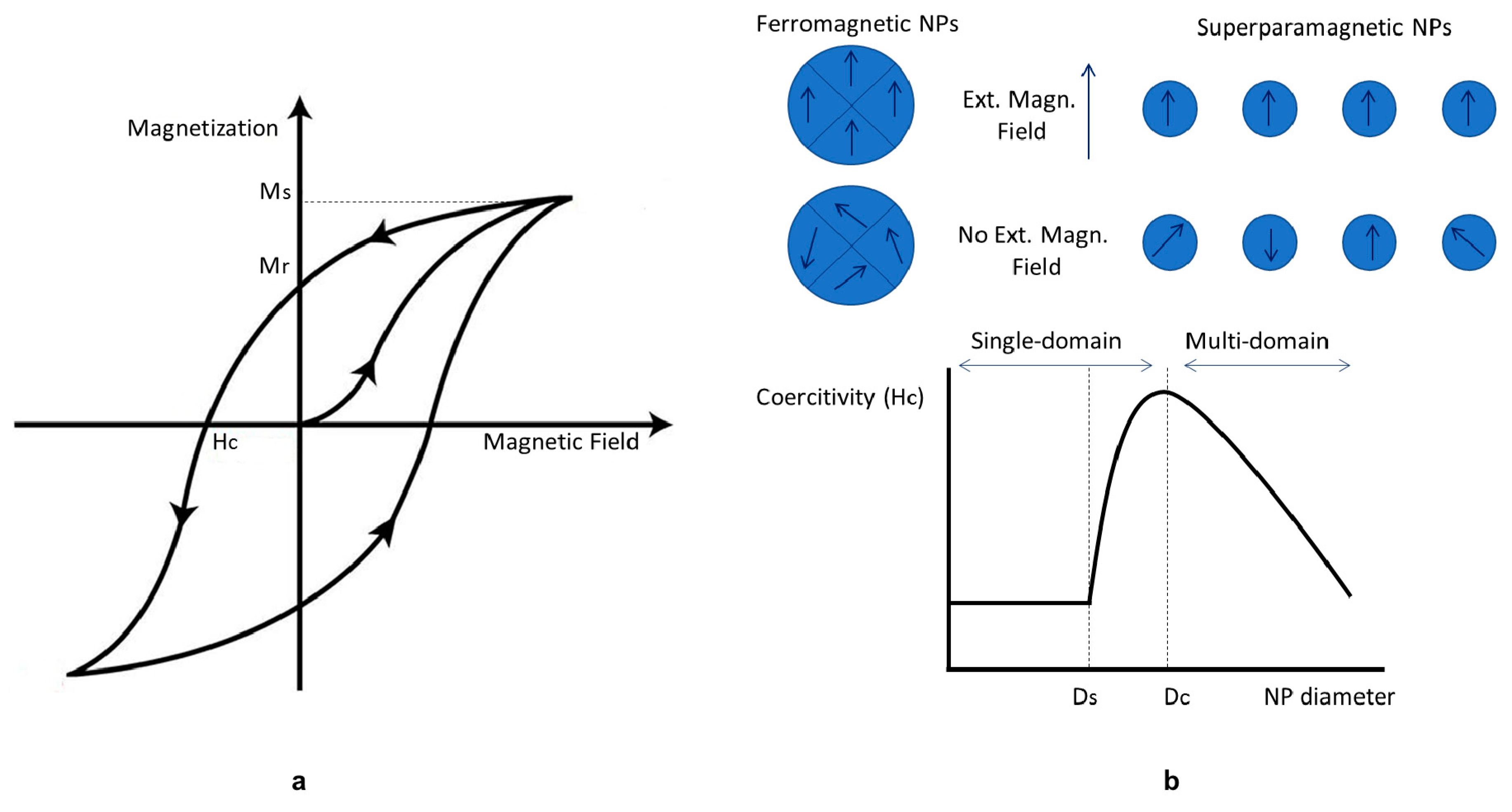
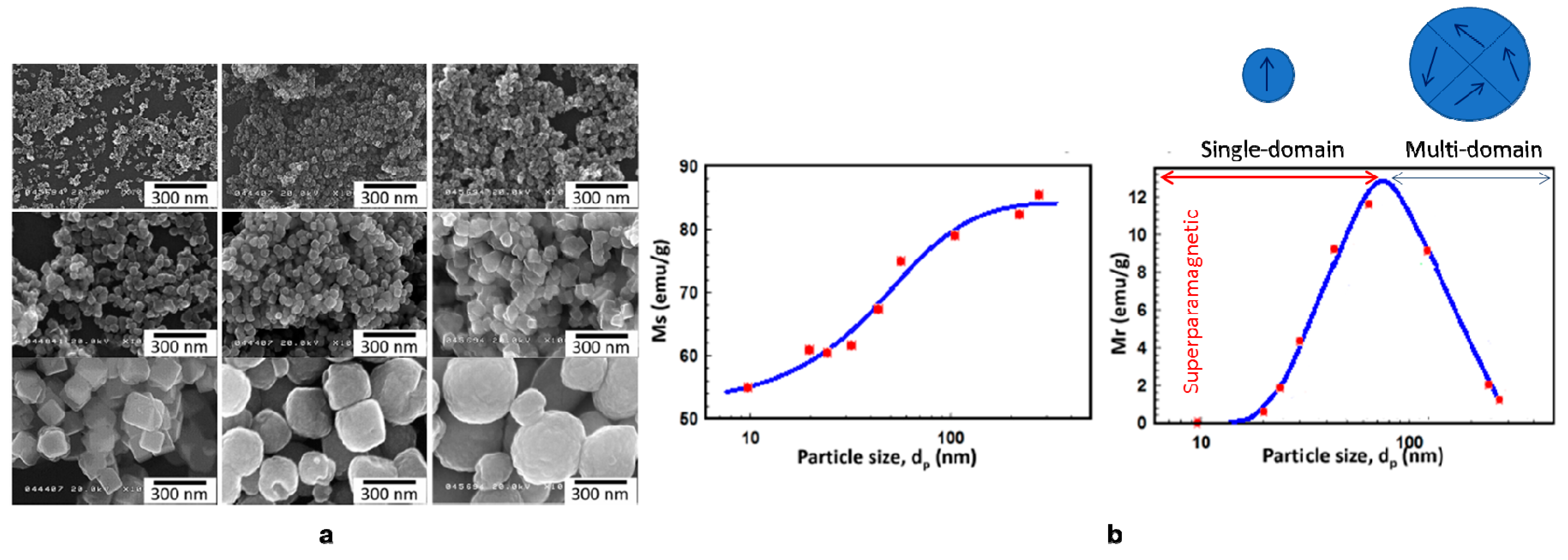
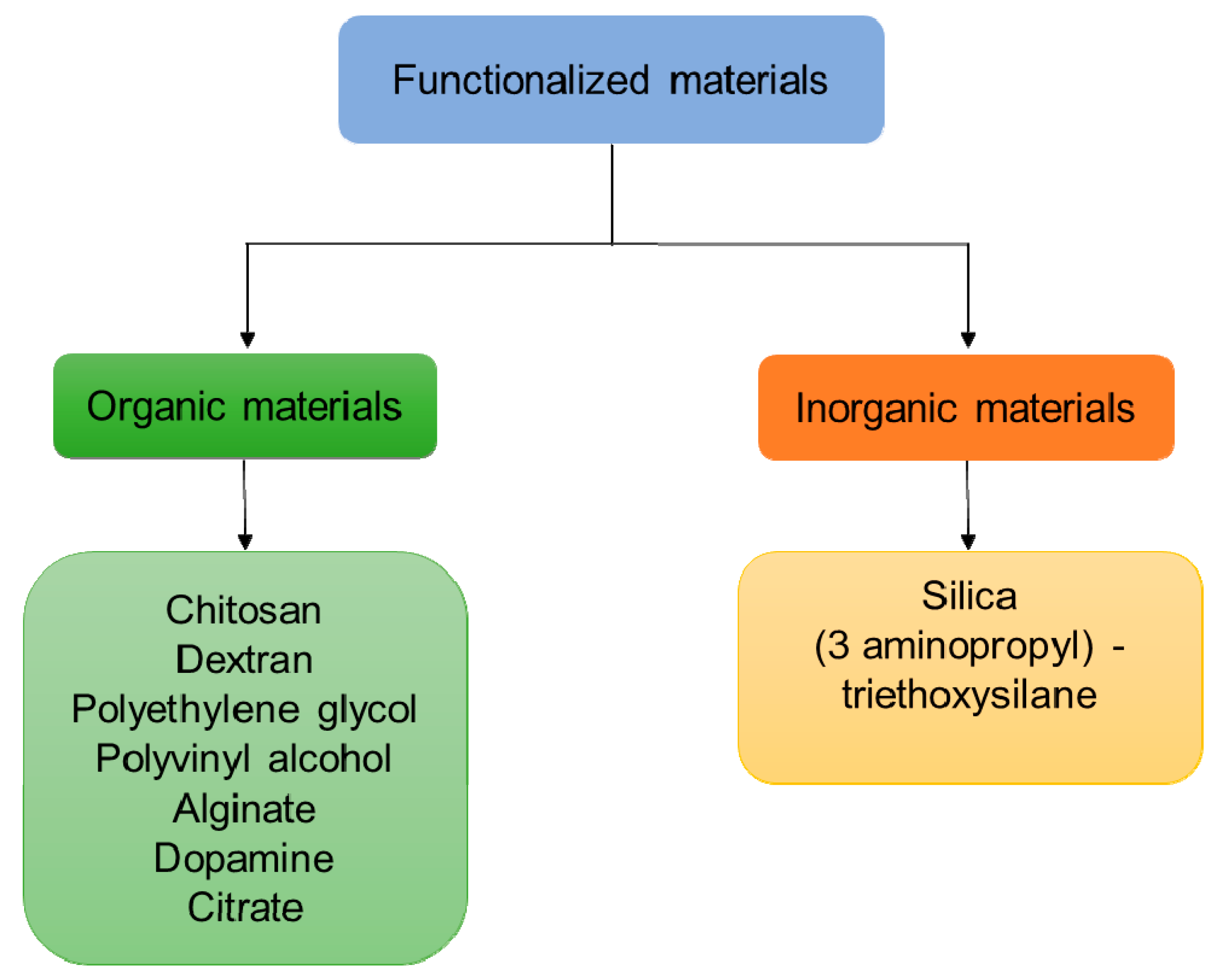
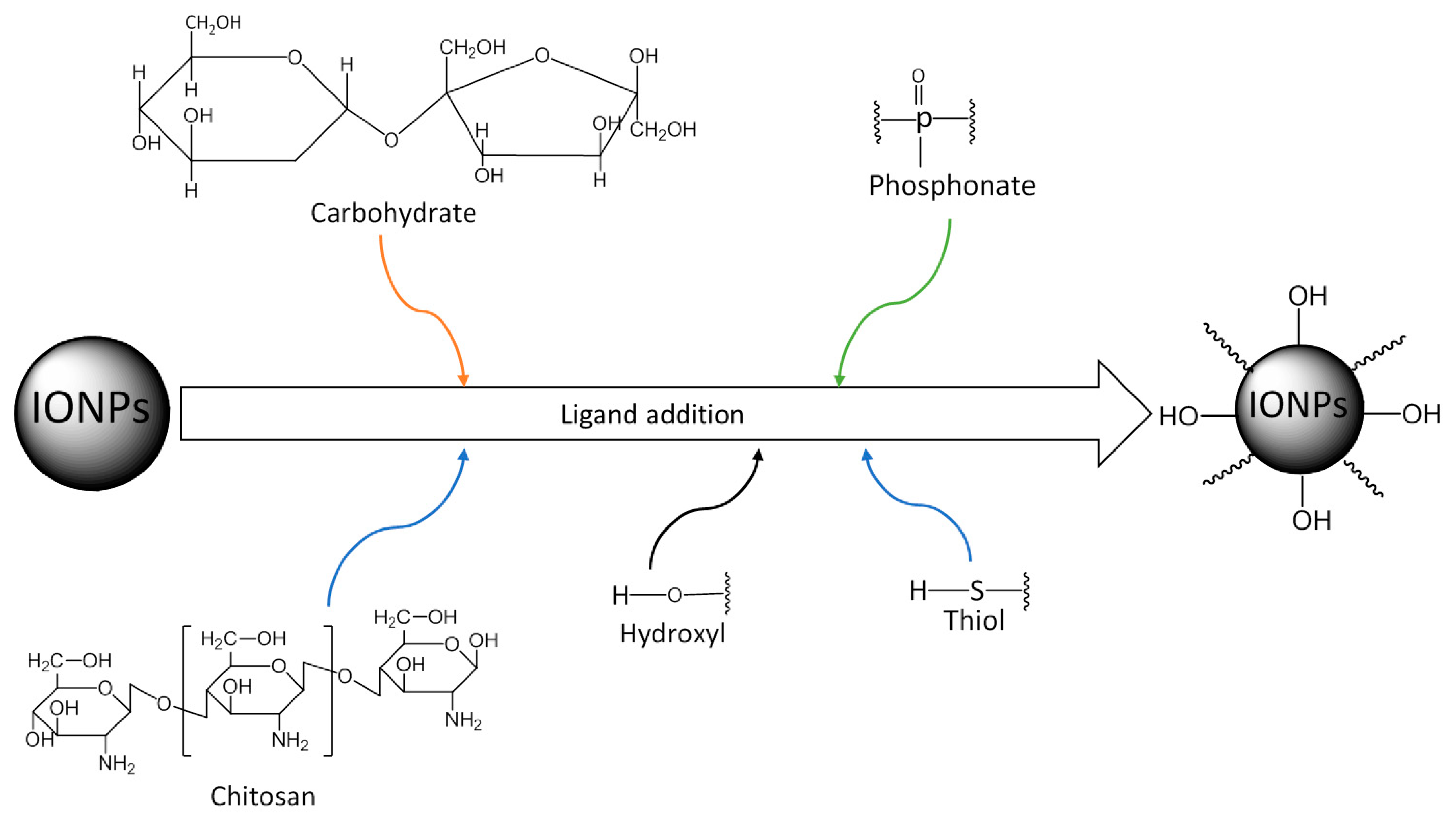
| Methods | Temperature | Time | Size | Magnetic Properties | References |
|---|---|---|---|---|---|
| Co-precipitation | 20–150 °C | minutes | 5–40 nm | Ms = 36.8 emu∙g−1 | [17,18,20,51] |
| Thermal decomposition | 100–350 °C | hours-days | 4–30 nm | Ms = 13.4–49.5 A∙m2∙kg−1 | [22,25,52] |
| Microemulsion | 20–80 °C | hours | 10–25 nm | Ms = 81 emu∙g−1 Mr = 10 emu∙g−1 Hc = 130 Oe | [28,53] |
| Hydrothermal | 150–280 °C | hours-days | 10–800 nm | Ms = 35–40 emu∙g−1 Hc = 0.6–15.7 Oe | [31,32,33,54] |
| Polyol | 130–220 °C | hours | 4–100 nm | Ms = 197–243 emu∙g−1 | [37,38] |
| Sol-gel | 25–200 °C | hours | 15–50 nm | Ms = 37.5 emu∙g−1 | [39,55,56] |
| Biomineralization | 80 °C | hours | ~140 nm | Ms = 92–100 A∙m2∙kg−1 | [43,44] |
| Sputter deposition | 100–800 °C | hours | 5–100 nm | Ms = 48–71 emu∙g−1 Mr = 2.5–5 emu∙g−1 Hc = 160–220 Oe | [45,46,47,48,49,50] |
| Techniques | Evaluation |
|---|---|
| Infrared spectroscopy (IR) | Nature of surface functionalization |
| Nuclear magnetic resonance spectroscopy (NMR) | Longitudinal and transverse relaxivity; Structure conformation |
| Superconducting quantum interference device (SQUID); Vibrating sample magnetometry (VSM) | Magnetic properties |
| Electron microscopy (transmission, TEM; scanning, SEM) | Morphology, crystallinity, size distribution, composition |
| X-ray diffraction (XRD) | Crystal structure, size |
| Dynamic light scattering (DLS) | Hydrodynamic diameter |
| Zeta potential measurement | Surface charge |
| Thermal analysis (differential scanning calorimetry, thermogravimetric analysis, etc.) | Surface coverage, thermal stability, nature of surface functionalization, carrier-drug interaction |
| Mass spectroscopy | Molecular weight |
| Fluorescence correlation spectroscopy | Dimension, binding kinetics of hydrodynamic |
| Surface-enhanced Raman scattering | Size distribution, electronic characteristics |
| Circular dichroism | Thermal constancy |
| Scanning tunnelling microscopy; Small-angle X-ray scattering | Shape heterogeneity, size and size navigation |
| Atomic force microscopy | Shape heterogeneity |
| Type of IONPs | Synthesis Method | Physicochemical Properties | Magnetometric Properties | Reference |
|---|---|---|---|---|
| Fe3O4 | Thermal decomposition of iron oleate in NaCl | Octapodes; 20–30 nm | Ms = 51–71 emu∙g−1 Tb = 240–290 K (SQUID) | [72] |
| Fe3O4 | Liquid precipitation + controlled crystal growth | Crystallite Cube; 10–300 nm | Ms = 54.7–84.7 emu∙g−1 Dc = 76 nm (SQUID) | [78] |
| Fe3O4 in SiO2 matrix | Co-precipitation + Stober modification | Cubic inverse spinel structure; 6–17 nm | Ms = 0.001–0.0015 emu Hc = 24–26 Oe (SQUID) | [79] |
| Fe3O4 | Thermal decomposition of iron oleate | Cuboid shape; 7–11 nm | Ms 5 K = 11.96 emu∙g−1 Ms 310 K = 11.01 emu∙g−1 Tb = 181 K Hc = 375 Oe (5 K) (VSM) | [84] |
| FemOn | Precipitation method | Spherical shape; 28.8–68.1 nm | Ms = 43.8 emu∙g−1 (VSM) | [85] |
| FemOn-SiO2 | Co-precipitation | Irregular nanoflakes; 98–101 nm | Ms = 11 emu∙g−1 (VSM) | [82] |
| SiO2-FemOn | Co-precipitation | Core-shell structure; 98–101 nm | Ms = 37.2 emu∙g−1 (VSM) | [82] |
| Synthesis Method | Advantage | Disadvantage |
|---|---|---|
| Stöber method | Controllable silica shell and uniform size, high crystallinity | Lack of understanding of its kinetics and mechanism |
| Microemulsion | Control of particle size | Poor yield, time consuming |
| Aeresol pyrolysis | Hermitically coated | Complex experimental condition |
| Polymers | Advantage | Application | Reference |
|---|---|---|---|
| Chitosan | Biocompatible, hydrophilic | MRI contrast agent, Drug delivery agent | [93] |
| Dextran | Enhance blood circulation, stabilizes colloidal suspension | MRI contrast agent, Molecular diagnostic agent | [100] |
| Gelatin | Gelling agent, biocompatible emulsifier | Magnetic resonance imaging | [101] |
| Polyethylene glycol | Internalization efficiency of the NPs | Magnetic hyperthermia agent, MRI theranostics | [102,103] |
| Polyvinyl alcohol | Prevents particles coagulation | Drug delivery agents, cytotoxicity studies | [104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials 2019, 12, 465. https://doi.org/10.3390/ma12030465
Ansari SAMK, Ficiarà E, Ruffinatti FA, Stura I, Argenziano M, Abollino O, Cavalli R, Guiot C, D’Agata F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials. 2019; 12(3):465. https://doi.org/10.3390/ma12030465
Chicago/Turabian StyleAnsari, Shoeb Anwar Mohammed Khawja, Eleonora Ficiarà, Federico Alessandro Ruffinatti, Ilaria Stura, Monica Argenziano, Ornella Abollino, Roberta Cavalli, Caterina Guiot, and Federico D’Agata. 2019. "Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System" Materials 12, no. 3: 465. https://doi.org/10.3390/ma12030465
APA StyleAnsari, S. A. M. K., Ficiarà, E., Ruffinatti, F. A., Stura, I., Argenziano, M., Abollino, O., Cavalli, R., Guiot, C., & D’Agata, F. (2019). Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Materials, 12(3), 465. https://doi.org/10.3390/ma12030465








