Preparation and Characterization of Poly(δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Pδ-VL)
2.3. Preparation of Pδ-VL/TiO2 Nanocomposite by SC Route
2.4. Preparation of Pδ-VL/TiO2 Nanocomposite by PM Route
2.5. Creation of Interconnected Pores
2.6. Characterization
2.6.1. FTIR Analysis
2.6.2. XRD Analysis
2.6.3. DSC Analysis
2.6.4. SEM Analysis
2.6.5. Porosity and Pore Size Distribution
2.6.6. Cell Adhesion and Growth Test
2.6.7. Mechanical Properties Analysis
3. Results and Discussions
3.1. FTIR Analysis
3.2. XRD Analysis
3.3. Thermal Behavior of Pδ-VL/TiO2 Nanocomposite
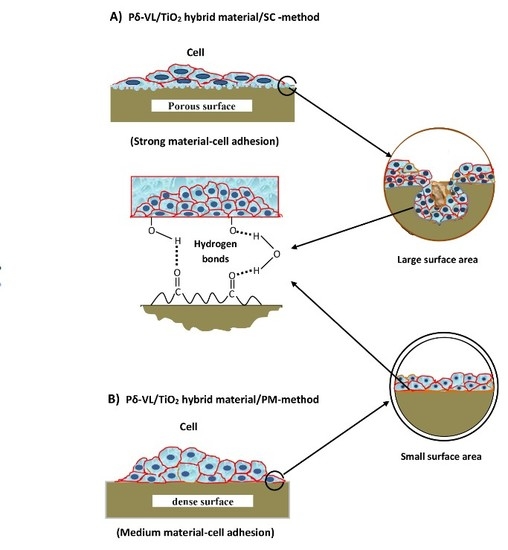
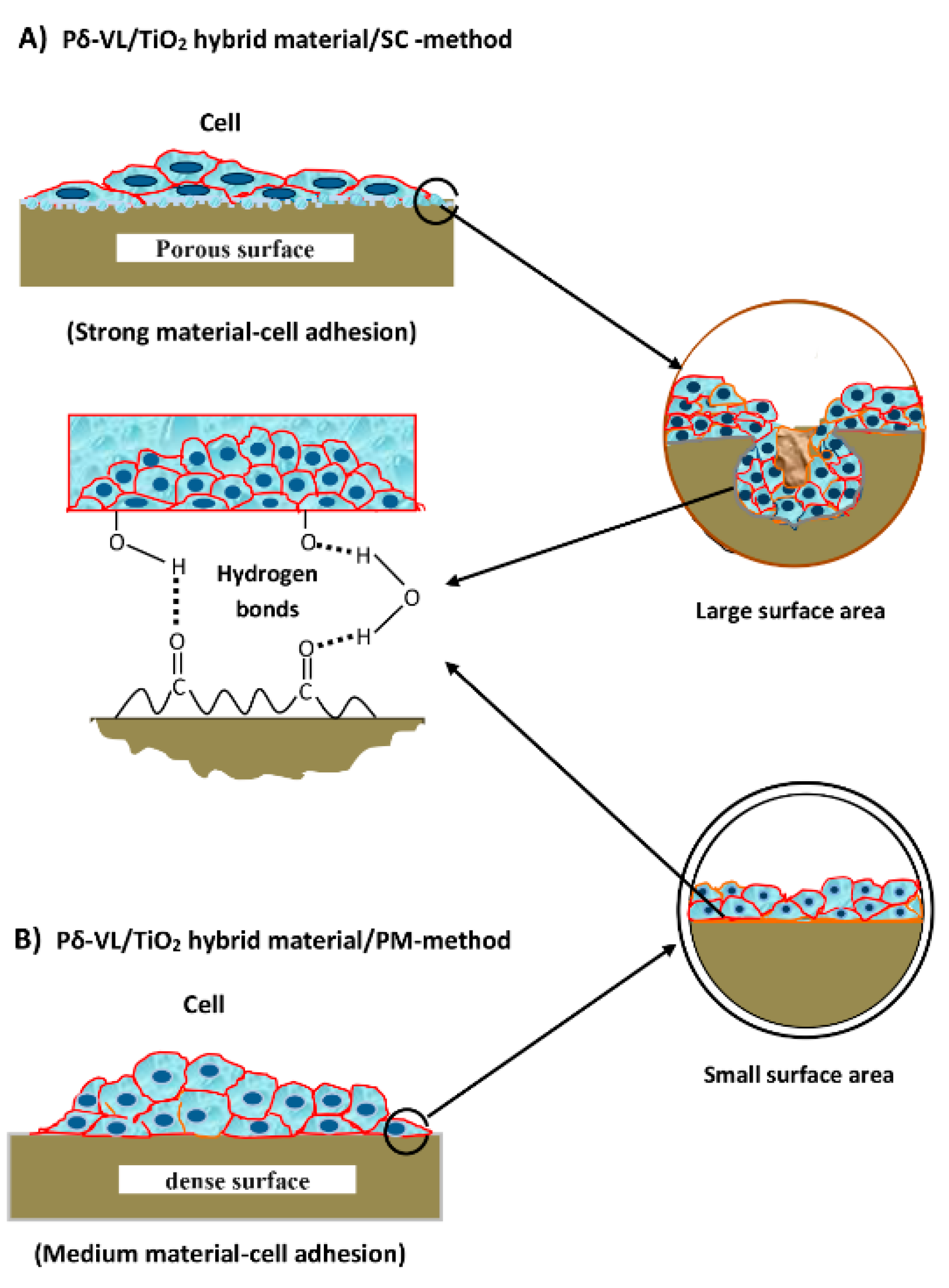
3.4. Assessment of Cell Adhesion and Growth
3.5. Dynamic Mechanical Properties
3.6. SEM Analysis
3.7. Porosity and Pore Size Distribution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural polymers: Polysaccharides and their derivatives for biomedical applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- McMahon, R.E.; Wang, L.; Skoracki, R.; Mathur, A.B. Development of nanomaterials for bone repair and regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.Y.; Doble, M. Design of biocomposite materials for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Aubin, M.; Prud’homme, R.E. Preparation and properties of poly (valerolactone). Polymer 1981, 22, 1223–1226. [Google Scholar] [CrossRef]
- Gagliardi, M.; Di Michele, F.; Mazzolai, B.; Bifone, A. Chemical synthesis of a biodegradable pegylated copolymer from ε-caprolactone and γ-valerolactone: Evaluation of reaction and functional properties. J. Polym. Res. 2015, 22, 17. [Google Scholar] [CrossRef]
- Khalil, M.; Al-Shamary, D.; Al-Deyab, S. Synthesis of poly (δ-valerolactone) by activated monomer polymerization, its characterization and potential medical application. Asian. J. Biochem. Pharm. Res 2015, 5, 137–147. [Google Scholar]
- Vaida, C.; Takwa, M.; Martinelle, M.; Hult, K.; Keul, H.; Möller, M. Γ-Acyloxy-ε-Caprolactones: Synthesis, Ring-Opening Polymerization vs. Rearrangement by Means of Chemical and Enzymatic Catalysis. Macromol. Symp. 2008. [Google Scholar] [CrossRef]
- D’auria, I.; Mazzeo, M.; Pappalardo, D.; Lamberti, M.; Pellecchia, C. Ring-opening polymerization of cyclic esters promoted by phosphido-diphosphine pincer group 3 complexes. J. Polym. Sci. A: Polym. Chem. 2011, 49, 403–413. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Grobelny, Z.; Matlengiewicz, M.; Skrzeczyna, K.; Swinarew, A.; Golba, S.; Jurek-Suliga, J.; Michalak, M.; Swinarew, B. Ring-opening polymerization of lactones initiated with metal hydroxide-activated macrocyclic ligands: Determination of mechanism and structure of polymers. Int. J. Polym. Anal. Charact. 2015, 20, 457–468. [Google Scholar] [CrossRef]
- Nair, L.; Jagadeeshan, S.; Nair, S.A.; Kumar, G.V. Evaluation of triblock copolymeric micelles of δ-valerolactone and poly (ethylene glycol) as a competent vector for doxorubicin delivery against cancer. J. Nanobiotechnol. 2011, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Varma, I.K.; Albertsson, A.-C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar] [CrossRef]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Recent developments in lipase-catalyzed synthesis of polyesters. Macromol. Rapid. Commun. 2009, 30, 237–266. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Lipase-catalyzed polyester synthesis–A green polymer chemistry. Proc. Jpn. Acad. Ser. B. 2010, 86, 338–365. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Kobayashi, S. Polymer synthesis by enzymatic catalysis. Curr. Opin. Chem. Biol. 2010, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Zhang, Y.; Liu, C.; Shi, W.; Li, Q. Lipase/esterase-catalyzed ring-opening polymerization: A green polyester synthesis technique. Proc. Biochem. 2011, 46, 1900–1908. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Krishnan, U.M.; Sethuraman, S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014, 32, 449–461. [Google Scholar] [CrossRef]
- Boucher, H. Development and characterization of a polyester-based implant for controlled drug release. Master’s Thesis, University of Toronto, Toronto, ON, Canada, November 2017. [Google Scholar]
- Xu, C.; Huang, Y.; Tang, L.; Hong, Y. Low-initial-modulus biodegradable polyurethane elastomers for soft tissue regeneration. ACS Appl. Mater. Interf. 2017, 9, 2169–2180. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhoundi, M.S.A.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A.H. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dental. Press. J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Forster, A.M.; Johnson, P.M.; Eidelman, N.; Quinn, G.; Schumacher, G.; Zhang, X.; Wu, W.-l. Improving performance of dental resins by adding titanium dioxide nanoparticles. Dent. Mater. 2011, 27, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Lu, X.; Tian, H.; Jiang, H.; Lv, G.; Guo, D.; Wu, C.; Chen, B. The incorporation of daunorubicin in cancer cells through the use of titanium dioxide whiskers. Biomaterials 2009, 30, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
- Paunesku, T.; Vogt, S.; Lai, B.; Maser, J.; Stojićević, N.; Thurn, K.T.; Osipo, C.; Liu, H.; Legnini, D.; Wang, Z. Intracellular distribution of TiO2−DNA oligonucleotide nanoconjugates directed to nucleolus and mitochondria indicates sequence specificity. Nano Lett. 2007, 7, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, N.M.; Rozhkova, E.; Rajh, T. Dynamics of localized charges in dopamine-modified TiO2 and their effect on the formation of reactive oxygen species. J. Am. Chem. Soc. 2009, 131, 2893–2899. [Google Scholar] [CrossRef]
- Lu, Z.; Ye, M.; Li, N.; Zhong, W.; Yin, Y. Self-assembled TiO2 nanocrystal clusters for selective enrichment of intact phosphorylated proteins. Angew. Chem. Int. Ed. Engl. 2010, 49, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Jiang, H.; Lu, X.; Zhu, Y.; Chen, B. New strategy of photodynamic treatment of TiO2 nanofibers combined with celastrol for HepG2 proliferation in vitro. Nanoscale 2011, 3, 3115–3122. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Increased osteoblast functions on nanophase titania dispersed in poly-lactic-co-glycolic acid composites. Nanotechnology 2005, 16, S601. [Google Scholar] [CrossRef]
- Goto, K.; Tamura, J.; Shinzato, S.; Fujibayashi, S.; Hashimoto, M.; Kawashita, M.; Kokubo, T.; Nakamura, T. Bioactive bone cements containing nano-sized titania particles for use as bone substitutes. Biomaterials 2005, 26, 6496–6505. [Google Scholar] [CrossRef]
- Sivakumar, S.; Pillai, P.K.; Mukundan, P.; Warrier, K. Sol–gel synthesis of nanosized anatase from titanyl sulfate. Mater. Lett. 2002, 57, 330–335. [Google Scholar] [CrossRef]
- Park, S.D.; Cho, Y.H.; Kim, W.W.; Kim, S.-J. Understanding of homogeneous spontaneous precipitation for monodispersed TiO2 ultrafine powders with rutile phase around room temperature. J. Solid State Chem. 1999, 146, 230–238. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- McCormick, J.R.; Zhao, B.; Rykov, S.A.; Wang, H.; Chen, J.G. Thermal stability of flame-synthesized anatase TiO2 nanoparticles. J. Phys. Chem. B 2004, 108, 17398–17402. [Google Scholar] [CrossRef]
- Docters, T.; Chovelon, J.; Herrmann, J.; Deloume, J. Syntheses of TiO2 photocatalysts by the molten salts method: Application to the photocatalytic degradation of prosulfuron. Appl. Catal. B Environ. 2004, 50, 219–226. [Google Scholar] [CrossRef]
- Avvakumov, G.V.; Senna, M.; Kosova, N.V. Soft Mechanochemical Synthesis: A Basis for New Chemical Technologies; Springer Science & Business Media: New York, NY, USA, 2001. [Google Scholar]
- Billik, P.; Plesch, G. Mechanochemical synthesis of anatase and rutile nanopowders from TiOSO4. Mater. Lett. 2007, 61, 1183–1186. [Google Scholar] [CrossRef]
- Savaiano, J.K.; Webster, T.J. Altered responses of chondrocytes to nanophase PLGA/nanophase titania composites. Biomaterials 2004, 25, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.S.; Hayes, W.C.; Keaveny, T.M.; Boskey, A.; Einhorn, T.A.; Lannotti, J.P. Orthopaedic Basic Science; Simon, S.R., Ed.; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 1994; pp. 127–185. [Google Scholar]
- Webster, T.J. Nanophase ceramics as improved bone tissue engineering materials. Am. Ceram. Soc. Bull. 2003, 82, 23–28. [Google Scholar]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res. 2000, 51, 475–483. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Kay, S.; Thapa, A.; Haberstroh, K.M.; Webster, T.J. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002, 8, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, P.X. Poly (α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Maquet, V. Bioresorbable and bioactive polymer/bioglass composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Blaker, J.; Gough, J.; Maquet, V.; Notingher, I.; Boccaccini, A. In vitro evaluation of novel bioactive composites based on bioglass-filled polylactide foams for bone tissue engineering scaffolds. J. Biomed. Mater. Res. A 2003, 67, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteil chenmittels Röntgenstrahlen, Kolloid Chemie EinLehrbuch; Springer: New York, NY, USA, 1912; pp. 387–409. [Google Scholar]
- Patterson, A. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Endogan Tanir, T.; Hasirci, V.; Hasirci, N. Preparation and characterization of Chitosan and PLGA-based scaffolds for tissue engineering applications. Polym. Compos. 2015, 36, 1917–1930. [Google Scholar] [CrossRef]
- Semlali, A.; Jacques, E.; Rouabhia, M.; Milot, J.; Laviolette, M.; Chakir, J. Regulation of epithelial cell proliferation by bronchial fibroblasts obtained from mild asthmatic subjects. Allergy 2010, 65, 1438–1445. [Google Scholar] [CrossRef]
- Semlali, A.; Chakir, J.; Goulet, J.P.; Chmielewski, W.; Rouabhia, M. Whole cigarette smoke promotes human gingival epithelial cell apoptosis and inhibits cell repair processes. J. Periodont. Res. 2011, 46, 533–541. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 868:2003, Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness); International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Ren, Y.; Wei, Z.; Wu, T.; Bian, Y.; Leng, X.; Zhou, C.; Li, Y. Synthesis of highly branched poly (δ-valerolactone)s: A comparative study between comb and linear analogues. RSC Adv. 2016, 6, 45791–45801. [Google Scholar] [CrossRef]
- Furuhashi, Y.; Sikorski, P.; Atkins, E.; Iwata, T.; Doi, Y. Structure and morphology of the aliphatic polyester poly (δ-valerolactone) in solution-grown, chain-folded lamellar crystals. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2622–2634. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. Nat. Sci. 2008, 42, 357–361. [Google Scholar]
- Kasyapi, N.; Bhowmick, A.K. Nanolamellar triblock of poly-d,l-lactide–δ-valerolactone–d,l-lactide with tuneable glass transition temperature and crystallinity for use as a drug-delivery vesicle. RSC Adv. 2014, 4, 27439–27451. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Kiran, A.; Kumar, T.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocomposite coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [PubMed]











| System | TiO2 (g) | Pδ-VL (g) | TiO2 (wt %) |
|---|---|---|---|
| Pδ-VL/TiO2-1 | 0.005 | 0.495 | 1.0 |
| Pδ-VL/TiO2-2 | 0.010 | 0.490 | 2.0 |
| Pδ-VL/TiO2-3 | 0.015 | 0.485 | 3.0 |
| Pδ-VL/TiO2-4 | 0.020 | 0.480 | 4.0 |
| Pδ-VL/TiO2-5 | 0.025 | 0.475 | 5.0 |
| Specimen | Yield Point (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Storage Modulus, E’ (GPa) at 37 °C | Shore A | Shore D |
|---|---|---|---|---|---|---|
| Pδ-VL | 9.02 | 14.20 | 118 | 0.72 | 92.7 | 42.7 |
| Pδ-VL/TiO2-1 | 8.31 | 12.30 | 120 | 0.91 | 93.6 | 43.2 |
| Pδ-VL/TiO2-2 | 8.18 | 10.72 | 125 | 1.05 | 94.0 | 43.8 |
| Pδ-VL/TiO2-3 | 8.32 | 10.04 | 137 | 1.12 | 94.5 | 44.9 |
| Pδ-VL/TiO2-4 | 7.80 | 9.06 | 143 | 1.43 | 95.0 | 46.0 |
| Pδ-VL/TiO2-5 | 6.73 | 8.12 | 160 | 1.63 | 95.1 | 46.2 |
| Sample | Porosity (%) |
|---|---|
| Pδ-VL/TiO2-1 | 78.2 ± 4.2 |
| Pδ-VL/TiO2-2 | 75.3 ± 3.2 |
| Pδ-VL/TiO2-3 | 74.8 ± 3.2 |
| Pδ-VL/TiO2-4 | 74.2 ± 3.6 |
| Pδ-VL/TiO2-4 | 73.8 ± 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, W.S.; Al-Odayni, A.-B.; Alrahlah, A.; Alghamdi, A.A.; Aouak, T. Preparation and Characterization of Poly(δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering. Materials 2019, 12, 528. https://doi.org/10.3390/ma12030528
Saeed WS, Al-Odayni A-B, Alrahlah A, Alghamdi AA, Aouak T. Preparation and Characterization of Poly(δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering. Materials. 2019; 12(3):528. https://doi.org/10.3390/ma12030528
Chicago/Turabian StyleSaeed, Waseem Sharaf, Abdel-Basit Al-Odayni, Ali Alrahlah, Abdulaziz Ali Alghamdi, and Taieb Aouak. 2019. "Preparation and Characterization of Poly(δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering" Materials 12, no. 3: 528. https://doi.org/10.3390/ma12030528
APA StyleSaeed, W. S., Al-Odayni, A.-B., Alrahlah, A., Alghamdi, A. A., & Aouak, T. (2019). Preparation and Characterization of Poly(δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering. Materials, 12(3), 528. https://doi.org/10.3390/ma12030528