The Penetration Ability of Calcium Silicate Root Canal Sealers into Dentinal Tubules Compared to Conventional Resin-Based Sealer: A Confocal Laser Scanning Microscopy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Specimen Grouping and Root Canal Obturation
2.3. Sample Sectioning
2.4. Confocal Laser Scanning Microscopy Assay
2.5. Statistical Analysis
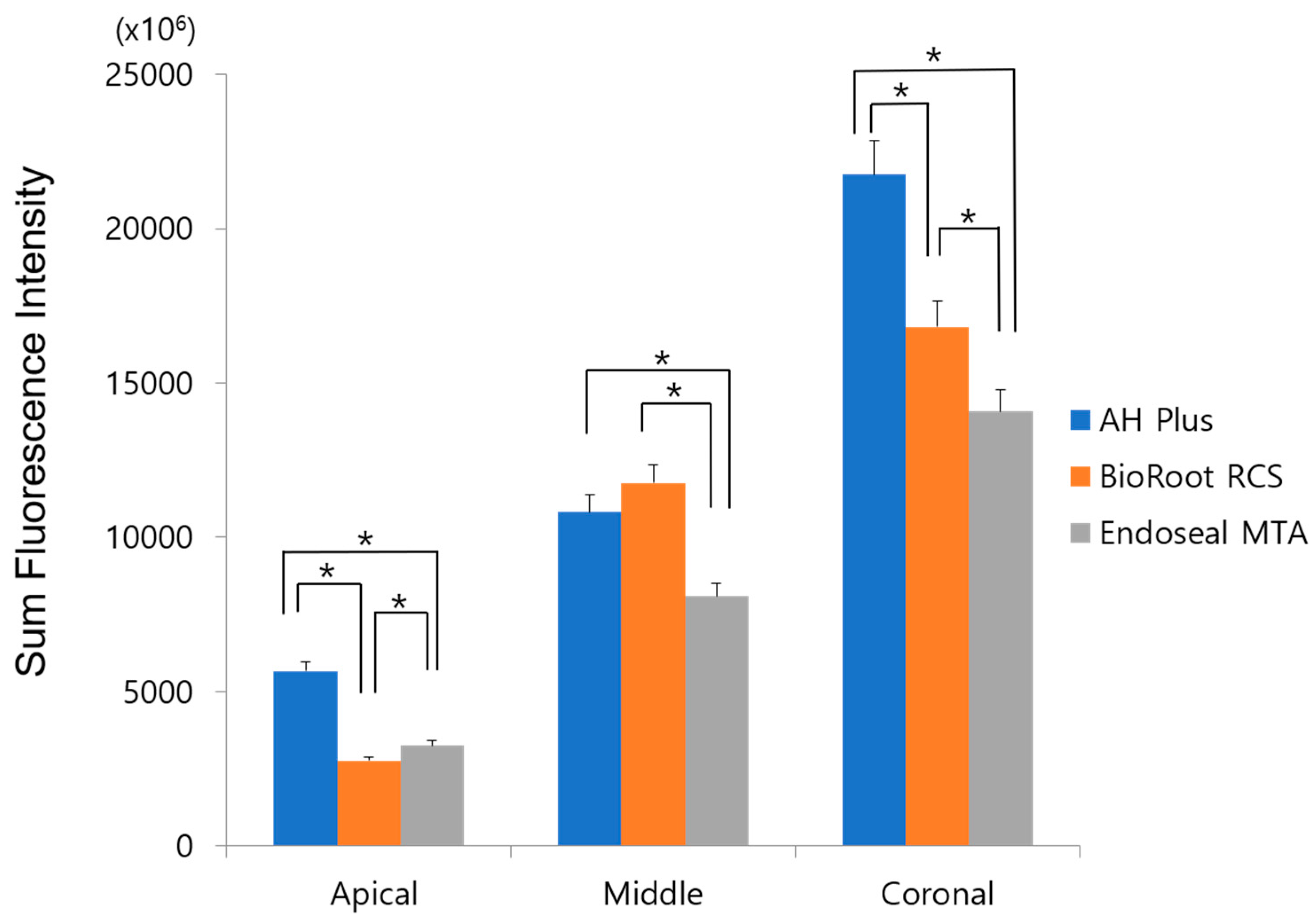
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirkevang, L.L.; Orstavik, D.; Horsted-Bindslev, P.; Wenzel, A. Periapical status and quality of root fillings and coronal restorations in a Danish population. Int. Endod. J. 2000, 33, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N.; Alves, F.R.; Campos, L.C. Periradicular status related to the quality of coronal restorations and root canal fillings in a Brazilian population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Tavares, P.B.; Bonte, E.; Boukpessi, T.; Siqueira, J.F., Jr.; Lasfargues, J.J. Prevalence of apical periodontitis in root canal-treated teeth from an urban French population: Influence of the quality of root canal fillings and coronal restorations. J. Endod. 2009, 35, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Shaw, L.; Barthelemy, J.; Krejci, I.; Wataha, J.C. Long-term sealing ability of Pulp Canal Sealer, AH-Plus, GuttaFlow and Epiphany. Int. Endod. J. 2008, 41, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mamootil, K.; Messer, H.H. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int. Endod. J. 2007, 40, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.E.; Marker, K.L.; Drake, D.R.; Dawson, D.V.; Walton, R.E. Resin-based versus gutta-percha-based root canal obturation: Influence on bacterial leakage in an in vitro model system. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Sielker, S.; Hanisch, M.R.; Libricht, V.; Schafer, E.; Dammaschke, T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS ONE 2018, 13, e0194467. [Google Scholar] [CrossRef]
- Camps, J.; Jeanneau, C.; El Ayachi, I.; Laurent, P.; About, I. Bioactivity of a calcium silicate–based endodontic cement (BioRoot RCS): Interactions with human periodontal ligament cells in vitro. J. Endod. 2015, 41, 1469–1473. [Google Scholar] [CrossRef]
- Dimitrova-Nakov, S.; Uzunoglu, E.; Ardila-Osorio, H.; Baudry, A.; Richard, G.; Kellermann, O.; Goldberg, M. In vitro bioactivity of BioRoot RCS, via A4 mouse pulpal stem cells. Dent. Mater. 2015, 31, 1290–1297. [Google Scholar] [CrossRef]
- Viapiana, R.; Moinzadeh, A.T.; Camilleri, L.; Wesselink, P.R.; Tanomaru Filho, M.; Camilleri, J. Porosity and sealing ability of root fillings with gutta-percha and BioRoot RCS or AH Plus sealers. Evaluation by three ex vivo methods. Int. Endod. J. 2016, 49, 774–782. [Google Scholar] [CrossRef]
- Da Silva, E.; Zaia, A.A.; Peters, O.A. Cytocompatibility of calcium silicate-based sealers in a three-dimensional cell culture model. Clin. Oral Investig. 2017, 21, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.S.; Park, Y.B.; Kwon, Y.S.; Shon, W.J.; Lee, K.W.; Min, K.S. Physical properties and biocompatibility of an injectable calcium-silicate-based root canal sealer: In vitro and in vivo study. BMC Oral Health 2015, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.; Park, J.W.; Jung, I.Y.; Shin, S.J. Comparison of the percentage of voids in the canal filling of a calcium silicate-based sealer and gutta percha cones using two obturation techniques. Materials 2017, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Hwang, Y.C.; Rosa, V.; Yu, M.K.; Lee, K.W.; Min, K.S. Root canal filling quality of a premixed calcium silicate endodontic sealer applied using gutta-percha cone-mediated ultrasonic activation. J. Endod. 2018, 44, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kwak, S.W.; Ha, J.H.; Lee, W.; Kim, H.C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg. Chem. Appl. 2017, 2017, 2582849. [Google Scholar] [CrossRef] [PubMed]
- Alsubait, S.A.; Al Ajlan, R.; Mitwalli, H.; Aburaisi, N.; Mahmood, A.; Muthurangan, M.; Almadhri, R.; Alfayez, M.; Anil, S. Cytotoxicity of different concentrations of three root canal sealers on human mesenchymal stem cells. Biomolecules 2018, 8, 68. [Google Scholar] [CrossRef]
- Loison-Robert, L.S.; Tassin, M.; Bonte, E.; Berbar, T.; Isaac, J.; Berdal, A.; Simon, S.; Fournier, B.P.J. In vitro effects of two silicate-based materials, Biodentine and BioRoot RCS, on dental pulp stem cells in models of reactionary and reparative dentinogenesis. PLoS ONE 2018, 13, e0190014. [Google Scholar] [CrossRef]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50, e120–e136. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.F.; Felippe, M.S.; Felippe, W.T. Biomineralization ability and interaction of mineral trioxide aggregate and white portland cement with dentin in a phosphate-containing fluid. J. Endod. 2009, 35, 731–736. [Google Scholar] [CrossRef]
- Yoo, J.S.; Chang, S.W.; Oh, S.R.; Perinpanayagam, H.; Lim, S.M.; Yoo, Y.J.; Oh, Y.R.; Woo, S.B.; Han, S.H.; Zhu, Q.; et al. Bacterial entombment by intratubular mineralization following orthograde mineral trioxide aggregate obturation: A scanning electron microscopy study. Int. J. Oral Sci. 2014, 6, 227–232. [Google Scholar] [CrossRef]
- Atmeh, A.R.; Chong, E.Z.; Richard, G.; Festy, F.; Watson, T.F. Dentin-cement interfacial interaction: Calcium silicates and polyalkenoates. J. Dent. Res. 2012, 91, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, M.; Vella, P.; Damidot, D.; Sammut, C.V.; Camilleri, J. In situ assessment of the setting of tricalcium silicate-based sealers using a dentin pressure model. J. Endod. 2015, 41, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Prullage, R.K.; Urban, K.; Schafer, E.; Dammaschke, T. Material Properties of a Tricalcium Silicate-containing, a Mineral Trioxide Aggregate-containing, and an Epoxy Resin-based Root Canal Sealer. J. Endod. 2016, 42, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Uzunoglu-Ozyurek, E.; Erdogan, O.; AktemurTurker, S. Effect of Calcium Hydroxide Dressing on the Dentinal Tubule Penetration of 2 Different Root Canal Sealers: A Confocal Laser Scanning Microscopic Study. J. Endod. 2018, 44, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Celikten, B.; Uzuntas, C.F.; Orhan, A.I.; Orhan, K.; Tufenkci, P.; Kursun, S.; Demiralp, K.O. Evaluation of root canal sealer filling quality using a single-cone technique in oval shaped canals: An In vitro Micro-CT study. Scanning 2016, 38, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Peng, B. Assessment of a new root canal sealer’s apical sealing ability. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e79–e82. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Kim, S.; Park, J.W.; Kim, E.; Shin, S.J. Different Setting Conditions Affect Surface Characteristics and Microhardness of Calcium Silicate-Based Sealers. Scanning 2018, 2018, 7136345. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Baek, S.H.; Kum, K.Y.; Shon, W.J.; Woo, K.M.; Lee, W. Dynamic intratubular biomineralization following root canal obturation with pozzolan-based mineral trioxide aggregate sealer cement. Scanning 2016, 38, 50–56. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chung, J.; Na, H.S.; Park, E.; Kwak, S.; Kim, H.C. Comparison of bacterial leakage resistance of various root canal filling materials and methods: Confocal laser-scanning microscope study. Scanning 2015, 37, 422–428. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lee, B.S.; Lin, F.H.; Yun Lin, A.; Lan, W.H.; Lin, C.P. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials 2004, 25, 787–793. [Google Scholar] [CrossRef]
- Jafari, F.; Jafari, S. Composition and physicochemical properties of calcium silicate based sealers: A review article. J. Clin Exp Dent. 2017, 9, e1249–e1255. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.M.; Shon, W.J.; Baek, S.H.; Bae, K.S.; Kum, K.Y.; Lee, W. Effect of final irrigation regimen on sealer penetration in curved root canals. J. Endod. 2010, 36, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.R.; Tordik, P.A.; Imamura, G.M.; Baginski, T.A.; Goodell, G.G. A confocal laser scanning microscope investigation of the epiphany obturation system. J. Endod. 2007, 33, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Piai, G.G.; Duarte, M.A.H.; Nascimento, A.L.D.; Rosa, R.A.D.; So, M.V.R.; Vivan, R.R. Penetrability of a new endodontic sealer: A confocal laser scanning microscopy evaluation. Microsc. Res. Tech. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jardine, A.P.; Rosa, R.A.; Santini, M.F.; Wagner, M.; So, M.V.; Kuga, M.C.; Pereira, J.R.; Kopper, P.M. The effect of final irrigation on the penetrability of an epoxy resin-based sealer into dentinal tubules: A confocal microscopy study. Clin. Oral Investig. 2016, 20, 117–123. [Google Scholar] [CrossRef] [PubMed]





| Table | Manufacturer | Composition | Batch Number |
|---|---|---|---|
| AH Plus | Dentsply DeTrey GmbH, Konstanz, Germany | Epoxide paste: diepoxide, calcium tungstate, zirconium oxide, aerosil, and pigment; amine paste: 1-adamantane amine, N,N′-dibenzyl-5-oxa-nonandiamine-1,9, TCD-diamine, calcium tungstate, zirconium oxide, aerosil, and silicon oil | 1703000226 |
| BioRoot RCS | Septodont, Saint-Maur-des-Fossés Cedex, France | Tricalcium silicate, zirconium oxide (opacifier), and excipients in powder form, and calcium chloride and excipients as an aqueous liquid | B16422 |
| Endoseal MTA | Maruchi, Wonju, Korea | Calcium silicates, calcium aluminates, calcium aluminoferrite, calcium sulfates, radiopacifier, and thickening agents | CD180327D |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, B.-S.; Kim, Y.-M.; Lee, D.; Kim, S.-Y. The Penetration Ability of Calcium Silicate Root Canal Sealers into Dentinal Tubules Compared to Conventional Resin-Based Sealer: A Confocal Laser Scanning Microscopy Study. Materials 2019, 12, 531. https://doi.org/10.3390/ma12030531
Kim Y, Kim B-S, Kim Y-M, Lee D, Kim S-Y. The Penetration Ability of Calcium Silicate Root Canal Sealers into Dentinal Tubules Compared to Conventional Resin-Based Sealer: A Confocal Laser Scanning Microscopy Study. Materials. 2019; 12(3):531. https://doi.org/10.3390/ma12030531
Chicago/Turabian StyleKim, Yemi, Ban-Suk Kim, Yong-Min Kim, Donghee Lee, and Sin-Young Kim. 2019. "The Penetration Ability of Calcium Silicate Root Canal Sealers into Dentinal Tubules Compared to Conventional Resin-Based Sealer: A Confocal Laser Scanning Microscopy Study" Materials 12, no. 3: 531. https://doi.org/10.3390/ma12030531
APA StyleKim, Y., Kim, B.-S., Kim, Y.-M., Lee, D., & Kim, S.-Y. (2019). The Penetration Ability of Calcium Silicate Root Canal Sealers into Dentinal Tubules Compared to Conventional Resin-Based Sealer: A Confocal Laser Scanning Microscopy Study. Materials, 12(3), 531. https://doi.org/10.3390/ma12030531






