Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles
Abstract
1. Introduction
2. Biogenic Mesocrystals and Their Nanoparticle Components
2.1. The Sea Urchin Spicule
2.2. The Mollusk Shell Nacre Layer
3. Hydrogels and Biomineralization
4. Biomineral-Associated Protein Families Are “Smart” Hydrogelators
5. Evidence for Protein-Driven Particle Assembly and Organization
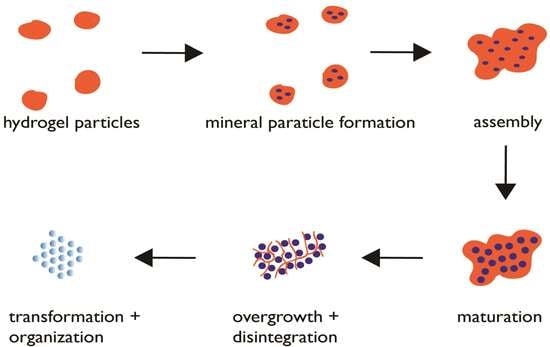
6. Proposed Mechanism of Protein-Directed Nanoparticle Assembly into a Crystal Format
7. The Future: Where Do We Go from Here?
Funding
Acknowledgments
Conflicts of Interest
References
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; pp. 1–134. ISBN 0-19-504977-2. [Google Scholar]
- Mann, S. Biomineralization. Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: New York, NY, USA, 2001; pp. 6–9, 24–108. [Google Scholar]
- Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; Burke, R.D.; et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xu, G.; Hu, Y.; Pan, C.; Xie, L.; Zhang, R. Identification of genes directly involved in shell formation and their functions in pearl oyster, Pinctada fucata. PLoS ONE 2011, 6, 1–13. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Woodcroft, B.; Moase, P.; Rose, R.A.; Kube, M.; Reinhart, R.; Rokhsar, D.S.; Montagnani, C.; Joube, C.; et al. Parallel evolution of nacre building gene sets in mollusks. Mol. Biol. Evol. 2010, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.L.; Mass, T.; Haramaty, L.; Zelzion, E.; Bhattacharya, D.; Falkowski, P.G. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proc. Natl. Acad. Sci. USA 2013, 110, 3788–3793. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Iwashima, A.; Kimura, M.; Kogure, T.; Nagasawa, H. The molecular evolution of the Pif family proteins in various species of mollusks. Mar. Biotechnol. 2013, 15, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Su, J.; Zheng, G.; Liang, J.; Zhang, G.; Wang, H.; Xie, L.; Zhang, R. Patterns of expression in the matrix proteins responsible for nucleation and growth of aragonite crystals in flat pearls of Pinctada fucata. PLoS ONE 2013, 8, e66564. [Google Scholar] [CrossRef]
- Marie, B.; Joubert, C.; Tayale, A.; Zanella-Cleon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef]
- Mann, L.; Poustka, A.J.; Mann, M. The sea urchin (Strongylocentrotus purpuratus) test and spine proteomes. Proteome Sci. 2008, 6, 1–10. [Google Scholar] [CrossRef]
- Mann, K.; Wilt, F.H.; Poustka, A.J. Proteomic analysis of the sea urchin (Strongylocentrotus purpuratus) spicule matrix. Proteome Sci. 2010, 8, 1–12. [Google Scholar] [CrossRef]
- Evans, J.S. Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis. Minerals 2017, 7, 62. [Google Scholar] [CrossRef]
- Evans, J.S. “Liquid-like” biomineralization protein assemblies: A key to the regulation of non-classical nucleation. Cryst. Eng. Commun. 2013, 15, 8388–8394. [Google Scholar] [CrossRef]
- Evans, J.S. “Tuning in” to mollusk shell nacre- and prismatic-associated protein terminal sequences. Implications for biomineralization and the construction of high-performance inorganic-organic composites. Chem. Rev. 2008, 108, 4455–4462. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Lambert, G.; Addadi, L.; Weiner, S. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Stephens, C.J.; Ladden, S.F.; Meldrum, F.C.; Christenson, H.K. Amorphous calcium carbonate is stabilized in confinement. Adv. Mater. 2010, 20, 2108–2115. [Google Scholar] [CrossRef]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef]
- Gal, A.; Habraken, W.; Gur, D.; Fratzl, P.; Weiner, S.; Addadi, L. Calcite crystal growth by a solid-state transformation of stabilized amorphous calcium carbonate nanospheres in a hydrogel. Angew. Chem. 2013, 52, 4967–4970. [Google Scholar] [CrossRef]
- Gong, Y.U.T.; Killian, C.E.; Olson, I.C.; Appathurai, N.P.; Amasino, A.L.; Martin, M.C.; Holt, L.J.; Wilt, F.H.; Gilbert, P.U.P.A. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2012, 109, 6088–6093. [Google Scholar] [CrossRef]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic evidence for liquid-liquid separation in supersaturated calcium carbonate solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef]
- Radha, A.V.; Forbes, T.Z.; Killian, C.E.; Gilbert, P.U.P.A.; Navrotsky, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef]
- Colfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Wucher, B.; Yue, W.; Kulak, A.N.; Meldrum, F.C. Designer crystals: Single crystals with complex morphologies. Chem. Mater. 2007, 19, 1111–1119. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 4341. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Ma, Y.; Davis, S.A.; Meldrum, F.; Schilde, U.; Gourrier, A.; Jaeger, C.; Coelfen, H. Structure-property relationships of a biological mesocrystal in the adult sea urchin spine. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, L.; Sturm, E.V.; Salazar-Alvarez, G.; Coelfen, H. Mesocrystals in biominerals and colloidal arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.M.; Willinger, M.G.; Pina, C.M.; Checa, A.G. Transformation of ACC into aragonite and the origin of the nanogranular structure of nacre. Sci. Rep. 2017, 7, 12728. [Google Scholar] [CrossRef]
- Zhou, G.T.; Yao, Q.Z.; Ni, J.; Jin, G. Formation of aragonite mesocrystals and implication for biominerlization. Am. Mineral. 2009, 94, 293–302. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, 498–508. [Google Scholar] [CrossRef]
- Levy, Y.; Onuchic, J.N. Mechanisms of protein assembly: Lessons from minimalist models. Acc. Chem. Res. 2006, 39, 135–142. [Google Scholar] [CrossRef]
- Papapostolou, D.; Smith, A.M.; Atkins, E.D.T.; Oliver, S.J.; Ryadnov, M.G.; Serpell, L.C.; Woolfson, D.N. Engineering nanoscale order into a designed protein fiber. Proc. Natl. Acad. Sci. USA 2007, 104, 10853–10858. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.A.; Teichmann, S.A. Structure, dynamics, assembly and evolution of protein complexes. Ann. Rev. Biochem. 2015, 84, 551–575. [Google Scholar] [CrossRef]
- Beniash, E.; Addadi, L.; Weiner, S. Cellular control over spicule formation in sea urchin embryos: A structural approach. J. Struct. Biol. 1999, 125, 50–62. [Google Scholar] [CrossRef]
- Wilt, F.H. Biomineralization of the spicules of sea urchin embryo. Zoolog. Sci. 2002, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Wilt, F.H.; Ettensohn, C.E. Morphogenesis and biomineralization of the sea urchin larval endoskeleton. In Handbook of Biomineralization: Biological Aspects and Structure Formation; Baeuerlein, E., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 183–210. [Google Scholar]
- Zhang, G.; Li, X. Uncovering aragonite nanoparticle self-assembly in nacre—A natural armor. Cryst. Growth Des. 2012, 12, 4306–4310. [Google Scholar] [CrossRef]
- Li, X.; Chang, W.C.; Chao, Y.J.; Wang, R.; Chang, M. Nanoscale structural and mechanical characterization of a natural nanocomposite material. The shell of the red abalone. Nanoletters 2004, 4, 613–617. [Google Scholar] [CrossRef]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. RSC Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z. Unveiling the formation mechanism of pseudo-single-crystal aragonite platelets in nacre. Phys. Rev. Lett. 2009, 102, 075502–075506. [Google Scholar] [CrossRef]
- Zheng, G.; Xu, J. From colloidal nanoparticles to a single crystal: New insights into the formation of nacre’s aragonite tablets. J. Struct. Biol. 2013, 182, 36–43. [Google Scholar] [CrossRef]
- Metzler, R.A.; Evans, J.S.; Killian, C.E.; Zhou, D.; Churchill, T.H.; Appathurai, P.N.; Coppersmith, S.N.; Gilbert, P.U.P.A. Lamellar self-assembly and aragonite polymorph selection by a single intrinsically disordered protein fragment. J. Am. Chem. Soc. 2010, 132, 6329–6334. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Mouries, L.; Ribeiro, A.M.J.; Barathelemy, P.J.; Milet, C.; Lopez, E. Soluble silk-like organic matrix in the nacreous layer of the bivalve Pinctada maxima. Eur. J. Biochem. 2002, 269, 4994–5003. [Google Scholar] [CrossRef]
- Samchenko, Y.; Ulberg, Z.; Korotych, O. Multipurpose smart hydrogel systems. Adv. Colloid Interfacial Sci. 2011, 147, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–260. [Google Scholar] [CrossRef]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef]
- Xia, L.W.; Xie, R.; Ju, X.J.; Wang, W.; Chen, Q.; Chu, L.Y. Nano-structured smart hydrogels with rapid response and high elasticity. Nat. Commun. 2013, 4, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-hydrogel: A hybrid biomaterial system for localized drug delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Loh, X.J. Nanoparticle-hydrogel composites. Concept, design, and applications of these promising, multifunctional materials. Adv. Sci. (Weinh) 2015, 2, 1–12. [Google Scholar] [CrossRef]
- Cantaert, B.; Beniash, E.; Meldrum, F.C. Nanoscale confinement controls the crystallization of calcium phosphate: Relevance to bone formation. Chemistry 2013, 19, 14918–14924. [Google Scholar] [CrossRef]
- Ping, H.; Xie, H.; Wan, Y.; Zhang, Z.; Zhang, J.; Xiang, M.; Xie, J.; Wang, H.; Wang, W.; Fu, Z. Confinement controlled mineralization of calcium carbonate within collagen fibrils. J. Mater. Chem. B 2016, 4, 880–886. [Google Scholar] [CrossRef]
- Risan, J.; Jain, G.; Pendola, M.; Evans, J.S. Intracrystalline incorporation of nacre protein hydrogels modifies the mechanical properties of calcite crystals: A microcompression study. J. Mater. Chem. B 2018, 6, 4191–4196. [Google Scholar] [CrossRef]
- Jain, G.; Pendola, M.; Huang, Y.C.; Gebauer, D.; Koutsoumpeli, E.; Johnson, S.; Evans, J.S. Selective synergism created by interactive nacre framework-associated proteins possessing EGF and vWA motifs. Implications for mollusk shell formation. Biochemistry 2018, 57, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Pendola, M.; Evans, J.S. Non-invasive µCT visualization of mineralization directed by sea urchin- and nacre-specific proteins. Cryst. Growth Des. 2018, 18, 1768–1775. [Google Scholar] [CrossRef]
- Pendola, M.; Evans, J.S. Insights into mollusk shell formation: Interlamellar and lamellar specific nacre protein hydrogels differ in ion interaction signatures. J. Phys. Chem. B 2018, 122, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Huang, Y.C.; Colas, J.J.; Gebauer, D.; Johnson, S.; Evans, J.S. Functional prioritization and hydrogel regulation phenomena created by a combinatorial pearl-associated 2-protein biomineralization model system. Biochemistry 2017, 56, 3607–3618. [Google Scholar] [CrossRef] [PubMed]
- Perovic, I.; Davidyants, A.; Evans, J.S. Aragonite-associated mollusk shell protein aggregates to form mesoscale “smart” hydrogels. ACS Omega 2016, 1, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Pendola, M.; Jain, G.; Davidyants, A.; Huang, Y.C.; Gebauer, D.; Evans, J.S. A nacre protein forms mesoscale hydrogels that “hijack” the biomineralization process within a seawater environment. Cryst. Eng. Commun. 2016, 18, 7675–7679. [Google Scholar] [CrossRef]
- Chang, E.P.; Roncal-Herrero, T.; Morgan, T.; Dunn, K.E.; Rao, A.; Kunitake, J.A.M.R.; Lui, S.; Bilton, M.; Estroff, L.A.; Kroeger, R.; et al. Synergistic biomineralization phenomena created by a nacre protein model system. Biochemistry 2016, 55, 2401–2410. [Google Scholar] [CrossRef]
- Chang, E.P.; Perovic, I.; Rao, A.; Cölfen, H.; Evans, J.S. Insect cell glycosylation and its impact on the functionality of a recombinant intracrystalline nacre protein, AP24. Biochemistry 2016, 55, 1024–1035. [Google Scholar] [CrossRef]
- Chang, E.P.; Evans, J.S. Pif97, a von Willebrand and Peritrophin biomineralization protein organizes mineral nanoparticles and creates intracrystalline nanochambers. Biochemistry 2015, 54, 5348–5355. [Google Scholar] [CrossRef]
- Chang, E.P.; Williamson, G.; Evans, J.S. Focused ion beam tomography reveals the presence of micro, meso, and microporous intracrystalline regions introduced into calcite by the gastropod nacre protein AP7. Cryst. Growth Des. 2015, 15, 1577–1582. [Google Scholar] [CrossRef]
- Perovic, I.; Chang, E.P.; Verch, A.; Rao, A.; Cölfen, H.; Kroeger, R.; Evans, J.S. An oligomeric C-RING nacre protein influences pre-nucleation events and organizes mineral nanoparticles. Biochemistry 2014, 53, 7259–7268. [Google Scholar] [CrossRef]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kroeger, R.; Estroff, L.A.; Evans, J.S. Engineering of crystal surfaces and subsurfaces by an intracrystalline biomineralization protein. Biochemistry 2014, 53, 4317–4319. [Google Scholar] [CrossRef]
- Chang, E.P.; Russ, J.A.; Verch, A.; Kroeger, R.; Estroff, L.A.; Evans, J.S. Engineering of crystal surfaces and subsurfaces by framework biomineralization protein phases. Cryst. Eng. Commun. 2014, 16, 7406–7409. [Google Scholar] [CrossRef]
- Perovic, I.; Chang, E.P.; Lui, M.; Rao, A.; Cölfen, H.; Evans, J.S. A framework nacre protein, n16.3, self-assembles to form protein oligomers that participate in the post-nucleation spatial organization of mineral deposits. Biochemistry 2014, 53, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Perovic, I.; Mandal, T.; Evans, J.S. A pseudo EF-hand pearl protein self-assembles to form protein complexes that amplify mineralization. Biochemistry 2013, 52, 5696–5703. [Google Scholar] [CrossRef]
- Amos, F.F.; Ndao, M.; Ponce, C.B.; Evans, J.S. A C-RING-like domain participates in protein self-assembly and mineral nucleation. Biochemistry 2011, 50, 8880–8887. [Google Scholar] [CrossRef]
- Amos, F.F.; Ponce, C.B.; Evans, J.S. Formation of framework nacre polypeptide supramolecular assemblies that nucleate polymorphs. Biomacromolecules 2011, 12, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Ponce, C.B.; Evans, J.S. Oligomer formation, metalation, and the existence of aggregation-prone and mobile sequences within the intracrystalline protein family, Asprich. Faraday Discuss. 2012, 159, 449–462. [Google Scholar] [CrossRef]
- Pendola, M.; Jain, G.; Huang, Y.-C.; Gebauer, D.; Evans, J.S. Secrets of the sea urchin spicule revealed: Protein cooperativity is responsible for ACC transformation, intracrystalline incorporation, and guided mineral particle assembly in biocomposite material formation. ACS Omega 2018, 3, 11823–11830. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Koutsoumpeli, E.; Johnson, S.; Evans, J.S. Glycosylation fosters interactions between model sea urchin spicule matrix proteins. Implications for embryonic spiculogenesis and biomineralization. Biochemistry 2018, 57, 3032–3035. [Google Scholar] [CrossRef] [PubMed]
- Pendola, M.; Davidyants, A.; Jung, Y.S.; Evans, J.S. Sea urchin spicule matrix proteins form mesoscale hydrogels that exhibit selective ion interactions. ACS Omega 2017, 2, 6151–6158. [Google Scholar] [CrossRef]
- Jain, G.; Pendola, M.; Huang, Y.C.; Gebauer, D.; Evans, J.S. A model sea urchin spicule matrix protein, rSpSM50, is a hydrogelator that modifies and organizes the mineralization process. Biochemistry 2017, 56, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Rao, A.; Cölfen, H.; Evans, J.S. A model sea urchin spicule matrix protein self-associates to form mineral-modifying protein hydrogels. Biochemistry 2016, 55, 4410–4421. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergström, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallization. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Berg, J.K.; Kellermeier, M.; Gebauer, D. Sweet on biomineralization: Effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Mineral. 2014, 26, 537–552. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergström, L.; Tai, C.W.; Sham, T.K.; et al. Proto-calcite and Proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef]
- Kellermeier, M.; Cölfen, H.; Gebauer, D. Investigating the early stages of mineral precipitation by potentiometric titration and analytical ultracentrifugation. In Methods in Enzymology: Research Methods in Biomineralization Science; De Yoreo, J.J., Ed.; Academic Press: New York, NY, USA, 2013; Volume 532, pp. 45–69. [Google Scholar]
- Evans, J.S. Aragonite-associated biomineralization proteins contain interactive and aggregation-prone motifs. Bioinformatics 2012, 28, 3182–3185. [Google Scholar] [CrossRef]
- Ingersoll, E.P.; Wilt, F.H. Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev. Biol. 1998, 196, 95–106. [Google Scholar] [CrossRef]



© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.S. Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles. Materials 2019, 12, 581. https://doi.org/10.3390/ma12040581
Evans JS. Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles. Materials. 2019; 12(4):581. https://doi.org/10.3390/ma12040581
Chicago/Turabian StyleEvans, John Spencer. 2019. "Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles" Materials 12, no. 4: 581. https://doi.org/10.3390/ma12040581
APA StyleEvans, J. S. (2019). Composite Materials Design: Biomineralization Proteins and the Guided Assembly and Organization of Biomineral Nanoparticles. Materials, 12(4), 581. https://doi.org/10.3390/ma12040581