Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PAA-AM/FA Superabsorbent Composite
2.3. Measurement of Water Absorbency
2.4. Measurement of Water Retention
2.4.1. Water Retention under Pressure
2.4.2. Water Retention at High Temperature
2.5. Characterization
3. Results and Discussion
3.1. Chemical Composition Analysis of Fly Ash
3.2. Optimization of Polymerization Conditions
3.2.1. Effect of Polymerization Temperature on Water Absorbency of PAA-AM/FA in 0.9% NaCl Aqueous Solution
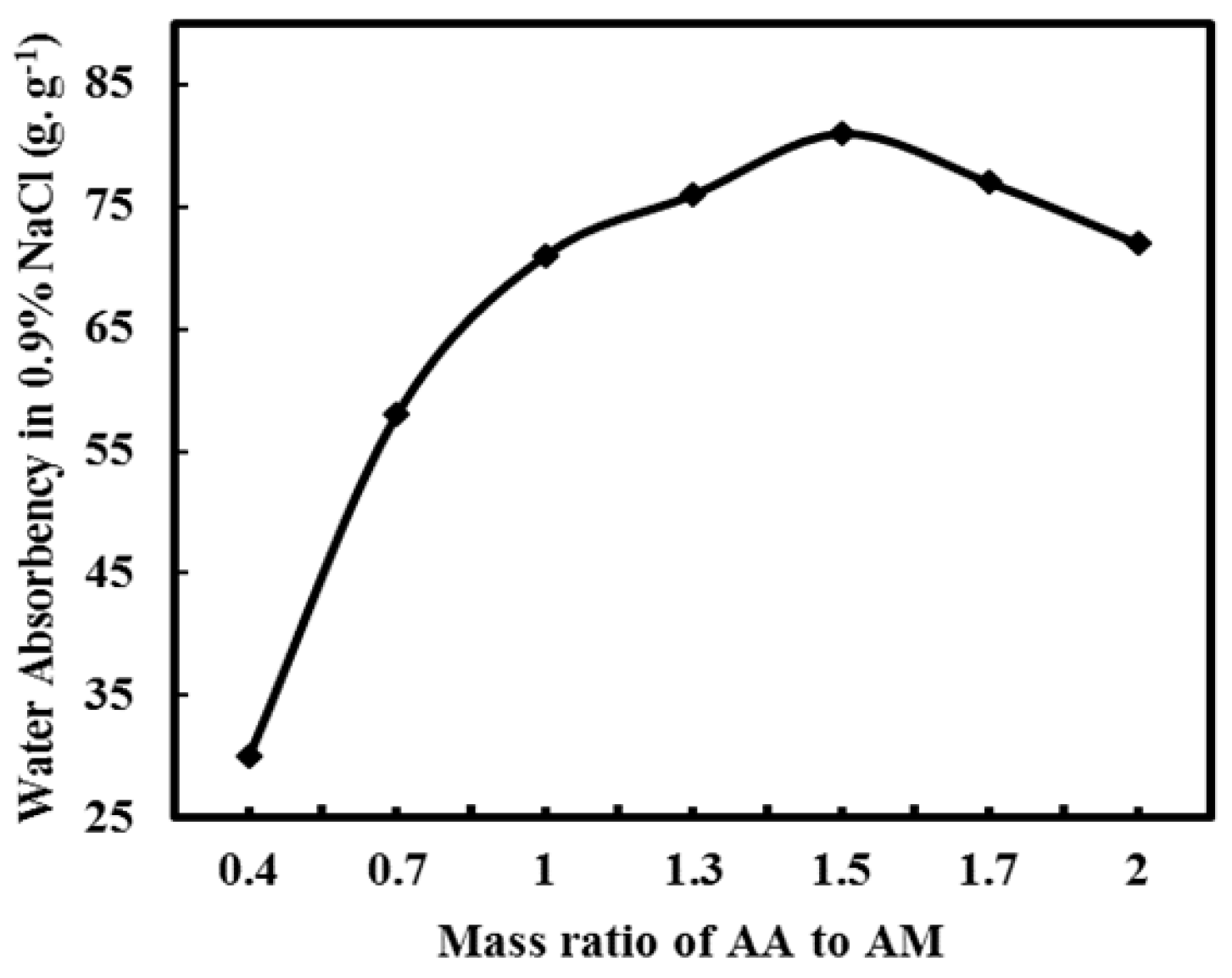
3.2.2. Effect of Mass Ratio of AA/AM on Water Absorbency of PAA-AM/FA in 0.9% NaCl Aqueous Solution
3.2.3. Effect of Neutralization Degree of AA on Water Absorbency of PAA-AM/FA in 0.9% NaCl Aqueous Solution
3.2.4. Effect of Crosslinker Concentration on Water Absorbency of PAA-AM/FA in 0.9% NaCl Aqueous Solution
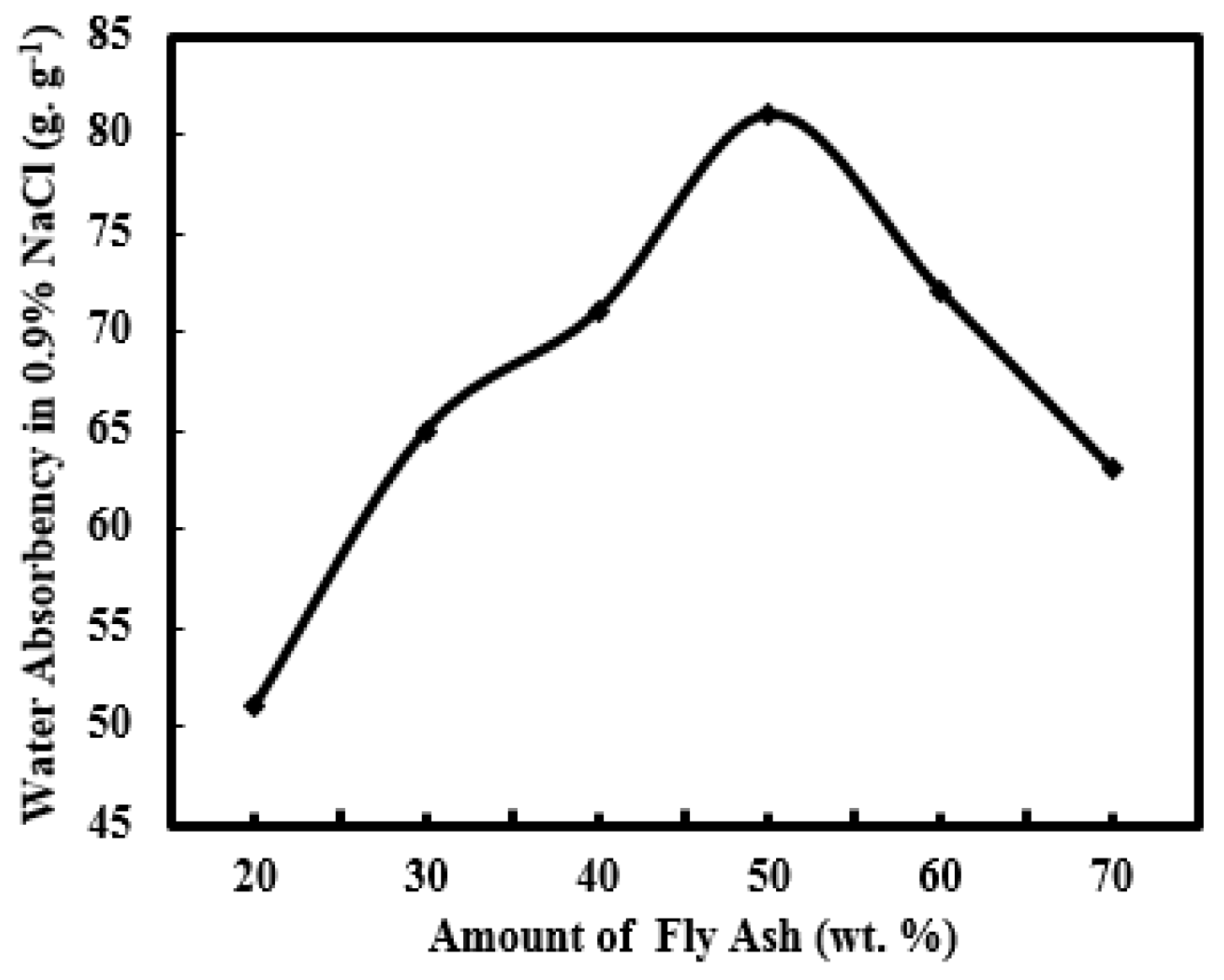
3.2.5. Effect of FA Concentration on Water Absorbency of PAA-AM/FA in 0.9% NaCl Aqueous Solution
3.3. FT-IR Spectra
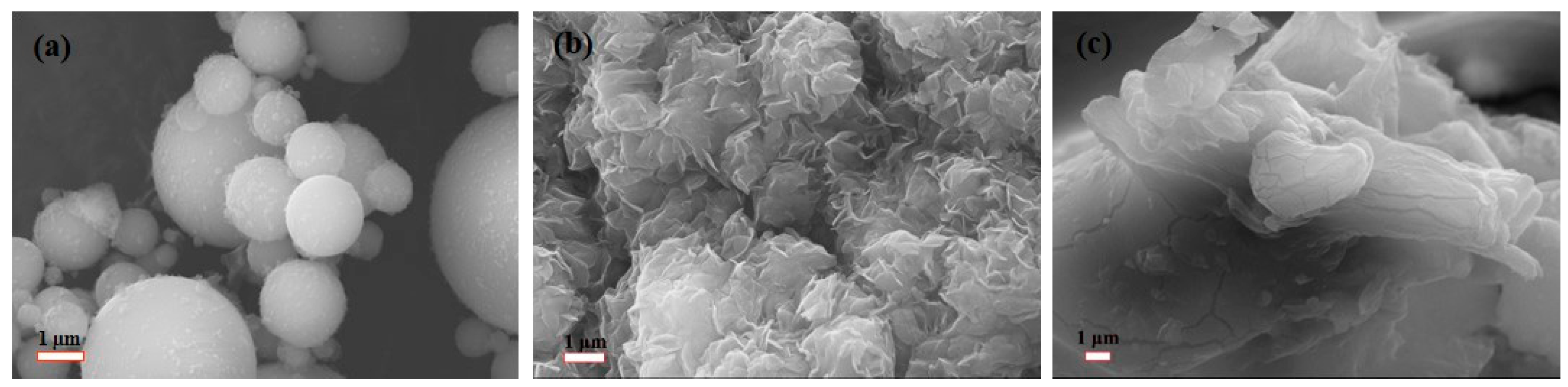
3.4. Morphology Analysis
3.5. Water Absorbency Study
3.6. Water Retention
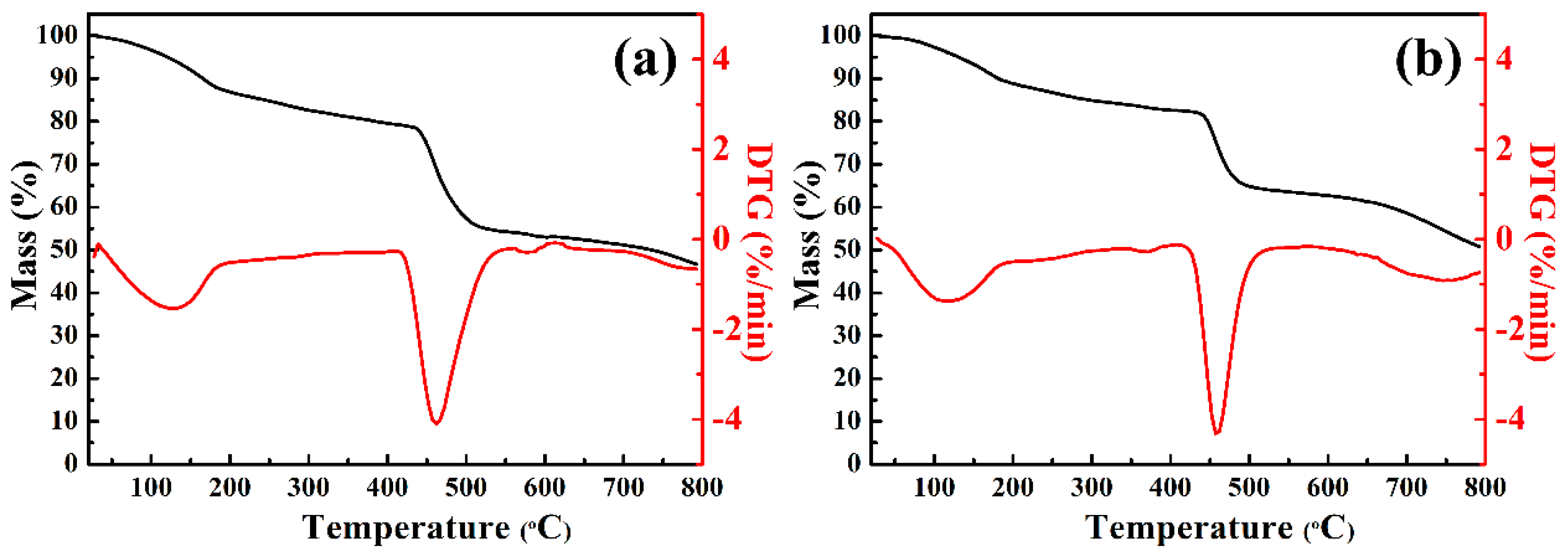
3.7. Thermal Stability
3.8. Adsorption of Rhodamine B in water by PAA-AM/FA
3.9. Preparation of Hybrid Organic-Inorganic Composite PAA-AM/FA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zimmer, A.; Bergmann, C.P. Fly ash of mineral coal as ceramic tiles raw material. Waste Manage. 2007, 27, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Haynes, R.J. Reclamation and revegetation of fly ash disposal sites-Challenges and research needs. J. Environ. Manag. 2009, 90, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Rhee, K.Y. Superabsorbent nanocomposite (alginate-g-PAMPS/MMT): Synthesis, characterization and swelling behavior. Carbohydr. Polym. 2012, 90, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Si, C.; Fatehi, P. Enhancement in biological treatment of pulping wastewater by fly ash. Chemosphere 2018, 210, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Asl, S.M.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Clean. Prod. 2019, 208, 1131–1147. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sen, S. Efficient removal of textile dye using nanosized fly ash derived zeolite-x: Kinetics and process optimization study. J. Taiwan Inst. Chem. E. 2018, 16, 43–53. [Google Scholar] [CrossRef]
- Ahad, M.Z.; Ashraf, M.; Kumar, R.; Ullah, M. Thermal, Physico-Chemical, and Mechanical Behaviour of Mass Concrete with Hybrid Blends of Bentonite and Fly Ash. Materials 2019, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.G.; Jia, L.; Wang, Y.L.; Zhao, R.; Mei, X.S.; Liu, Y.Y.; Jin, Y. Study on Adsorption Mechanism and Failure Characteristics of CO2 Adsorption by Potassium-Based Adsorbents with Different Supports. Materials 2018, 11, 2424. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, P.; Zhang, J.; Wang, A. Study on superabsorbent composite XVI. Synthesis, characterization and swelling behaviors of poly(sodium acrylate)/vermiculite superabsorbent composites. Eur. Polym. J. 2007, 43, 1691–1698. [Google Scholar] [CrossRef]
- Lomthong, T.; Hanphakphoom, S.; Kongsaeree, P.; Srisuk, N.; Guicherd, M.; Cioci, G.; Duquesne, S.; Marty, A.; Kitpreechavanich, V. Enhancement of poly(L-lactide)-degrading enzyme production by Laceyella sacchari LP175 using agricultural crops as substrates and its degradation of poly(L-lactide) polymer. Polym. Degrad. Stab. 2017, 143, 64–73. [Google Scholar] [CrossRef]
- Black-Solis, J.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.; Corona-Rangel, M.L.; Bautista-Baños, S. Preharvest use of biodegradable polyester nets added with cinnamon essential oil and the effect on the storage life of tomatoes and the development of Alternaria alternata. Sci. Hortic-Amsterdam 2019, 245, 65–73. [Google Scholar] [CrossRef]
- Amonpattaratkit, P.; Khunmanee, S.; Kim, D.H.; Park, H. Synthesis and Characterization of Gelatin-Based Crosslinkers for the Fabrication of Superabsorbent Hydrogels. Materials 2017, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2013, 7, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Riche, F.; Schneebeli, M.; Tschanz, S.A. Design-based stereology to quantify structural properties of artificial and natural snow using thin sections. Cold Reg. Sci. Technol. 2012, 79, 67–74. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Zillohu, A.U.; Abdelaziz, R.; Hedayati, M.K.; Elbahri, M. A Novel Nanohybrid Nanofibrous Adsorbent for Water Purification from Dye Pollutants. Materials 2016, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.-G.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and Characterization of Superabsorbent Polymers Based on Starch Aldehydes and Carboxymethyl Cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Luo, M.-T.; Huang, C.; Li, H.-L.; Guo, H.-J.; Chen, X.-F.; Xiong, L.; Chen, X.-D. Bacterial cellulose based superabsorbent production: A promising example for high value-added utilization of clay and biology resources. Carbohydr. Polym. 2019, 208, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.G.; Gessner, F.; Schmitt, C.C.; Sartori, R. Influence of the Layer Charge and Clay Particle Size on the Interactions between the Cationic Dye Methylene Blue and Clays in an Aqueous Suspension. J. Colloid Interf. Sci. 2002, 255, 254–259. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Adsorption characteristics of brilliant green dye on kaolin. J. Hazard. Mater. 2009, 161, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, A.; Wang, A. Study on superabsorbent composite. VI. Preparation, characterization and swelling behaviors of starch phosphate-graft-acrylamide/attapulgite superabsorbent composite. Carbohydr. Polym. 2006, 65, 150–158. [Google Scholar] [CrossRef]
- Li, A.; Wang, A.; Chen, J. Studies on poly(acrylic acid)attapulgite superabsorbent composite. I. Synthesis and characterization. J. Appl. Polym. Sci. 2004, 92, 1596–1603. [Google Scholar] [CrossRef]
- Wu, J.; Lin, J.; Zhou, M.; Wei, C. Synthesis and properties of starch-graft-polyacrylamide/clay superabsorbent composite. Macromol. Rapid Commun. 2000, 21, 1032–1034. [Google Scholar] [CrossRef]
- Lin, J.; Wu, J.; Yang, Z.; Pu, M. Synthesis and Properties of Poly(acrylic acid)/Mica Superabsorbent Nanocomposite. Macromol. Rapid Commun. 2001, 22, 422–424. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, M.; Shangguan, L. Synthesis of a Novel and Salt Sensitive Superabsorbent Hydrogel Using Soybean Dregs by UV-Irradiation. Materials 2018, 11, 2198. [Google Scholar] [CrossRef]
- Temuujin, J.; Surenjav, E.; Ruescher, C.H.; Vahlbruch, J. Processing and uses of fly ash addressing radioactivity (critical review). Chemosphere 2019, 216, 866–882. [Google Scholar] [CrossRef]
- Gao, J.; Wang, A.; Li, Y.; Fu, Y.; Wu, J.; Wang, Y.; Wang, Y. Synthesis and characterization of superabsorbent composite by using glow discharge electrolysis plasma. React. Funct. Polym. 2008, 68, 1377–1383. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F. A new approach for blending waste plastics processing: Superabsorbent resin synthesis. J. Clean. Prod. 2018, 197, 501–510. [Google Scholar] [CrossRef]
- Zhang, J.; Li, A.; Wang, A. Study on superabsorbent composite. V. Synthesis, swelling behaviors and application of poly(acrylic acid-co-acrylamide)/sodium humate/attapulgite superabsorbent composite. Polym. Adv. Technol. 2005, 16, 813–820. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, J.; Zhang, Q.; Li, S.; Chen, H. Synthesis and Characterization of Prussian Blue Modified Magnetite Nanoparticles and Its Application to the Electrocatalytic Reduction of H2O2. Chem. Mater. 2005, 17, 3154–3159. [Google Scholar] [CrossRef]
- Hassan, M.S. Removal of reactive dyes from textile wastewater by immobilized chitosan upon grafted Jute fibers with acrylic acid by gamma irradiation. Radiat. Phys. Chem. 2015, 115, 55–61. [Google Scholar] [CrossRef]
- Hu, S.; Guan, X.; Ding, Q. Research on optimizing components of microfine high-performance composite cementitious materials. Cem. Concr. Res. 2002, 32, 1871–1875. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2007, 70, 166–173. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Aouaz, N.; Dairi, N. Environmental-sensitive chitosan-g-polyacrylamide/carboxymethylcellulose superabsorbent composites for wastewater purification I: synthesis and properties. Polym. Bull. 2016, 73, 815–840. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Bybordi, A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahaman, M.S.; Yeum, J.H. Electrospun novel super-absorbent based on polysaccharide-polyvinyl alcohol-montmorillonite clay nanocomposites. Carbohydr. Polym. 2015, 115, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Kim, T.-H.; Lee, B. Syntheses of carboxymethylcellulose/graphene nanocomposite superabsorbent hydrogels with improved gel properties using electron beam radiation. Macromol. Res. 2016, 24, 143–151. [Google Scholar] [CrossRef]
- Ghazy, M.B.; El-Hai, F.A.; Mohamed, M.F.; Essawy, H.A. Potassium fulvate as co-interpenetrating agent during graft polymerization of acrylic acid from cellulose. Int. J. Biol. Macromol. 2016, 91, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, H.; Li, Y.; Lu, Q.; Wang, Q.; Yang, W. Synthesis, characterization, and property testing of PGS/P(AMPS-co-AM) superabsorbent hydrogel initiated by glow-discharge electrolysis plasma. Colloid Polym. Sci. 2016, 294, 257–270. [Google Scholar] [CrossRef]
- Olad, A.; Pourkhiyabi, M.; Gharekhani, H.; Doustdar, F. Semi-IPN superabsorbent nanocomposite based on sodium alginate and montmorillonite: Reaction parameters and swelling characteristics. Carbohydr. Polym. 2018, 190, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Limparyoon, N.; Seetapan, N.; Kiatkamjornwong, S. Acrylamide/2-acrylamido-2-methylpropane sulfonic acid and associated sodium salt superabsorbent copolymer nanocomposites with mica as fire retardants. Polym. Degrad. Stab. 2011, 96, 1054–1063. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite superabsorbent composites. Carbohydr. Polym. 2007, 68, 367–374. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaith, B.S.; Kumar, V.; Som, S.; Kalia, S.; Swart, H.C. A study of the biodegradation behaviour of poly(methacrylic acid/aniline)-grafted gum ghatti by a soil burial method. RSC Adv. 2014, 4, 25637–25649. [Google Scholar] [CrossRef]








| Superabsorbent Composite | Q (g·g−1) | |
|---|---|---|
| Distilled Water | 0.9% NaCl | |
| PAA-AM/FA | 976 | 81 |
| PAA-AM | 1019 | 49 |
| Superabsorbent Composites | Centrifuging Time (min) | |||
|---|---|---|---|---|
| 10 | 20 | 30 | 50 | |
| PAA-AM/FA | 99% | 98% | 97% | 96% |
| PAA-AM | 98% | 96% | 94% | 93% |
| Superabsorbent Composites | Heating Time (h) | |||||
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 11 | |
| PAA-AM/FA | 99% | 97% | 95% | 92% | 89% | 86% |
| PAA-AM | 96% | 94% | 91% | 87% | 80% | 73% |
| Compound | Na2O | K2O | Al2O3 | SiO2 | P2O5 | SO3 | CaO | TiO2 | MgO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass (wt. %) | 3.045 | 2.108 | 21.82 | 52.88 | 0.220 | 0.827 | 8.08 | 0.86 | 1.929 | 5.62 |
| Superabsorbent Composite | Q (g·g−1) | Reference | |
|---|---|---|---|
| Distilled Water | 0.9% NaCl | ||
| PAA-AM/MMT | 1024 | 56 | [31] |
| PAA/SH/CTS | 183 | 41 | [37] |
| PAA/CTS/Cell | 390 | 40 | [38] |
| PAM/CMC/CTS | 500 | 58 | [39] |
| PAA-AM/NaAlg/RHA | 830 | 36 | [40] |
| PULL/PVA/MMT | 143 | 40 | [41] |
| PCMC/AC | 142.4 | 69 | [42] |
| PAA/KF/Cell | 422 | 49 | [43] |
| PAMPS-AM/PGS | 652.6 | 69 | [44] |
| PAA-AM | 1019 | 49 | This work |
| PAA-AM/FA | 976 | 81 | This work |
| PAA-AM/FA | PAA-AM | FA |
|---|---|---|
| 148 | 7.50 | 2.60 |
| Superabsorbent Composite | Dye Feed Concentration (mg·L−1) | Adsorption Performance mg·g−1 Resin | Reference |
|---|---|---|---|
| PHEMA-GMA | 1000 | 76.8 | [24] |
| PAA-VP | 40 | 4.1 | [40] |
| PAA-AM | 50 | 6.4 | [42] |
| Jute stick | 50 | 4.6 | [44] |
| PAM-HEMA | 0.25–3 | 0.15 | [45] |
| PAM-HEMA | 500 | 257 | [45] |
| PAA-AM/FA | 100 | 148 | This work |
| PAA-AM/FA | 500 | 366 | This work |
| PAA-AM | 50 | 7.5 | This work |
| Concentration (mg·L−1) | PAA-AM | FA | PAA-AM/FA |
|---|---|---|---|
| Initial Conc. | 100 | 100 | 100 |
| 2 min later | 89.0 | 95.8 | 1.90 |
| 10 min later | 85.0 | 95.1 | 0 |
| 1 h later | 84.3 | 94.7 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhang, Y.; Wang, P.; Yang, Z.; Yasin, A.; Zhang, L. Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite. Materials 2019, 12, 596. https://doi.org/10.3390/ma12040596
Zhu W, Zhang Y, Wang P, Yang Z, Yasin A, Zhang L. Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite. Materials. 2019; 12(4):596. https://doi.org/10.3390/ma12040596
Chicago/Turabian StyleZhu, Wenjuan, Yagang Zhang, Penglei Wang, Zhiyong Yang, Akram Yasin, and Letao Zhang. 2019. "Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite" Materials 12, no. 4: 596. https://doi.org/10.3390/ma12040596
APA StyleZhu, W., Zhang, Y., Wang, P., Yang, Z., Yasin, A., & Zhang, L. (2019). Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite. Materials, 12(4), 596. https://doi.org/10.3390/ma12040596






