“Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts
Abstract
:1. Introduction
2. Sol-Gel Chemistry
2.1. Fundamental Features
- Preparation of the solution of precursors.
- Hydrolysis and partial condensation of alkoxides to form a “sol”.
- Formation of the gel via polycondensation of hydrolyzed precursors.
- Drying. The gel forms a dense “xerogel” via collapse of the porous network caused by the evaporation of the solvent (or an aerogel for example through supercritical drying).
- Calcination to obtain mechanically stable materials.
2.2. Gelation: Hydrolysis and Polycondensation
2.3. Precursors (M and -OR)
2.4. Converting A Wet Gel into A Dry Solid
3. Sol-Gel Chemistry: The Evolution and New Perspectives
3.1. Sol-Gel Routes for Bio-Catalysts
3.2. Nonhydrolytic Sol-Gel Chemistry
3.3. Modified Pechini Method
4. Porosity: The Role of Water/TEOS Ratio
5. Supported Metal and Mixed Oxide Systems Synthesized by “Traditional” Sol-Procedures: Some Examples
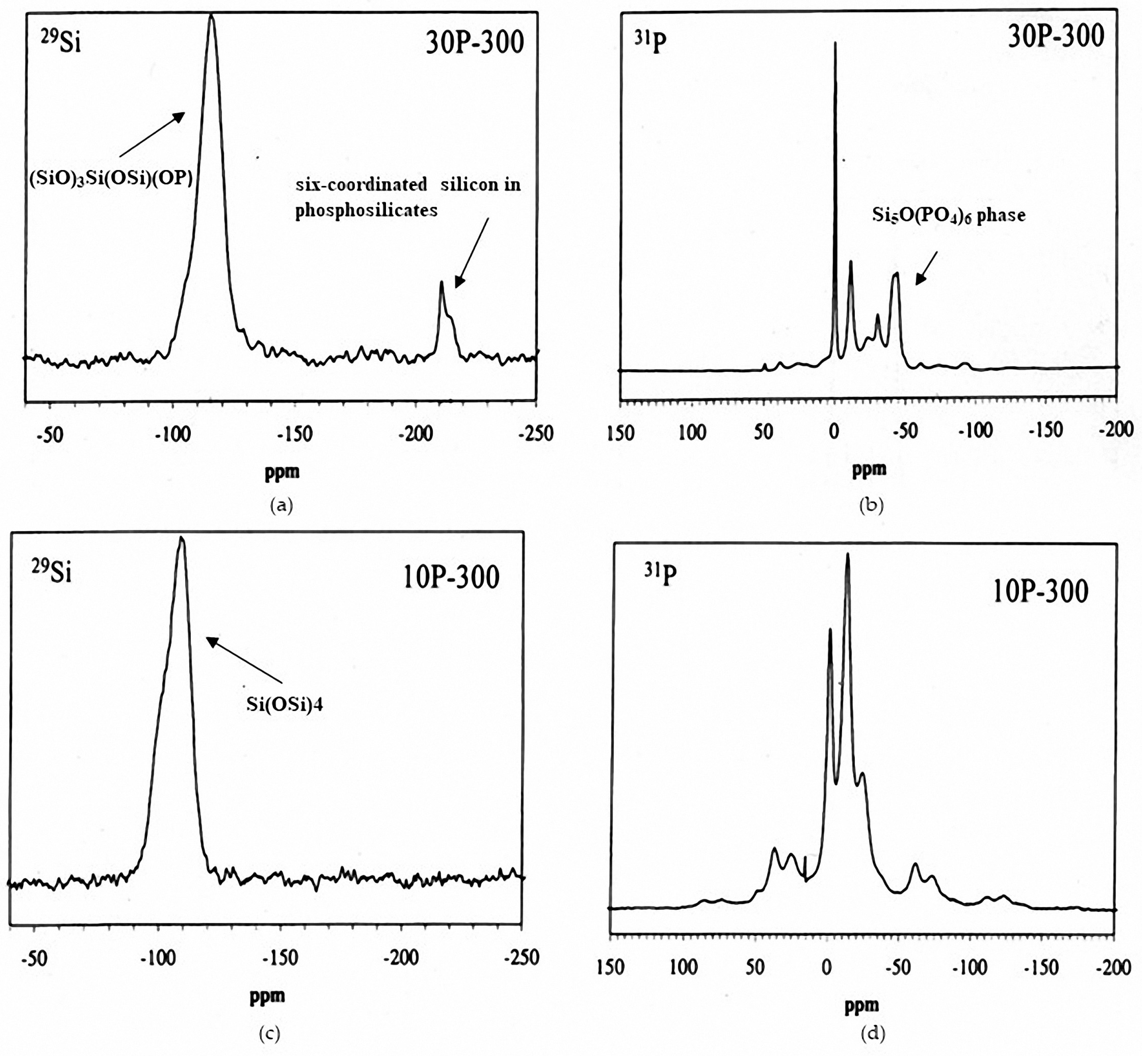
5.1. SiO2–P2O5
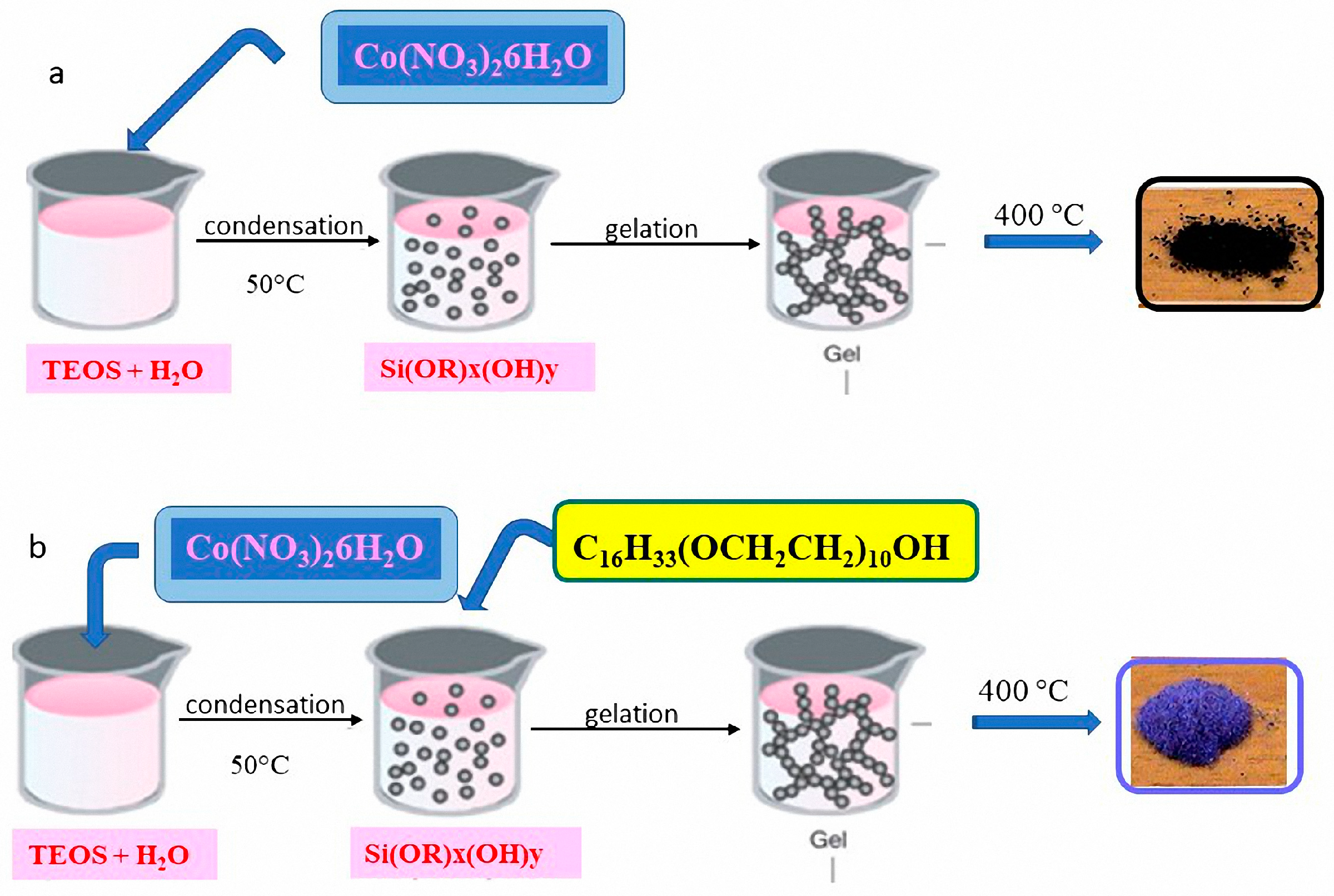
5.2. Co–SiO2
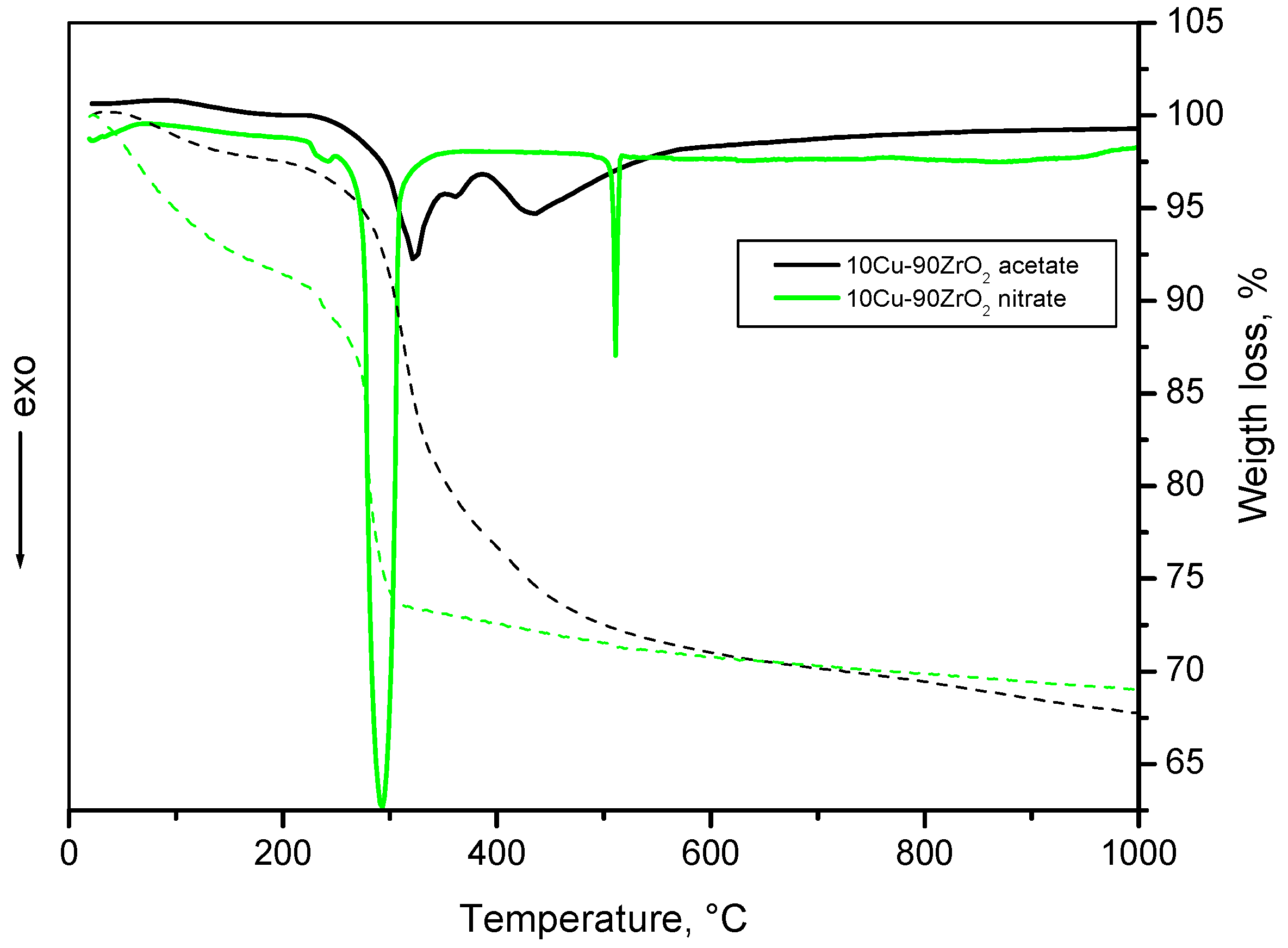
5.3. Cu–ZrO2
6. Conclusions
Funding
Conflicts of Interest
References
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar]
- Satterfield, C.N. Heterogeneous Catalysis in Industrial Practice, 2nd ed.; McGraw-Hill Inc.: New York, NY, USA, 1991. [Google Scholar]
- Sui, R.; Charpentier, P. Synthesis of metal oxide nanostructure by direct sol-gel chemistry in supercritical fluids. Chem. Rev. 2011, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.A.; Ko, E.I. Preparing catalytic materials by the sol-gel method. Ind. Eng. Res. 1995, 34, 421–433. [Google Scholar] [CrossRef]
- Cauqui, M.A.; Rodriguez-Izquierdo, J.M. Application of the sol-gel methods to catalyst preparation. J. Non-Cryst. Solids 1992, 147–148, 724–738. [Google Scholar] [CrossRef]
- Lee, D.W.; Yoo, B.R. Advanced metal oxide (supported) catalysts: Synthesis and applications. J. Ind. Eng. Chem. 2014, 20, 3947–3959. [Google Scholar] [CrossRef]
- Carrier, X.; Royer, S.; Marceau, E. Synthesis of metal oxide catalysts. In Metal Oxides in Heterogeneous Catalysis, 1st ed.; Védrine, J.C., Ed.; Elsevier: Amsterdam, The Netherland, 2018; pp. 43–103. [Google Scholar]
- Grasselli, R.K.; Sleight, A.W. Structure–Activity and Selectivity Relationships in Heterogeneous Catalysis, 1st ed.; Elsevier: New York, NY, USA, 1991. [Google Scholar]
- Minieri, L.; Esposito, S.; Russo, V.; Bonelli, B.; Di Serio, M.; Silvestri, B.; Vergara, A.; Aronne, A. A new sol-gel Ru–Nb–Si mixed-oxides bifunctional catalyst for the hydrogenation of levulinic acid in aqueous phase. ChemCatChem 2017, 9, 1476–1486. [Google Scholar] [CrossRef]
- Bañares, M.A. Supported metal oxide and other catalysts for ethane conversion: A review. Catal. Today 1999, 51, 319–348. [Google Scholar] [CrossRef]
- Bainbridge, M.; Clarkson, J.S.; Parnham, B.L.; Tabatabaei, J.; Tyers, D.V.; Waugh, K.C. Evidence for support effects in metal oxide supported cobalt catalysts. Catal. Struct. React. 2017, 3, 128–137. [Google Scholar] [CrossRef]
- Campanati, M.; Fornasari, G.; Vaccari, A. Fundamentals in the preparation of heterogeneous catalysts. Catal. Today 2003, 77, 299–314. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol-gel” chemistry as a technique for material synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press Inc: New York, NY, USA, 1990. [Google Scholar]
- Levy, D.; Zayat, M. The Sol-Gel Handbook: Synthesis, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol-gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Highly Ordered Mesoporous TiO2–Fe2O3 mixed oxide synthesized by sol-gel pathway: An efficient and reusable heterogeneous catalyst for dehalogenation reaction. ACS Appl. Mater. Interfaces 2012, 4, 5022–5028. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.S.; Shchukin, D.G.; Ustinovich, E.; Antonietti, M.; Caruso, R.A.; Shchukin, D. Titania and mixed titania/aluminum, gallium or indium oxide spheres: Sol-gel/template synthesis and photocatalytic properties. Adv. Funct. Mater. 2005, 15, 239–245. [Google Scholar] [CrossRef]
- Lannoy, A.; Bleta, R.; Machut, C.; Monflier, E.; Ponchel, A. Block copolymer–cyclodextrin supramolecular assemblies as soft templates for the synthesis of titania materials with controlled crystallinity, porosity and photocatalytic activity. RSC Adv. 2014, 4, 40061–40070. [Google Scholar] [CrossRef]
- Sarkar, K.; Rawolle, M.; Herzig, E.M.; Wang, W.; Buffet, A.; Roth, S.V.; Müller-Buschbaum, P. Custom-made morphologies of ZnO nanostructured films templated by a poly(styrene-block-ethylene oxide) diblock copolymer obtained by a sol-gel technique. ChemSusChem 2013, 6, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, S.; Baldinozzi, G.; Friedrich, D.; Kressman, S.; Strub, H.; Artero, V.; Laberty-Robert, C. Mesoporous thin film WO3 photoanode for photoelectrochemical water splitting: A sol-gel dip coating approach. Sustain. Energy Fuels 2017, 1, 145–153. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Alifanti, M.; Baps, B.; Blangenois, N.; Naud, J.; Grange, P.; Delmon, B. Characterization of CeO2–ZrO2 mixed oxides. Comparison of the citrate and sol-gel preparation methods. Chem. Mater. 2003, 15, 395–403. [Google Scholar] [CrossRef]
- Ebelmen, J.J. Untersuchungen über die verbindung der barsaure und kieselsaure mit aether. Ann. Chim. Phys. Ser. 1846, 57, 319–355. [Google Scholar]
- Schroeder, H. Oxide layer deposited from organic solution. Phys. Thin Film 1969, 5, 87–141. [Google Scholar]
- Graham, T. On the properties of silicic acid and other analogous substances. J. Chem. Soc. 1864, 17, 318–327. [Google Scholar] [CrossRef]
- Hurd, C.B. Theories for the mechanism of the setting of silicic acid gels. Chem. Rev. 1938, 22, 403–422. [Google Scholar] [CrossRef]
- Nijenhuis, K.T. Thermoreversible Networks: Viscoelastic Properties and Structure of Gels; Springer: Berlin, Germany, 1997. [Google Scholar]
- Sakka, S.; Kozuka, H. Handbook of Sol-Gel Science and Technology. 1 Sol-Gel Processing, 1st ed.; Kluwer Academic Publishing: Norwell, MA, USA, 2005. [Google Scholar]
- Pope, E.J.A.; Mackenzie, J.D. Sol-gel processing of silica: The role of the catalysts. J. Non-Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Hook, R.J. A 29Si NMR study of the sol-gel polymerisation rates of substituted ethoxysilanes. J. Non-Cryst. Solids 1996, 195, 1–15. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Ilharco, L.M.; Pagliaro, M. Role of the alkyl-alkoxide precursors on the structure and catalytic properties of hybrid sol-gel catalysts. Chem. Mater. 2005, 17, 6686–6694. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Progr. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Kandimalla, V.B.; Tripathi, V.S.; Ju, H. Immobilization of biomolecules in sol-gels: Biological and analytical applications. Crit. Rev. Anal. Chem. 2006, 36, 73–106. [Google Scholar] [CrossRef]
- Harper, J.C.; Lopez, D.M.; Larkin, E.C.; Economides, M.K.; McIntyre, S.K.; Alam, T.M.; Tartis, M.S.; Werner-Washburne, M.; Brinker, C.J.; Brozik, S.M.; et al. Encapsulation of S. cerevisiae in poly(glycerol) silicate derived matrices: Effect of matrix additives and cell metabolic phase on long-term viability and rate of gene expression. Chem. Mater. 2011, 23, 2555–2564. [Google Scholar]
- Bhatia, R.R.; Brinker, C.J.; Gupta, A.K.; Singh, A.P. Aqueous sol-gel process for protein encapsulation. Chem. Mater. 2000, 12, 2434–2441. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Barnes, C.E.; Pinkas, J. The power of non-hydrolytic sol-gel chemistry: A review. Catalysts 2017, 7, 168. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Abbott, J.G.; Barnes, C.E.; Pinkas, J. Synthesis of homogeneous silicophosphate xerogels by non-hydrolytic condensation reactions. Microporous Mesoporous Mater. 2014, 197, 204–212. [Google Scholar] [CrossRef]
- Styskalik, A.; Skoda, D.; Moravec, Z.; Barnes, C.E.; Pinkas, J. Surface reactivity of non-hydrolytic silicophosphate xerogels: A simple method to create Brønsted or Lewis acid sites on porous supports. New J. Chem. 2016, 40, 3705–3715. [Google Scholar] [CrossRef]
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: A review. Appl. Catal. A Gen. 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form A Capacitor. US Patent 3330697A, 7 November 1967. [Google Scholar]
- Lin, J.; Yu, M.; Lin, C.; Liu, X. Multiform oxide optical materials via the versatile Pechini-type sol-gel process—Synthesis and characteristics. J. Phys. Chem. C 2007, 111, 5835–5845. [Google Scholar] [CrossRef]
- Motta, M.; Deimling, C.V.; Saeki, M.J.; Lisboa-Filho, P.N. Chelating agent effects in the synthesis of mesoscopic-size superconducting particles. J. Sol-Gel Sci. Technol. 2008, 46, 201–207. [Google Scholar] [CrossRef]
- Strawbridge, I.; Craievich, A.F.; James, P.F. The effect of the H2O/TEOS ratio on the structure of gels derived by the acid catalyzed hydrolysis of tetraethoxysilane. J. Non-Cryst. Solids 1985, 72, 139–157. [Google Scholar] [CrossRef]
- Ro, J.C.; Chung, I.J. Structures and properties of silica gels prepared by the sol-gel method. J. Non-Cryst. Solids 1991, 130, 8–17. [Google Scholar]
- Elferink, W.J.; Nair, B.N.; De Vos, R.M.; Keizer, K.; Verweij, H. Sol-gel synthesis and characterization of microporous silica membranes: II. Tailor-making porosity. J. Colloid Interface Sci. 1996, 180, 127–134. [Google Scholar] [CrossRef]
- Yoldas, B.E. Monolithic glass formation by chemical polymerization. J. Mater. Sci. 1979, 14, 1843–1849. [Google Scholar] [CrossRef]
- Sakka, S.; Kamiya, K. The sol-gel transition in the hydrolysis of metal alkoxides in relation to the formation of glass fibers and films. J. Non-Cryst. Solids 1982, 48, 31–46. [Google Scholar] [CrossRef]
- Esposito, S.; Sannino, F.; Pansini, M.; Bonelli, B.; Garrone, E. Modes of interaction of simazine with the surface of model amorphous silicas in water. J. Phys. Chem. C 2013, 117, 11203–11210. [Google Scholar] [CrossRef]
- Sannino, F.; Ruocco, S.; Marocco, A.; Esposito, S.; Pansini, M. Simazine removal from waters by adsorption on porous silicas tailored by sol-gel technique. Microporous Mesoporous Mater. 2013, 180, 178–186. [Google Scholar] [CrossRef]
- Esposito, S.; Sannino, F.; Armandi, M.; Bonelli, B.; Garrone, E. Modes of interaction of simazine with the surface of amorphous silica in water. Part II: Adsorption at temperatures higher than ambient. J. Phys. Chem. C 2013, 117, 27047–27051. [Google Scholar] [CrossRef]
- Sannino, F.; Pansini, M.; Marocco, A.; Bonelli, B.; Garrone, E.; Esposito, S. The role of outer surface/inner bulk Brønsted acidic sites in the adsorption of a large basic molecule (simazine) on H-Y zeolite. Phys. Chem. Chem. Phys. 2015, 17, 28950–28957. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Garrone, E.; Marocco, A.; Pansini, M.; Martinelli, P.; Sannino, F. Application of highly porous materials for simazine removal from aqueous solutions. Environ. Technol. 2016, 37, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Védrine, J.C. Metal oxides in heterogeneous oxidation catalysis: State of the art and challenges for a more sustainable world. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Busca, G. Acid catalysts in industrial hydrocarbon chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Krawietz, T.R.; Lin, P.; Lotterhos, K.E.; Torres, P.D.; Barich, D.H.; Clearfield, A.; Haw, J.F. Solid phosphoric acid catalyst: A multinuclear NMR and theoretical study. J. Am. Chem. Soc. 1998, 120, 8502–8511. [Google Scholar] [CrossRef]
- Fougret, C.; Hölderich, W. Ethylene hydration over metal phosphates impregnated with phosphoric acid. Appl. Catal. A Gen. 2001, 207, 295–301. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Kooshki, B.; Ruoho, A.E. Nitric acid in the presence of supported P2O5 on silica gel: An efficient and novel reagent for oxidation of sulfides to the corresponding sulfoxides. Tetrahedron Lett. 2005, 46, 5503–5506. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Ruoho, A.E. Nitric acid in the presence of P2O5 supported on silica gel—A useful reagent for nitration of aromatic compounds under solvent-free conditions. Tetrahedron Lett. 2005, 46, 8307–8310. [Google Scholar] [CrossRef]
- Eshghi, H.; Gordi, Z. Microwave-Assisted Efficient One-Pot Synthesis of Nitriles from Aldehydes in the Presence of P2O5/SiO2 in Solvent-Free Media. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 619–623. [Google Scholar] [CrossRef]
- Eshghi, H.; Shafieyoon, P. P2O5/SiO2 as a mild and efficient reagent for acylation of alcohols, phenols and amines under solvent-free conditions. J. Chem. Res. 2004, 2004, 802–805. [Google Scholar] [CrossRef]
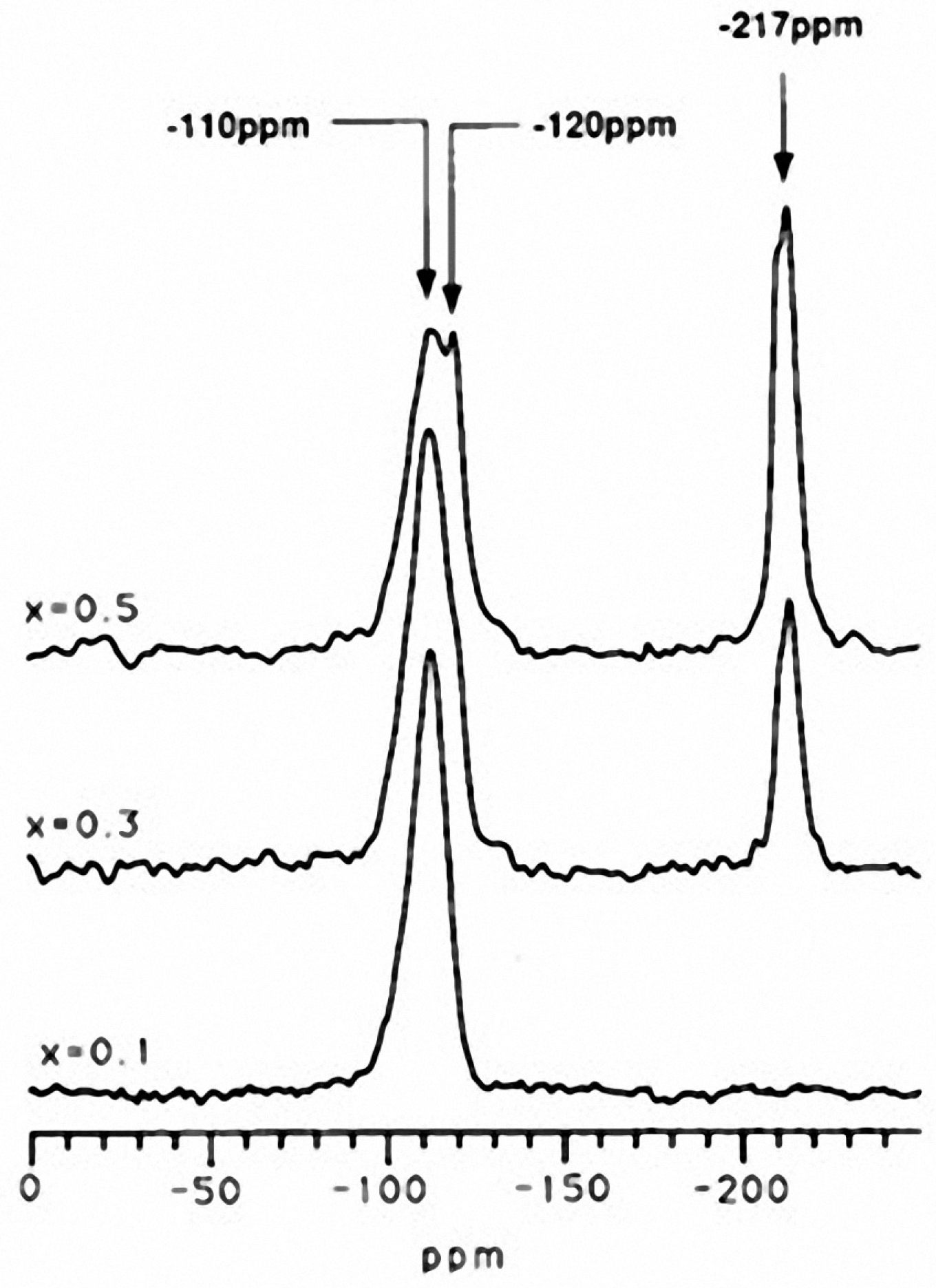
- Clayden, N.J.; Aronne, A.; Esposito, S.; Pernice, P. Solid state NMR study of phosphosilicate gels. J. Non-Cryst. Solids 2004, 345–346, 601–604. [Google Scholar] [CrossRef]
- Livage, J.; Barboux, P.; Vandenborre, M.T.; Schmutz, C.; Taulelle, F. Sol-gel synthesis of phosphates. J. Non-Cryst. Solids 1992, 147–148, 18–23. [Google Scholar] [CrossRef]
- Szu, S.P.; Klein, L.C.; Greenblatt, M. Effect of precursors on the structure of posphosilicate gels: 29Si and 31P MAS-NMR study. J. Non-Cryst. Solids 1992, 143, 21–30. [Google Scholar] [CrossRef]
- Schrotter, J.C.; Cardenas, A.; Smaihi, M.; Hovnanian, N. Silicon and phosphorus alkoxide mixture: sol-gel study by spectroscopy technics. J. Sol-Gel Sci. Technol. 1995, 4, 195–204. [Google Scholar] [CrossRef]
- D’Apuzzo, M.; Aronne, A.; Esposito, S.; Pernice, P. Sol-gel synthesis of humidity-sensitive P2O5–SiO2 amorphous films. J. Sol-Gel Sci. Technol. 2000, 17, 247–254. [Google Scholar] [CrossRef]
- Clayden, N.J.; Esposito, S.; Pernice, P.; Aronne, A. Solid state 29Si and 31P NMR study of gel derived phosphosilicate glasses. J. Mater. Chem. 2001, 11, 936–943. [Google Scholar]
- Clayden, N.J.; Esposito, S.; Pernice, P.; Aronne, A. Solid state 1H NMR study, humidity sensitivity and protonic conduction of gel derived phosphosilicate glasses. J. Mater. Chem. 2002, 12, 3746–3753. [Google Scholar] [CrossRef]
- Clayden, N.J.; Esposito, S.; Aronne, A. Chemical heterogeneity in phosphosilicate gels by NMR magnetisation exchange. J. Chem. Soc. Dalton Trans. 2001, 2003–2008. [Google Scholar] [CrossRef]
- Todan, L.; Anghel, E.M.; Osiceanu, P.; Turcu, R.V.F.; Atkinson, I.; Simon, S.; Zaharescu, M. Structural characterization of some sol-gel derived phosphosilicate glasses. J. Mol. Struct. 2015, 1086, 161–171. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Ramis, G.; Bagnasco, G.; Pernice, P.; Pagliuca, C.; Bevilacqua, M.; Aronne, A. Cobalt–silicon mixed oxide nanocomposites by modified sol-gel method. J. Solid State Chem. 2007, 180, 3341–3350. [Google Scholar] [CrossRef]
- Esposito, S.; Setaro, A.; Maddalena, P.M.; Aronne, A.; Pernice, P.; Laracca, M. Synthesis of cobalt doped silica thin film for low temperature optical gas sensor. J. Sol-Gel Sci. Technol. 2011, 60, 388–394. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Guan, N.; Schreier, E.; Richter, M.; Eckelt, R.; Fricke, R. NO SCR with propane and propene on Co-based alumina catalysts prepared by co-precipitation. Appl. Catal. B Environ. 2007, 73, 209–219. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Derrouiche, S.; Haller, S.D.; Pfefferle, L.D. Role of surface cobalt silicate in single-walled carbon nanotube synthesis from silica-supported cobalt catalysts. ACS Nano 2010, 4, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, Y.; Qui, Z.; Zhao, L.; Zhu, Y. Synthesis and characterization of cobalt-substituted SBA-15 and its high activity in epoxidation of styrene with molecular oxygen. Appl. Catal. B Environ. 2010, 101, 45–53. [Google Scholar] [CrossRef]
- Zhu, J.; Kailasam, K.; Fischer, A.; Thomas, A. Supported cobalt oxide nanoparticles as catalyst for aerobic oxidation of alcohols in liquid phase. ACS Catal. 2011, 1, 342–347. [Google Scholar] [CrossRef]
- Da Silva, A.L.M.; den Breejen, J.P.; Mattos, L.V.; Bitter, J.H.; de Jong, K.P.; Noronha, F.B. Cobalt particle size effects on catalytic performance for ethanol steam reforming—Smaller is better. J. Catal. 2014, 318, 67–74. [Google Scholar] [CrossRef]
- Esposito, S.; Bonelli, B.; Armandi, M.; Garrone, E.; Saracco, G. Nanoparticles of CoAPO-5: Synthesis and comparison with microcrystalline samples. Phys. Chem. Chem. Phys. 2015, 17, 10774–10780. [Google Scholar] [CrossRef] [PubMed]
- Artero, V.; Chavarot-Kerlidou, M.; Fontecave, M. Splitting water with cobalt: A review. Angew. Chem. Int. Ed. 2011, 50, 7238–7272. [Google Scholar] [CrossRef] [PubMed]
- Tüysüz, H.; Hwang, Y.J.; Khan, S.B.; Asiri, A.M.; Yang, P. Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res. 2013, 6, 47–54. [Google Scholar] [CrossRef]
- Song, W.; Poyraz, A.S.; Meng, Y.; Ren, Z.; Chen, S.Y.; Suib, S.L. Mesoporous Co3O4 with controlled porosity: Inverse micelle synthesis and high-performance catalytic CO oxidation at −60 °C. Chem. Mater. 2014, 26, 4629–4639. [Google Scholar] [CrossRef]
- Santos, G.A.; Santos, C.M.B.; da Silva, S.W.; Urquieta-González, E.A.; Confessori Sartoratto, P.P. Sol-gel synthesis of silica–cobalt composites by employing Co3O4 colloidal dispersions. Colloids Surf. A 2012, 395, 217–224. [Google Scholar] [CrossRef]
- Khodakov, A.Y. Fischer–Tropsch synthesis: Relations between structure of cobalt catalysts and their catalytic performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Rossetti, I.; Bonelli, B.; Ramis, G.; Bahadori, E.; Nasi, R.; Aronne, A.; Esposito, S. New insights into the role of the synthesis procedure on the performance of Co-based catalysts for ethanol steam reforming. Top. Catal. 2018, 61, 1734–1745. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval-Constant, A.; Bechara, R.; Villain, F. Pore-size control of cobalt dispersion and reducibility in mesoporous silicas. J. Phys. Chem. B 2001, 105, 9805–9811. [Google Scholar] [CrossRef]
- Puskas, I.; Fleisch, T.H.; Full, P.R.; Kaduk, J.A.; Marshall, C.L.; Meyers, B.L. Novel aspects of the physical chemistry of Co/SiO2 Fischer–Tropsch catalyst preparations: The chemistry of cobalt silicate formation during catalyst preparation or hydrogenation. Appl. Catal. A Gen. 2006, 311, 146–154. [Google Scholar] [CrossRef]
- Martínez, A.; López, C.; Márquez, F.; Díaz, I. Fischer–Tropsch synthesis of hydrocarbons over mesoporous Co/SBA-15 catalysts: The influence of metal loading, cobalt precursor, and promoters. J. Catal. 2003, 220, 486–499. [Google Scholar] [CrossRef]
- Vinu, A.; Dědeček, J.; Murugesan, V.; Hartmann, M. Synthesis and characterization of Co-SBA cubic mesoporous molecular sieves. Chem. Mater. 2002, 14, 2433–2435. [Google Scholar] [CrossRef]
- Rossetti, I.; Lasso, J.; Nichele, V.; Signoretto, M.; Finocchio, E.; Ramis, G.; Di Michele, A. Silica and zirconia supported catalysts for the low-temperature ethanol steam reforming. Appl. Catal. B Environ. 2014, 150–151, 257–267. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Comparison of ethanol steam reforming using Co and Ni catalysts supported on SBA-15 modified by Ca and Mg. Fuel Process. Technol. 2016, 146, 99–109. [Google Scholar] [CrossRef]
- Finocchio, E.; Rossetti, I.; Ramis, G. Redox properties of Co- and Cu-based catalysts for the steam reforming of ethanol. Int. J. Hydrogen Energy 2013, 38, 3213–3225. [Google Scholar] [CrossRef]
- El Haskouri, J.; Cabrera, S.; Gómez-García, C.J.; Guillem, C.; Latorre, J.; Beltrán, A.; Beltrán, D.; Marcos, M.D.; Amorós, P. High cobalt content mesoporous silicas. Chem. Mater. 2004, 16, 2805–2813. [Google Scholar] [CrossRef]
- Vrålstad, T.; Øye, G.; Rønning, M.; Glomm, W.R.; Stöcker, M.; Sjöblom, J. Interfacial chemistry of cobalt(II) during sol-gel synthesis of cobalt-containing mesoporous materials. Microporous Mesoporous Mater. 2005, 80, 291–300. [Google Scholar] [CrossRef]
- Olguin, G.; Yacou, C.; Smart, S.; da Costa, J.C.D. Influence of surfactant alkyl length in functionalizing sol-gel derived microporous cobalt oxide silica. RSC Adv. 2014, 4, 40181–40187. [Google Scholar] [CrossRef]
- Bagnasco, G.; Cammarano, C.; Turco, M.; Esposito, S.; Aronne, A.; Pernice, P. TPR/TPO characterization of cobalt–silicon mixed oxide nanocomposites prepared by sol-gel. Thermochim. Acta 2008, 471, 51–54. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Lubitz, W.; Tumas, W. Hydrogen: An overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Bagnasco, G.; Cammarano, C.; Pernice, P.; Aronne, A. Highly dispersed sol-gel synthesized Cu–ZrO2 materials as catalysts for oxidative steam reforming of methanol. Appl. Catal. A Gen. 2010, 372, 48–57. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Bagnasco, G.; Cammarano, C.; Pernice, P. New insight into the preparation of copper/zirconia catalysts by sol-gel method. Appl. Catal. A Gen. 2011, 403, 128–135. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Bockris, J.O.M. Hydrogen economy in the future. Int. J. Hydrogen Energy 1999, 24, 1–15. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Matsumura, Y.; Tanaka, K.; Tode, N.; Yazawa, T.; Haruta, M. Catalytic methanol decomposition to carbon monoxide and hydrogen over nickel supported on silica. J. Mol. Catal. A Chem. 2000, 152, 157–165. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming: A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Turco, M.; Bagnasco, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Ramis, G.; Busca, G. Production of hydrogen from oxidative steam reforming of methanol I. Preparation and characterization of Cu/ZnO/Al2O3 catalysts from a hydrotalcite-like LDH precursor. J. Catal. 2004, 228, 43–55. [Google Scholar] [CrossRef]
- Fierro, V.; Akdim, O.; Mirodatos, C. On-board hydrogen production in a hybrid electric vehicle by bio-ethanol oxidative steam reforming over Ni and noble metal-based catalysts. Green Chem. 2003, 5, 20–24. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takehira, K. Production of hydrogen from methanol over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation: Steam reforming and oxidative steam reforming. J. Mol. Catal. A Chem. 2007, 268, 185–194. [Google Scholar] [CrossRef]
- Ritzkopf, I.; Vukojević, S.; Weidenthaler, C.; Grunwaldt, J.D.; Schuth, F. Decreased CO production in methanol steam reforming over Cu/ZrO2 catalysts prepared by the microemulsion technique. Appl. Catal. A Gen. 2006, 302, 215–223. [Google Scholar] [CrossRef]
- Szizybalski, A.; Girgsdies, F.; Rabis, A.; Wang, Y.; Niederberger, N.; Ressler, T. In situ investigations of structure–activity relationships of a Cu/ZrO2 catalyst for the steam reforming of methanol. J. Catal. 2005, 233, 297–307. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Gutiérrez-Martínez, A.; Espinosa-Pesqueira, M.E.; Estanislao, M.L.; Palacios, J. Effect of the bimetallic Ni/Cu loading on the ZrO2 support for H2 production in the autothermal steam reforming of methanol. Catal. Today 2015, 250, 166–172. [Google Scholar] [CrossRef]
- López, P.; Mondragón-Galicia, G.; Espinosa-Pesqueira, M.E.; Mendoza-Anaya, D.; Fernández, M.E.; Gómez-Cortés, A.; Bonifacio, J.; Martínez-Barrera, G.; Pérez-Hernández, R. Hydrogen production from oxidative steam reforming of methanol: Effect of the Cu and Ni impregnation on ZrO2 and their molecular simulation studies international. Int. J. Hydrogen Energy 2012, 37, 9018–9027. [Google Scholar] [CrossRef]
- Dell’Agli, G.; Ferone, C.; Mascolo, G.; Pansini, M. Crystallization of monoclinic zirconia from metastable phases. Solid State Ion. 2000, 127, 223–230. [Google Scholar] [CrossRef]
- Wang, Y.; Caruso, R.A. Preparation and characterization of CuO–ZrO2 nanopowders. J. Mater. Chem. 2002, 12, 1442–1445. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Bhagwat, M.; Srinivas, D.; Ramaswamy, A.V. Structural and spectral features of nano-crystalline copper-stabilized zirconia. Catal. Today 2004, 97, 63–70. [Google Scholar] [CrossRef]
- Livage, J.; Henry, M.; Sanchez, C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non-Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Nabavi, M.; Doeuff, S.; Sanchez, C.; Livage, J. Chemical modification of metal alkoxides by solvents: A way to control sol-gel chemistry. J. Non-Cryst. Solids 1990, 121, 31–34. [Google Scholar] [CrossRef]
- Schubert, U. Organically modified transition metal alkoxides: Chemical problems and structural issues on the way to materials syntheses. Acc. Chem. Res. 2007, 40, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Livage, J.; Sanchez, C. Sol-gel chemistry. J. Non-Cryst. Solids 1992, 145, 11–19. [Google Scholar] [CrossRef]
- Stöcker, C.; Baiker, A. Zirconia aerogels: effect of the use of mono- and dicarboxylic acids in the sol-gel process on structural properties. J. Sol-Gel Sci. Technol. 1997, 10, 269–282. [Google Scholar] [CrossRef]
- Hayashi, H.; Suzuki, H.; Kaneko, S. Effect of chemical modification on hydrolysis and condensation reaction of zirconium alkoxide. J. Sol-Gel Sci. Technol. 1998, 12, 87–94. [Google Scholar] [CrossRef]
- Yao, C.; Wang, L.; Liu, Y.; Wu, G.; Cao, Y.; Dai, W.; He, H.; Fan, K. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Sagar, G.V.; Srikanth, C.S.; Rao, V.V. Characterization and catalytic functionalities of copper oxide catalysts supported on zirconia. J. Phys. Chem. B 2007, 111, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mao, D.; Xiao, J.; Guo, X.; Yu, J. CO2 hydrogenation to methanol over CuO–ZnO–TiO2–ZrO2: A comparison of catalysts prepared by sol-gel, solid-state reaction and solution-combustion. J. Sol-Gel Sci. Technol. 2018, 86, 719–730. [Google Scholar] [CrossRef]

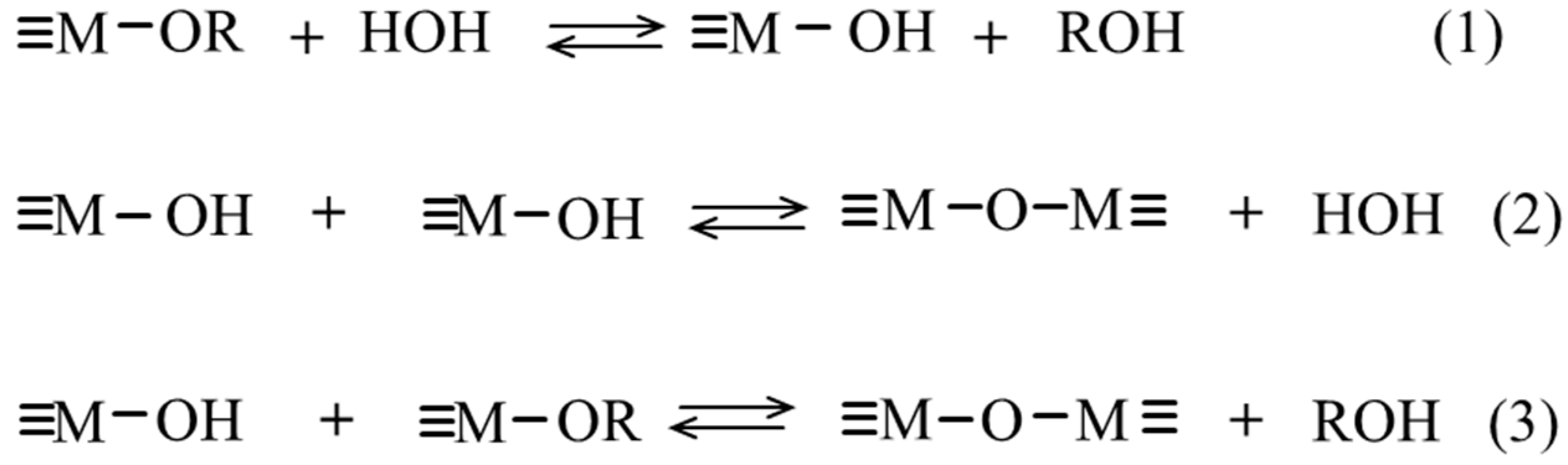


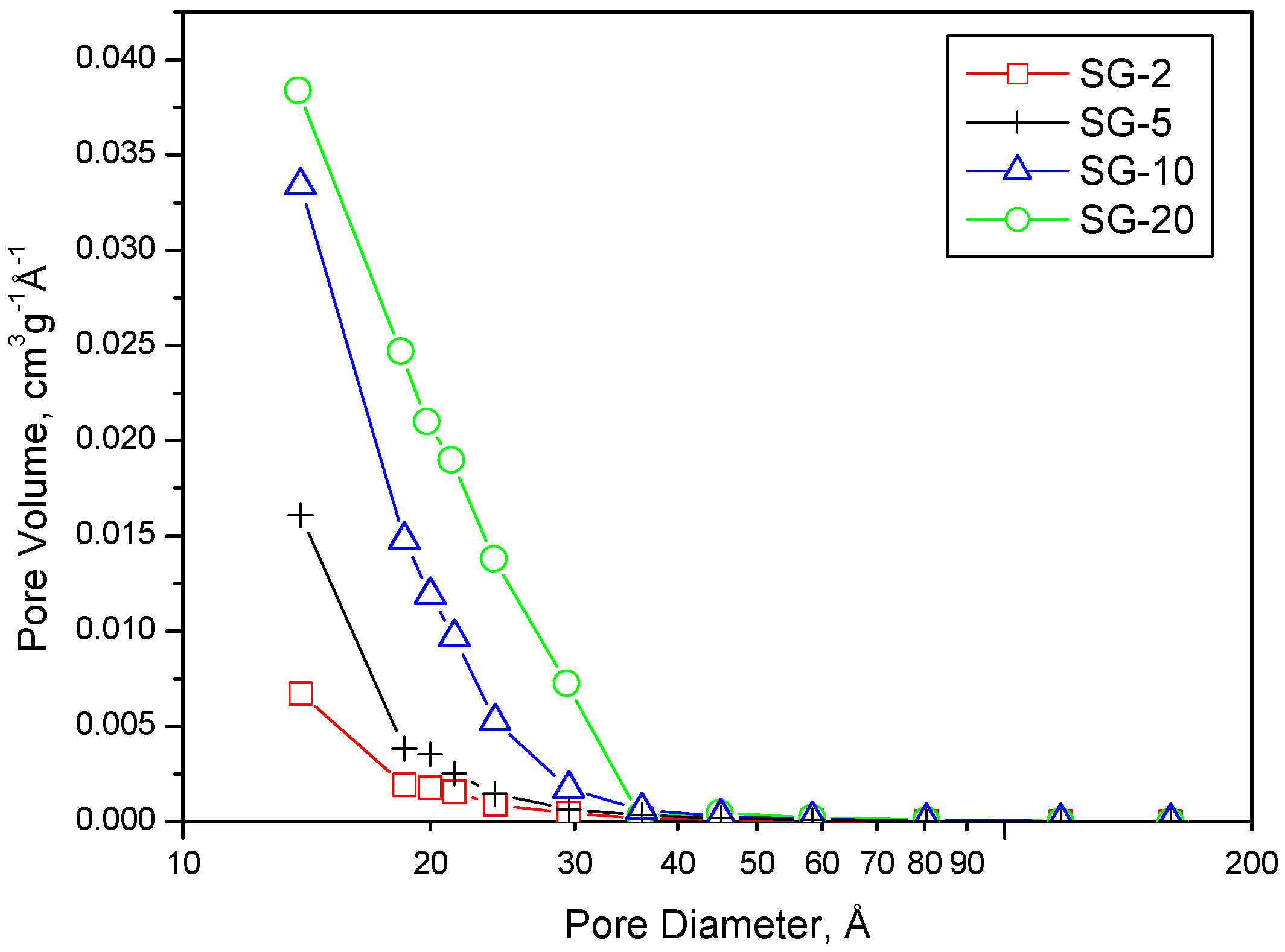


| Samples | Rw | Tgel (days) | SSA (m2 g−1) | Vp (cm3 g−1) | Vmp (cm3 g−1) |
|---|---|---|---|---|---|
| SG-2 | 2 | 60 | 410 | 0.183 | 0.151 |
| SG-5 | 5 | 4 | 528 | 0.242 | 0.186 |
| SG-10 | 10 | 9 | 588 | 0.285 | 0.131 |
| SG-20 | 20 | 15 | 705 | 0.364 | 0.056 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. https://doi.org/10.3390/ma12040668
Esposito S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials. 2019; 12(4):668. https://doi.org/10.3390/ma12040668
Chicago/Turabian StyleEsposito, Serena. 2019. "“Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts" Materials 12, no. 4: 668. https://doi.org/10.3390/ma12040668
APA StyleEsposito, S. (2019). “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials, 12(4), 668. https://doi.org/10.3390/ma12040668