Smart Control of Nitroxide-Mediated Polymerization Initiators’ Reactivity by pH, Complexation with Metals, and Chemical Transformations
Abstract
:1. Introduction
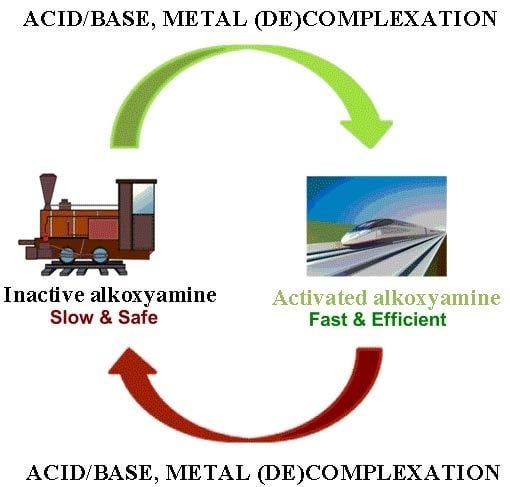
Nitroxide-Mediated Polymerization. The General Concept of “Smart Alkoxyamines”
2. Chemical Activation of C–ON Bond Homolysis
2.1. Activation by Protonation or Deprotonation
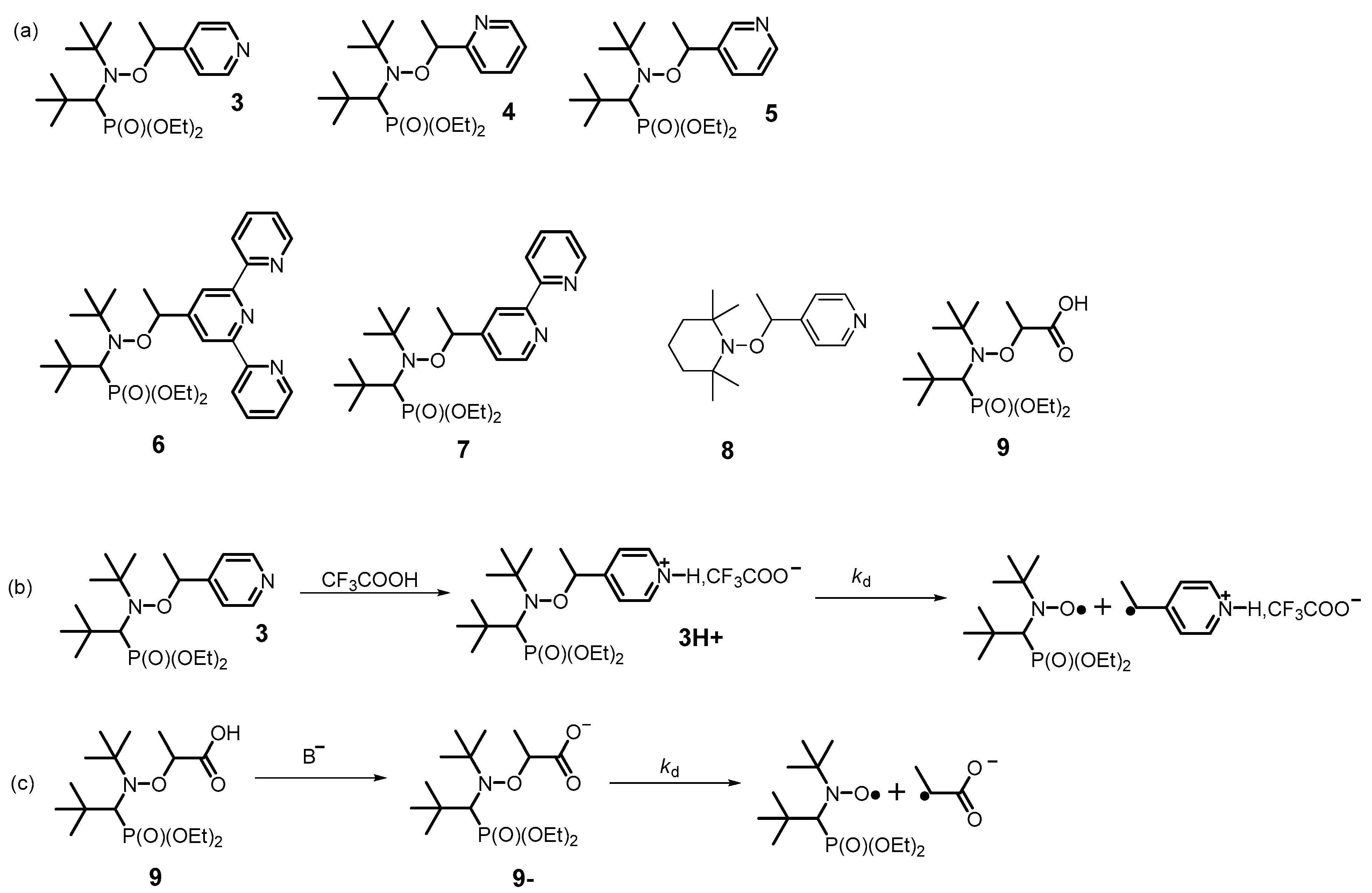
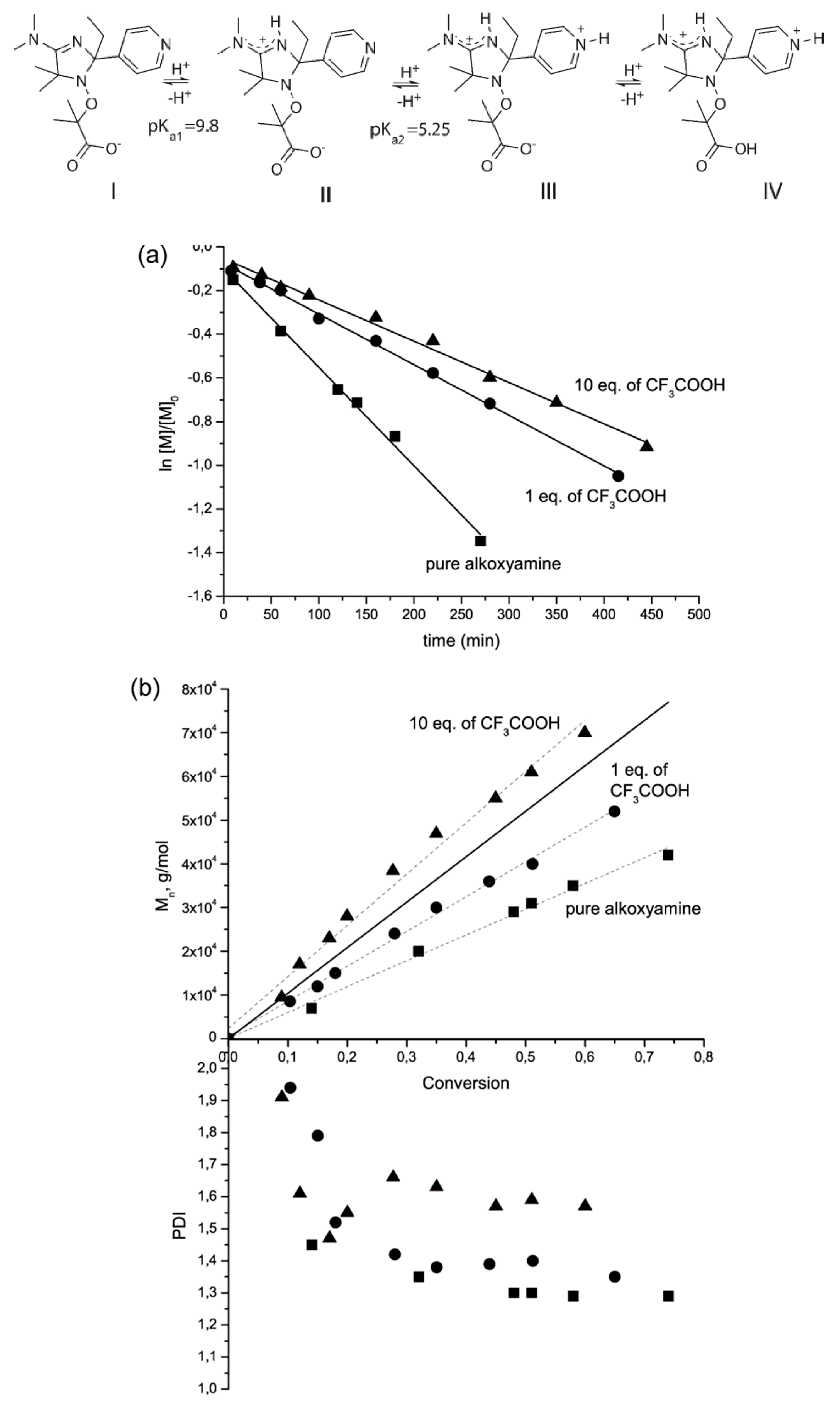
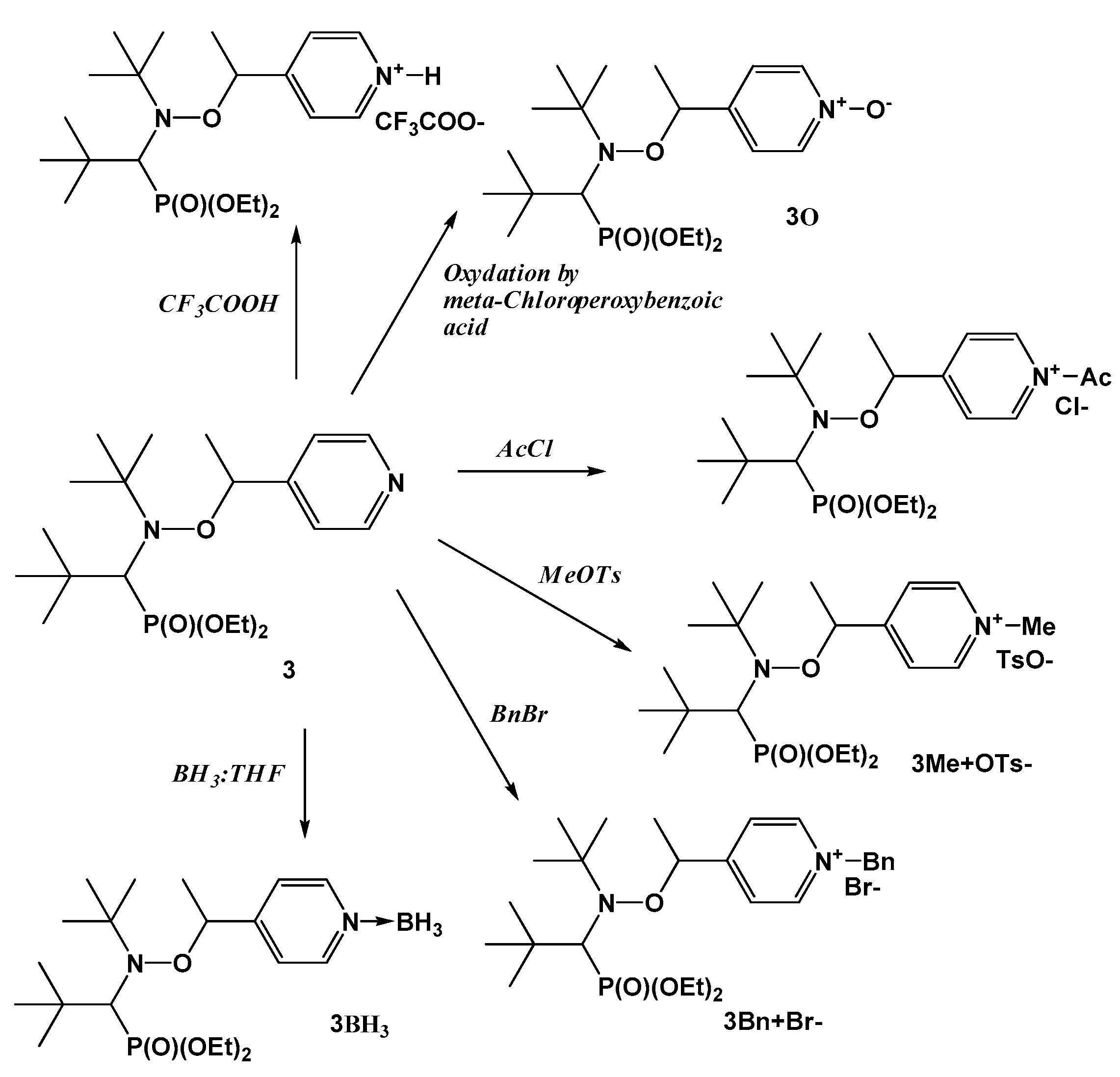
2.1.1. Protonation of the Alkyl Moiety
2.1.2. Protonation of the Nitroxyl Part
2.1.3. Theoretical Research
2.1.4. Nmp Using Initiators with Reactivity Activated by pH
2.2. Activation by Formation of Metal–Alkoxyamine Complexes
2.2.1. Formation of Metal–Alkoxyamine Complexes: Alkoxyamines with Cu(hfac)2, Zn(hfac)2, or Tb(hfac)3. Structure and Influence on kd
2.2.2. NMP of Various Monomers with Initiators in the Form of a Metal–Alkoxyamine Complex
2.3. Activation via Chemical Transformations
2.3.1. Lewis Acid and Quaternization
2.3.2. Activation by Oxidation
2.3.3. Biological Activation
2.3.4. Activation of Alkoxyamine Homolysis by 1,3-Dipolar Cycloaddition
2.4. Other Factors that Alter Alkoxyamine Reactivity. The Solvent Effect and Intramolecular Hydrogen Bonds
2.4.1. The Basic Solvent Effect
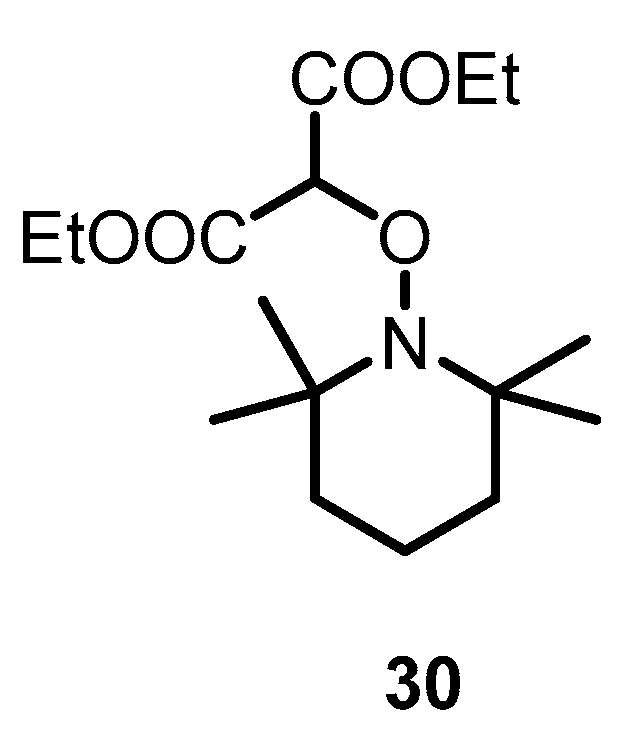
2.4.2. The Influence of the Counteranion
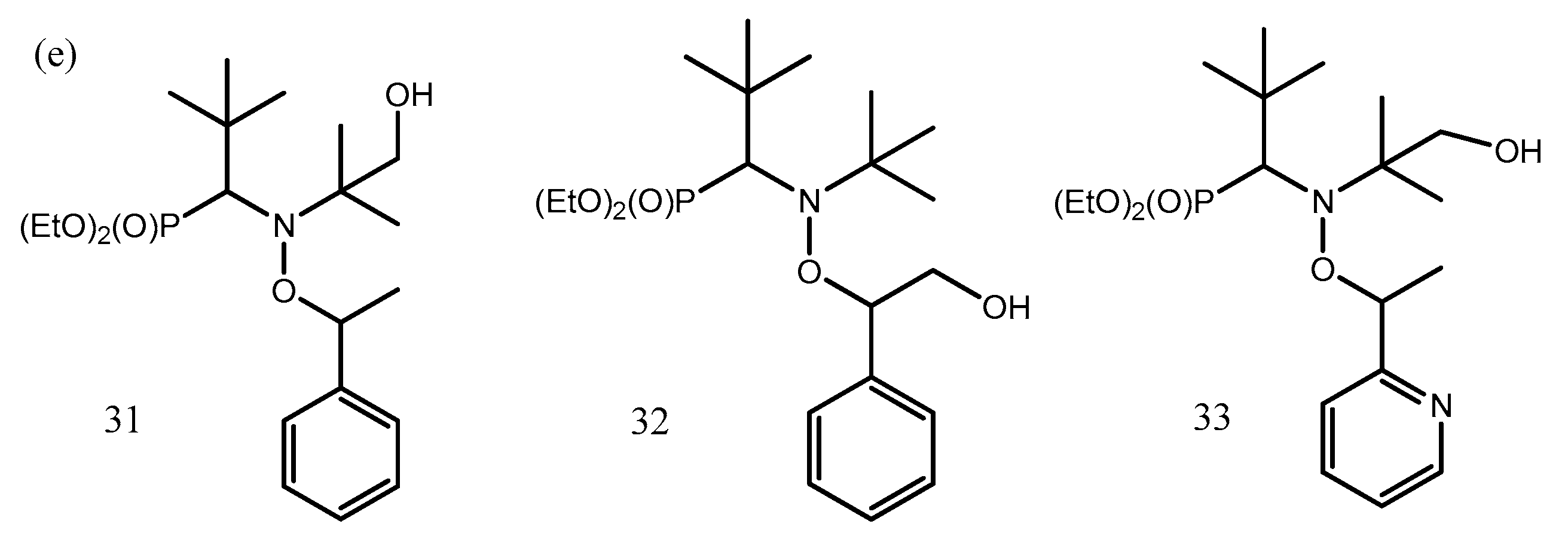
2.4.3. IHBs
2.5. Photochemical Activation of Alkoxyamine Homolysis
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solomon, D.; Rizzardo, E.; Cacioli, P. Eur Pat Appl EP135280 (1985). Chem. Abstr. 1985, 102, 221335q. [Google Scholar]
- Blinco, J.P.; Bottle, S.E.; Fairfull-Smith, K.E.; Simpson, E.; Thomas, K. Synthesis of nitroxides and alkoxyamines. In Nitroxide Mediated Polymerization; From Fundamentals to Applications in Materials Science; Gigmes, D., Ed.; Royal Society of Chemistry Publishing: Cambridge, UK, 2015; pp. 114–152. [Google Scholar]
- Tebben, L.; Studer, A. Nitroxides: Applications in synthesis and in polymer chemistry. Angew. Chem. Int. Ed. 2011, 50, 5034–5068. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Caro-Diaz, E.J.; Trzoss, L.; Theodorakis, E.A. Nature-inspired total synthesis of (−)-fusarisetin A. J. Am. Chem. Soc. 2012, 134, 5072–5075. [Google Scholar] [CrossRef] [PubMed]
- Studer, A. Tin-free radical chemistry using the persistent radical effect: Alkoxyamine isomerization, addition reactions and polymerizations. Chem. Soc. Rev. 2004, 33, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wetter, C.; Jantos, K.; Woithe, K.; Studer, A. Intermolecular radical addition and addition/cyclization reactions of alkoxyamines onto nonactivated alkenes. Organ. Lett. 2003, 5, 2899–2902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Q.; Rong, M.Z. Intrinsic self-healing of covalent polymers through bond reconnection towards strength restoration. Polym. Chem. 2013, 4, 4878–4884. [Google Scholar] [CrossRef]
- Schulte, B.; Tsotsalas, M.; Becker, M.; Studer, A.; De Cola, L. Dynamic microcrystal assembly by nitroxide exchange reactions. Angew. Chem. Int. Ed. 2010, 49, 6881–6884. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.K.; Meszynska, A.; Laure, C.; Charles, L.; Verchin, C.; Lutz, J.-F. Design and synthesis of digitally encoded polymers that can be decoded and erased. Nat. Commun. 2015, 6, 7237. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Brémond, P.; Franconi, J.-M.; Marque, S.R.; Massot, P.; Mellet, P.; Parzy, E.; Thiaudière, E. Alkoxyamines: A new family of pro-drugs against cancer. Concept for theranostics. Org. Biomol. Chem. 2014, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, F.; Dufils, P.-E.; Gigmes, D.; Guillaneuf, Y.; Marque, S.R.; Tordo, P.; Bertin, D. Nitroxide-mediated polymerization: The pivotal role of the kd value of the initiating alkoxyamine and the importance of the experimental conditions. Macromolecules 2006, 39, 5238–5250. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Grubbs, R.B. Nitroxide-mediated radical polymerization: Limitations and versatility. Polym. Rev. 2011, 51, 104–137. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Marque, S.R.; Bagryanskaya, E.G. Imidazoline and imidazolidine nitroxides as controlling agents in nitroxide-mediated pseudoliving radical polymerization. Russ. Chem. Rev. 2018, 87, 328. [Google Scholar] [CrossRef]
- Bertin, D.; Gigmes, D.; Marque, S.R.; Tordo, P. Kinetic subtleties of nitroxide mediated polymerization. Chem. Soc. Rev. 2011, 40, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Bagryanskaya, E.G.; Marque, S.R.A. RSC polymer chemistry series, n = 19. In Nitroxide Mediated Polymerization: From Fundamentals to Applications in Materials Sciences; Gigmes, D., Ed.; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Audran, G.; Brémond, P.; Marque, S.R. Labile alkoxyamines: Past, present, and future. Chem. Commun. 2014, 50, 7921–7928. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Mros, G.R. Characterization and safe handling of reactive initiator solutions. Process Saf. Prog. 2006, 25, 303–310. [Google Scholar] [CrossRef]
- Fischer, A.; Brembilla, A.; Lochon, P. Nitroxide-mediated radical polymerization of 4-vinylpyridine: Study of the pseudo-living character of the reaction and influence of temperature and nitroxide concentration. Macromolecules 1999, 32, 6069–6072. [Google Scholar] [CrossRef]
- Marx, L.; Hemery, P. Synthesis and evaluation of a new polar, TIPNO type nitroxide for “living” free radical polymerization. Polymer 2009, 50, 2752–2761. [Google Scholar] [CrossRef]
- Mazarin, M.; Girod, M.; Viel, S.; Phan, T.N.; Marque, S.R.; Humbel, S.; Charles, L. Role of the adducted cation in the release of nitroxide end group of controlled polymer in mass spectrometry. Macromolecules 2009, 42, 1849–1859. [Google Scholar] [CrossRef]
- Benaglia, M.; Chiefari, J.; Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Universal (switchable) RAFT agents. J. Am. Chem. Soc. 2009, 131, 6914–6915. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, M.; Chen, M.; Chong, Y.K.; Moad, G.; Rizzardo, E.; Thang, S.H. Polystyrene-block-poly (vinyl acetate) through the use of a switchable RAFT agent. Macromolecules 2009, 42, 9384–9386. [Google Scholar] [CrossRef]
- Brémond, P.; Marque, S.R. First proton triggered C–ON bond homolysis in alkoxyamines. Chem. Commun. 2011, 47, 4291–4293. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Bosco, L.; Brémond, P.; Marque, S.R.; Roubaud, V.; Siri, D. Chemically triggered C–ON bond homolysis of alkoxyamines. 8. Quaternization and steric effects. J. Org. Chem. 2013, 78, 9914–9920. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Brémond, P.; Ibanou, M.B.B.; Marque, S.R.; Roubaud, V.; Siri, D. Chemically triggered C–ON bond homolysis in alkoxyamines: Regioselectivity and chemoselectivity. Org. Biomol. Chem. 2013, 11, 7738–7750. [Google Scholar] [CrossRef] [PubMed]
- Nkolo, P.; Audran, G.; Bikanga, R.; Brémond, P.; Marque, S.R.; Roubaud, V. C–ON bond homolysis of alkoxyamines: When too high polarity is detrimental. Org. Biomol. Chem. 2017, 15, 6167–6176. [Google Scholar] [CrossRef] [PubMed]
- Bertin, D.; Gigmes, D.; Marque, S.R.; Siri, D.; Tordo, P.; Trappo, G. Effect of the carboxylate salt on the C–ON bond homolysis of SG1-based alkoxyamines. Chem. Phys. Chem. 2008, 9, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Edeleva, M.V.; Kirilyuk, I.A.; Zhurko, I.F.; Parkhomenko, D.A.; Tsentalovich, Y.P.; Bagryanskaya, E.G. pH-Sensitive C–ON bond homolysis of alkoxyamines of imidazoline series with multiple ionizable groups as an approach for control of Nitroxide mediated polymerization. J. Org. Chem. 2011, 76, 5558–5573. [Google Scholar] [CrossRef] [PubMed]
- Le Du, Y.; Binet, L.; Hémery, P.; Marx, L. Proton-controlled nitroxide mediated radical polymerization of styrene. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2871–2877. [Google Scholar] [CrossRef]
- Parkhomenko, D.A.; Edeleva, M.V.; Kiselev, V.G.; Bagryanskaya, E.G. pH-sensitive C–ON bond homolysis of alkoxyamines of imidazoline series: A theoretical study. J. Phys. Chem. B 2014, 118, 5542–5550. [Google Scholar] [CrossRef] [PubMed]
- Gryn’ova, G.; Smith, L.M.; Coote, M.L. Computational design of pH-switchable control agents for nitroxide mediated polymerization. Phys. Chem. Chem. Phys. 2017, 19, 22678–22683. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Luan, Y.; Yang, L.; Sun, H. A Perspective on reversibility in controlled polymerization systems: Recent progress and new opportunities. Molecules 2018, 23, 2870. [Google Scholar] [CrossRef] [PubMed]
- Souaille, M.; Fischer, H. Kinetic conditions for living and controlled free radical polymerizations mediated by reversible combination of transient propagating and persistent radicals: The ideal mechanism. Macromolecules 2000, 33, 7378–7394. [Google Scholar] [CrossRef]
- Pavlovskaya, M.; Kirilyuk, I.; Shchepalov, A.; Grigoriev, I.; Grishin, D. Polymerization of styrene and methyl methacrylate in the presence of 2, 2-diethyl-4, 5, 5-trimethyl-2, 5-dihydroimidazol-1-oxyl. Polym. Sci. Ser. B 2008, 50, 356–361. [Google Scholar] [CrossRef]
- Lazarev, M.; Kirilyuk, I.; Grigor’ev, I.; Grishin, D. Polymerization of styrene in the presence of nitroxide radicals of the dihydroimidazole series. Polym. Sci. Ser. B 2007, 49, 224–228. [Google Scholar] [CrossRef]
- Zubenko, D.; Kirilyuk, I.; Roshchupkina, G.; Zhurko, I.; Reznikov, V.; Marque, S.R.; Bagryanskaya, E. 2,5-Dihydro-1H-imidazole-based nitroxides as prospective mediators in living radical polymerization. Helv. Chim. Acta 2006, 89, 2341–2353. [Google Scholar] [CrossRef]
- Bagryanskaya, E.; Bertin, D.; Gigmes, D.; Kirilyuk, I.; Marque, S.R.; Reznikov, V.; Roshchupkina, G.; Zhurko, I.; Zubenko, D. Can the first addition of alkyl radicals play a role in the fate of NMP? Macromol. Chem. Phys. 2008, 209, 1345–1357. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Zhu, J.; Cheng, Z. Imidazoline Nitroxide-mediated radical polymerization of styrene. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 41–46. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Kirilyuk, I.A.; Zubenko, D.P.; Zhurko, I.F.; Marque, S.R.; Gigmes, D.; Guillaneuf, Y.; Bagryanskaya, E.G. Kinetic study of H-atom transfer in imidazoline-, imidazolidine-, and pyrrolidine-based alkoxyamines: Consequences for nitroxide-mediated polymerization. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 6579–6595. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Parkhomenko, D.A.; Morozov, D.A.; Dobrynin, S.A.; Trofimov, D.G.; Kanagatov, B.; Kirilyuk, I.A.; Bagryanskaya, E.G. Controlled/living polymerization of methyl methacrylate using new sterically hindered imidazoline nitroxides prepared via intramolecular 1, 3-dipolar cycloaddition reaction. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 929–943. [Google Scholar] [CrossRef]
- Bagryanskaya, E.; Brémond, P.; Edeleva, M.; Marque, S.R.; Parkhomenko, D.; Roubaud, V.; Siri, D. Chemically triggered C–ON Bond homolysis in alkoxyamines. Part 2: DFT Investigation and application of the pH Effect on NMP. Macromol. Rapid Commun. 2012, 33, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Bagryanskaya, E.; Bagryanskaya, I.; Brémond, P.; Edeleva, M.; Marque, S.R.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. C–ON bond homolysis of alkoxyamines triggered by paramagnetic copper (ii) salts. Inorg. Chem. Front. 2016, 3, 1464–1472. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.; Bagryanskaya, I.; Edeleva, M.; Marque, S.R.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. Zinc (II) Hexafluoroacetylacetonate complexes of alkoxyamines: NMR and Kinetic investigations. first step for a new way to prepare hybrid materials. Chem. Sel. 2017, 2, 3584–3593. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.G.; Bagryanskaya, I.Y.; Edeleva, M.; Kaletina, P.; Marque, S.R.; Parkhomenko, D.; Tretyakov, E.V.; Zhivetyeva, S.I. The effect of the oxophilic Tb (III) cation on CON bond homolysis in alkoxyamines. Inorg. Chem. Commun. 2018, 91, 5–7. [Google Scholar] [CrossRef]
- Fokin, S.; Ovcharenko, V.; Romanenko, G.; Ikorskii, V. Problem of a wide variety of products in the Cu (hfac) 2-nitroxide system. Inorg. Chem. 2004, 43, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Partenheimer, W.; Drago, R.S. Preparation and thermodynamic data for adducts of bases with some copper (II). beta.-diketonates. Inorg. Chem. 1970, 9, 47–52. [Google Scholar] [CrossRef]
- Pradilla-Sorzano, J.; Fackler Jr, J.P. Base adducts of. beta.-ketoenolates. V. Crystal and molecular structure of cis-bis (1, 1, 1, 6, 6, 6-hexafluoro-2, 4-pentanedionato) bis (pyridine) zinc (II) and copper (II). Inorg. Chem. 1973, 12, 1174–1182. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.; Edeleva, M.; Marque, S.R.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. Coordination-Initiated nitroxide-mediated polymerization (CI-NMP). Aust. J. Chem. 2018, 71, 334–340. [Google Scholar] [CrossRef]
- Brémond, P.; Koїta, A.; Marque, S.R.; Pesce, V.; Roubaud, V.; Siri, D. Chemically triggered C–ON bond homolysis of alkoxyamines. Quaternization of the alkyl fragment. Org. Let. 2011, 14, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Edeleva, M.; Morozov, D.; Parkhomenko, D.; Polienko, Y.; Iurchenkova, A.; Kirilyuk, I.; Bagryanskaya, E. Versatile approach to activation of alkoxyamine homolysis by 1, 3-dipolar cycloaddition for efficient and safe nitroxide mediated polymerization. Chem. Commun. 2019, 55, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E. Alkoxyamine-initiated living radical polymerization: Factors affecting alkoxyamine homolysis rates. Macromolecules 1995, 28, 8722–8728. [Google Scholar] [CrossRef]
- Guerret, O.; Couturier, J.; Chauvin, F.; El-Bouazzy, H.; Bertin, D.; Gigmes, D.; Marque, S.; Fischer, H.; Tordo, P. Influence of solvent and polymer chain length on the homolysis of SG1-based alkoxyamines. In Proceedings of ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1999; pp. 412–423. [Google Scholar]
- Audran, G.; Brémond, P.; Marque, S.R.; Obame, G. Chemically triggered C–ON bond homolysis of alkoxyamines. Part 4: Solvent effect. Polym. Chem. 2012, 3, 2901–2908. [Google Scholar] [CrossRef]
- Audran, G.; Brémond, P.; Marque, S.R.; Obame, G. Chemically triggered C–ON bond homolysis of alkoxyamines. 5. Cybotactic effect. J. Org. Chem. 2012, 77, 9634–9640. [Google Scholar] [CrossRef] [PubMed]
- Audran, G.; Brémond, P.; Marque, S.R.; Obame, G. Chemically triggered C–ON Bond homolysis in alkoxyamines. 6. Effect of the counteranion. J. Org. Chem. 2013, 78, 7754–7757. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Grellier, P.L.; Prior, D.V.; Duce, P.P.; Morris, J.J.; Taylor, P.J. Hydrogen bonding. Part 7. A scale of solute hydrogen-bond acidity based on log K values for complexation in tetrachloromethane. J. Chem. Soc. Perkin Trans. 1989, 2, 699–711. [Google Scholar] [CrossRef]
- Audran, G.; Bikanga, R.; Brémond, P.; Edeleva, M.; Joly, J.-P.; Marque, S.R.; Nkolo, P.; Roubaud, V. How intramolecular hydrogen bonding (IHB) controls the C–ON bond homolysis in alkoxyamines. Org. Biomol. Chem. 2017, 15, 8425–8439. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Guillaneuf, Y.; Bertin, D.; Gigmes, D.; Versace, D.-L.; Lalevee, J.; Fouassier, J.-P. Toward nitroxide-mediated photopolymerization. Macromolecules 2010, 43, 2204–2212. [Google Scholar] [CrossRef]
- Morris, J.; Telitel, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Lalevée, J.; Clément, J.-L.; Guillaneuf, Y.; Gigmes, D. Novel polymer synthesis methodologies using combinations of thermally-and photochemically-induced nitroxide mediated polymerization. Polym.Chem. 2015, 6, 754–763. [Google Scholar] [CrossRef]
- Huix-Rotllant, M.; Ferré, N. Theoretical Study of the photochemical initiation in nitroxide-mediated photopolymerization. J. Phys. Chem. A 2014, 118, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Beinhoff, M.; Frommer, J.; Carter, K.R. Photochemical attachment of reactive cross-linked polymer films to Si/SiO2 surfaces and subsequent polymer brush growth. Chem. Mater. 2006, 18, 3425–3431. [Google Scholar] [CrossRef]
- Yoshida, E. Effect of azoinitiators on nitroxide-mediated photo-living radical polymerization of methyl methacrylate. Colloid Polym. Sci. 2010, 288, 341–345. [Google Scholar] [CrossRef]
- Su, J.; Liu, X.; Hu, J.; You, Q.; Cui, Y.; Chen, Y. Photo-induced controlled radical polymerization of methyl methacrylate mediated by photosensitive nitroxides. Polym. Int. 2015, 64, 867–874. [Google Scholar] [CrossRef]
- Yoshida, E. Photo-controlled/living radical polymerization of tert-butyl methacrylate in the presence of a photo-acid generator using a nitroxide mediator. Colloid Polym. Sci. 2012, 290, 661–665. [Google Scholar] [CrossRef]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edeleva, M.; Audran, G.; Marque, S.; Bagryanskaya, E. Smart Control of Nitroxide-Mediated Polymerization Initiators’ Reactivity by pH, Complexation with Metals, and Chemical Transformations. Materials 2019, 12, 688. https://doi.org/10.3390/ma12050688
Edeleva M, Audran G, Marque S, Bagryanskaya E. Smart Control of Nitroxide-Mediated Polymerization Initiators’ Reactivity by pH, Complexation with Metals, and Chemical Transformations. Materials. 2019; 12(5):688. https://doi.org/10.3390/ma12050688
Chicago/Turabian StyleEdeleva, Mariya, Gerard Audran, Sylvain Marque, and Elena Bagryanskaya. 2019. "Smart Control of Nitroxide-Mediated Polymerization Initiators’ Reactivity by pH, Complexation with Metals, and Chemical Transformations" Materials 12, no. 5: 688. https://doi.org/10.3390/ma12050688
APA StyleEdeleva, M., Audran, G., Marque, S., & Bagryanskaya, E. (2019). Smart Control of Nitroxide-Mediated Polymerization Initiators’ Reactivity by pH, Complexation with Metals, and Chemical Transformations. Materials, 12(5), 688. https://doi.org/10.3390/ma12050688