Conductomeric Evaluation of the Release Kinetics of Active Substances from Pharmaceutical Preparations Containing Iron Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dissolution Test
2.3. Electric Conductivity Method Conditions
2.4. Calculations
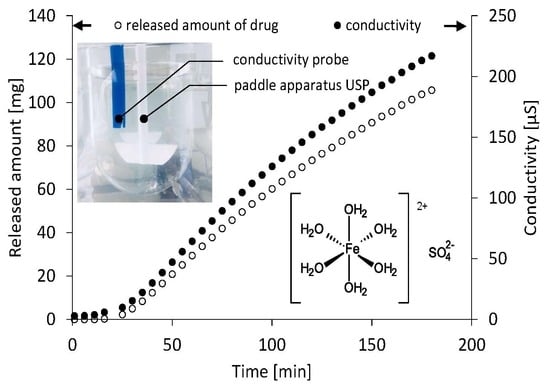
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vella, J.; Bussuttil, F.; Sammut Bartolo, N.; Sammut, C.; Ferrito, V.; Serracino Inglot, A.; Azzopardi, L.M.; La Ferla, G. A simple HPLC–UV method for the determination of ciprofloxacin in human plasma. J. Chromatogr. B 2015, 989, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, G.; Di Federico, L.; Iuliani, P. HPLC-DAD method for the simultaneous determination of zofenopril and hydrochlorothiazide in oral pharmaceutical formulations. J. Sep. Sci. 2010, 33, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, L.; Dufresne, G.; Deeks, J.; Clément, G.; Bertrand, J.; Turcotte, S.; Robichaud, A.; Beraldin, F.; Fouquet, A. Determination of vitamin D3 and 25-hydroxyvitamin D3 in foodstuffs by HPLC UV-DAD and LC–MS/MS. J. Food Compos. Anal. 2011, 24, 441–448. [Google Scholar] [CrossRef]
- Karlsson, G.; Winge, S.; Sandberg, H. Separation of monosaccharides by hydrophilic interaction chromatography with evaporative light scattering detection. J. Chromatogr. A 2005, 1092, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Sang-Ho, Y.; Jay-lin, J. Molecular weights and gyration radii of amylopectins determined by high-performance size-exclusion chromatography equipped with multi-angle laser-light scattering and refractive index detectors. Carbohydr. Polym. 2002, 49, 307–314. [Google Scholar] [CrossRef]
- Coufal, P.; Zuska, J.; Van de Goor, T.; Smith, V.; Gaš, B. Separation of twenty underivatized essential amino acids by capillary zone electrophoresis with contactless conductivity detection. Electrphoresis 2003, 24, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Li, C.; Fu, Y.; Zhao, C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharmaceut. Biomed. 2006, 41, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Boussèsa, C.; Fereyb, L.; Vedrinesa, E.; Gaudin, K. Using an innovative combination of quality-by-design and green analytical chemistry approaches for the development of a stability indicating UHPLC method in pharmaceutical products. J. Pharmaceut. Biomed. 2015, 115, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio, M.A.; Hu, J.; Zhou, P.; Cauchon, N. Simultaneous determination of formic acid and formaldehyde in pharmaceutical excipients using headspace GC/MS. J. Pharmaceut. Biomed. 2006, 41, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.; Naschitz, V.; Halperin, L.; Halperin, N.; Lin, L.; Chen, J.; Ghosh, S.; Liu, B. Analysis of ferrate(VI) compounds and super-iron Fe(VI) battery cathodes: FTIR, ICP, titrimetric, XRD, UV/VIS, and electrochemical characterization. J. Power Sources 2001, 101, 167–176. [Google Scholar] [CrossRef]
- Johansson, J.; Cauchi, M.; Sundgren, M. Multiple fiber-optic dual-beam UV/Vis system with application to dissolution testing. J. Pharmaceut. Biomed. 2002, 29, 469–476. [Google Scholar] [CrossRef]
- Abarcaa, A.; Canfranca, E.; Sierraa, I.; Marina, M.L. A validated flame AAS method for determining magnesium in a multivitamin pharmaceutical preparation. J. Pharmaceut. Biomed. 2001, 25, 941–945. [Google Scholar] [CrossRef]
- Lisik, A.; Prescha, A.; Cavlaz, L.E.; Grajeta, H.; Musiał, W. The evaluation of alternative method of ferrous ions assessment in pharmaceutical preparations. Monatsh. Chem. 2018, 149, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, N.P.; Nath, L.; Chwdhury, P. Kinetic modeling on drug release from kinetic controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.K.; Kshirsagar, R.V.; Patil, S.G. Mathematical models for drug release characterization: A review. World J. Pharm. Pharm. Sci. 2015, 4, 324–338. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Kalam, M.A.; Humayun, M.; Parvez, N.; Yadav, S.; Garg, A.; Amin, S.; Sultana, Y.; Ali, A. Release kinetics of modified pharmaceutical dosage forms: A rewiev. CJP Sci. 2007, 1, 30–35. [Google Scholar]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 160, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Narasimhand, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia Commission. Dissolution test for solid dosage forms (Chapter 2.9.3). In European Pharmacopoeia, 5th ed.; Council of Europe: Strasburg, Germany, 2005; p. 20903. [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Validation of Analytical Procedures: Text and Methodology Q2(R1); Current Step 4 Version Parent Guideline dated 27 October 1994 (Complementary Guideline on Methodology Dated 6 November 1996 Incorporated in November 2005). Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 16 October 2017).
- Holowka, E.P.; Bhatia, K.S. Controled Released Systems. In Drug Delivery: Materials Design and Clinical Perspective, 1st ed.; Springer-Verlag: New York, NY, USA, 2014; pp. 7–14. ISBN 978-1-4939-1998-7. [Google Scholar]



| Kinetic Model | Equation | Source |
|---|---|---|
| zero-order | [15,16,17] | |
| first-order | [15,16,17] | |
| Hixson–Crowell | [15,16,17] | |
| Korsmeyer–Peppas | [15,16,17] |
| n | Drug Transport Mechanism | Rate as a Function of Time (t) Transformation |
|---|---|---|
| 0.5 | Fickian diffusion | t−0.5 |
| 0.45 < n = 0.89 | Non-Fickian transport | tn−1 |
| 0.89 | Case II transport | t |
| >0.89 | Super case II transport | tn−1 |
| Composition | k1 (1/min) | k0 (mg/min) | t0.5 (min) | r2 | KS | r2 | Korsmeyer–Peppas KKP n | r2 | |
|---|---|---|---|---|---|---|---|---|---|
| FEP1, 1st stage | 0.174 ± 0.0160 | - | 52.00 (b) ± 2.67 | 0.9995 (a) ± 0.0011 | 0.0160 ± 0.0013 | 0.9933 (b) ± 0.4200 | 0.0512 ± 0.0082 | 0.574 ± 0.029 | 0.9960 ± 0.3633 |
| FEP1, 2nd stage | - | 0.315 ± 0.022 | |||||||
| FEP2 | - | 0.680 ± 0.020 | 89.77 ± 4.09 | 0.9916 ± 0.0027 | 0.0180 ± 0.0009 | 0.9959 (c) ± 0.1360 | 0.0023 ± 0.0008 | 1.192 ± 0.061 | 0.9837 ± 0.0678 |
| FEN3 | 0.0544 ± 0.0031 | - | 11.56 ± 0.54 | 0.9945 ± 0.0034 | 0.0437 ± 0.0045 | 0.9956 (d) ± 0.3719 | 0.1630 ± 0.016 | 0.472 ± 0.025 | 0.9408 ± 0.7519 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisik, A.; Musiał, W. Conductomeric Evaluation of the Release Kinetics of Active Substances from Pharmaceutical Preparations Containing Iron Ions. Materials 2019, 12, 730. https://doi.org/10.3390/ma12050730
Lisik A, Musiał W. Conductomeric Evaluation of the Release Kinetics of Active Substances from Pharmaceutical Preparations Containing Iron Ions. Materials. 2019; 12(5):730. https://doi.org/10.3390/ma12050730
Chicago/Turabian StyleLisik, Anna, and Witold Musiał. 2019. "Conductomeric Evaluation of the Release Kinetics of Active Substances from Pharmaceutical Preparations Containing Iron Ions" Materials 12, no. 5: 730. https://doi.org/10.3390/ma12050730
APA StyleLisik, A., & Musiał, W. (2019). Conductomeric Evaluation of the Release Kinetics of Active Substances from Pharmaceutical Preparations Containing Iron Ions. Materials, 12(5), 730. https://doi.org/10.3390/ma12050730