Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix design
2.3. Paste Preparation and the Creation of Aggregate by Crushing
2.4. Paste Preparation and the Creation of Aggregate by Pelletization
2.5. Curing Regimes and Labelling
2.6. Experimental Procedures and Techniques
3. Results and Discussion
3.1. Physical Properties of Aggregates
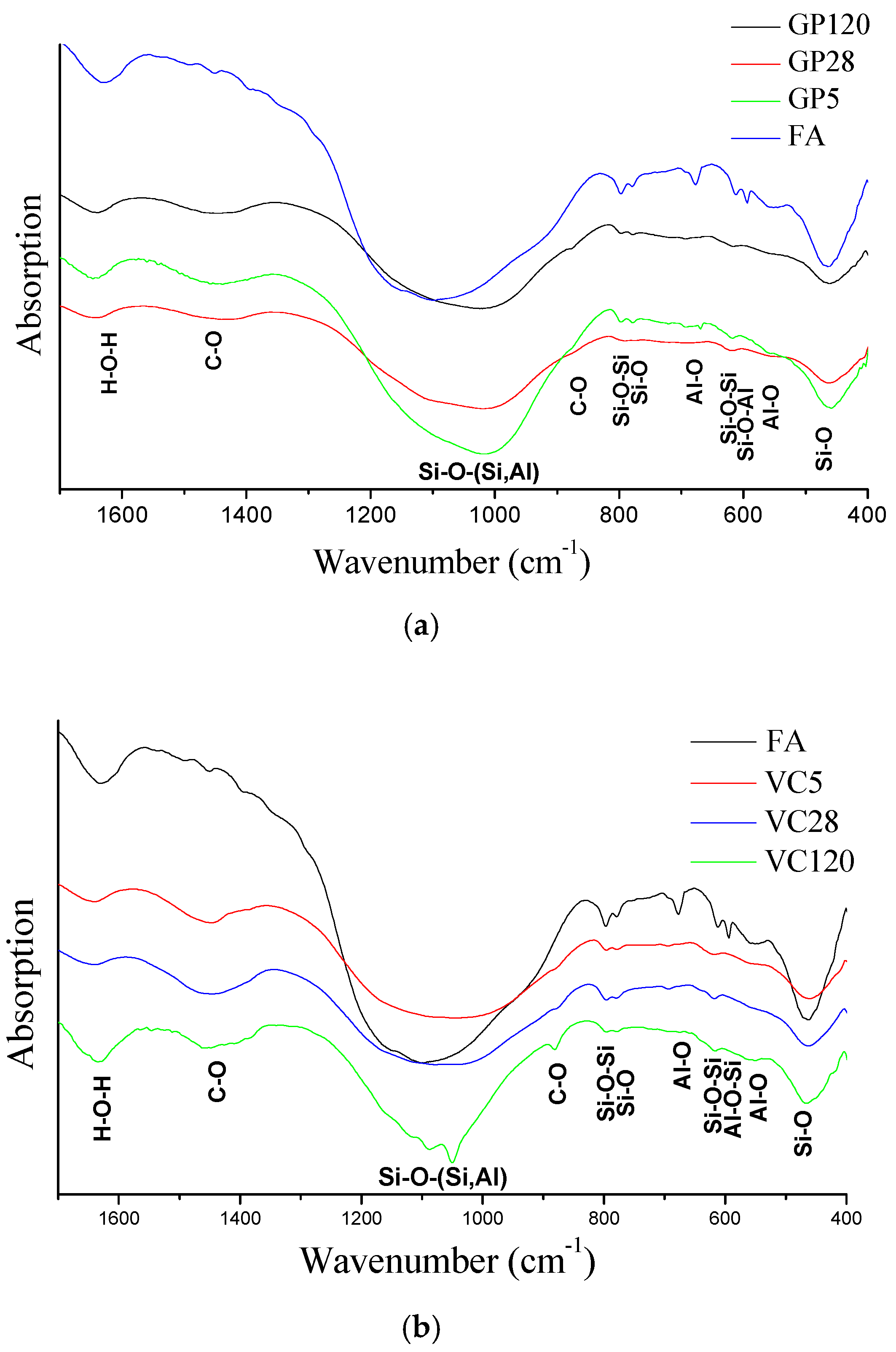
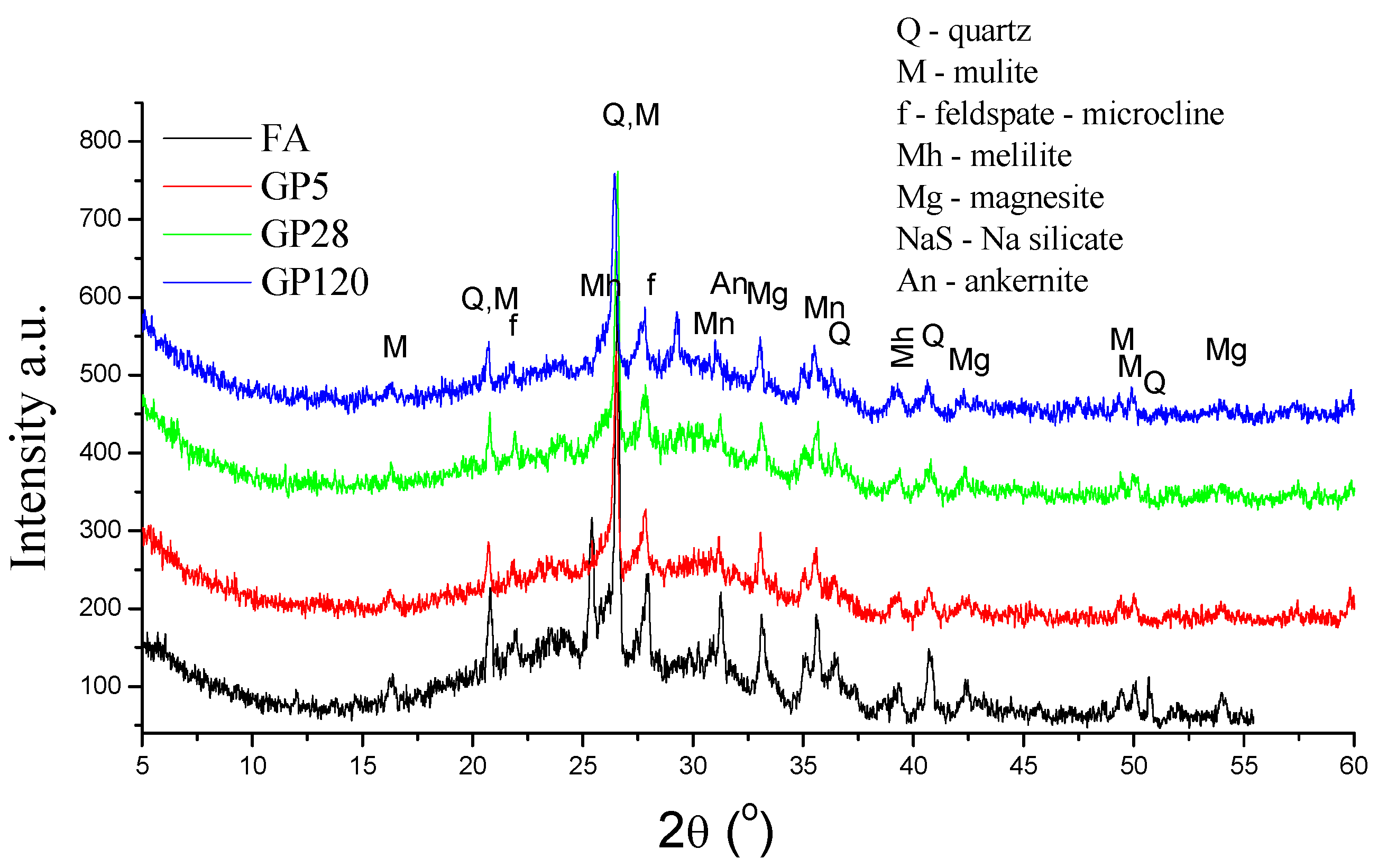
3.2. Mineralogical and Structural Characterization of FA and Alkali Activated Fly Ash Aggregates
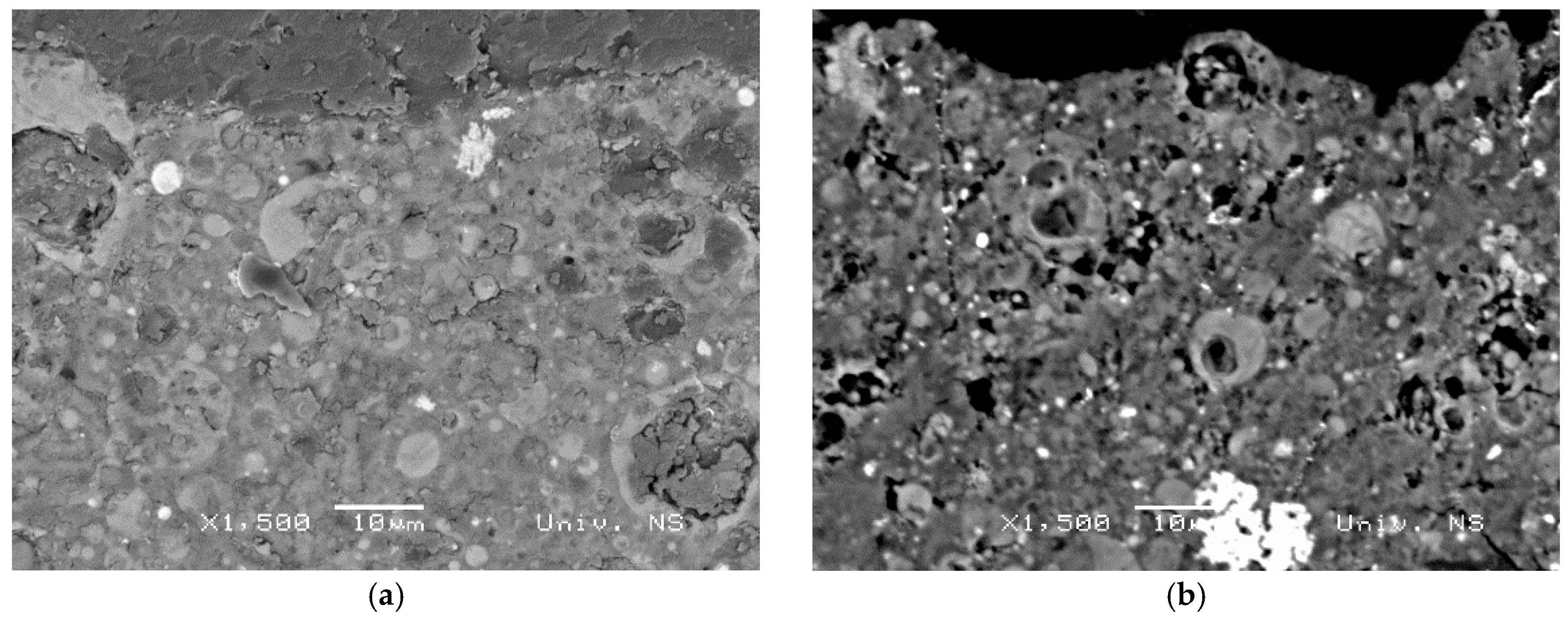
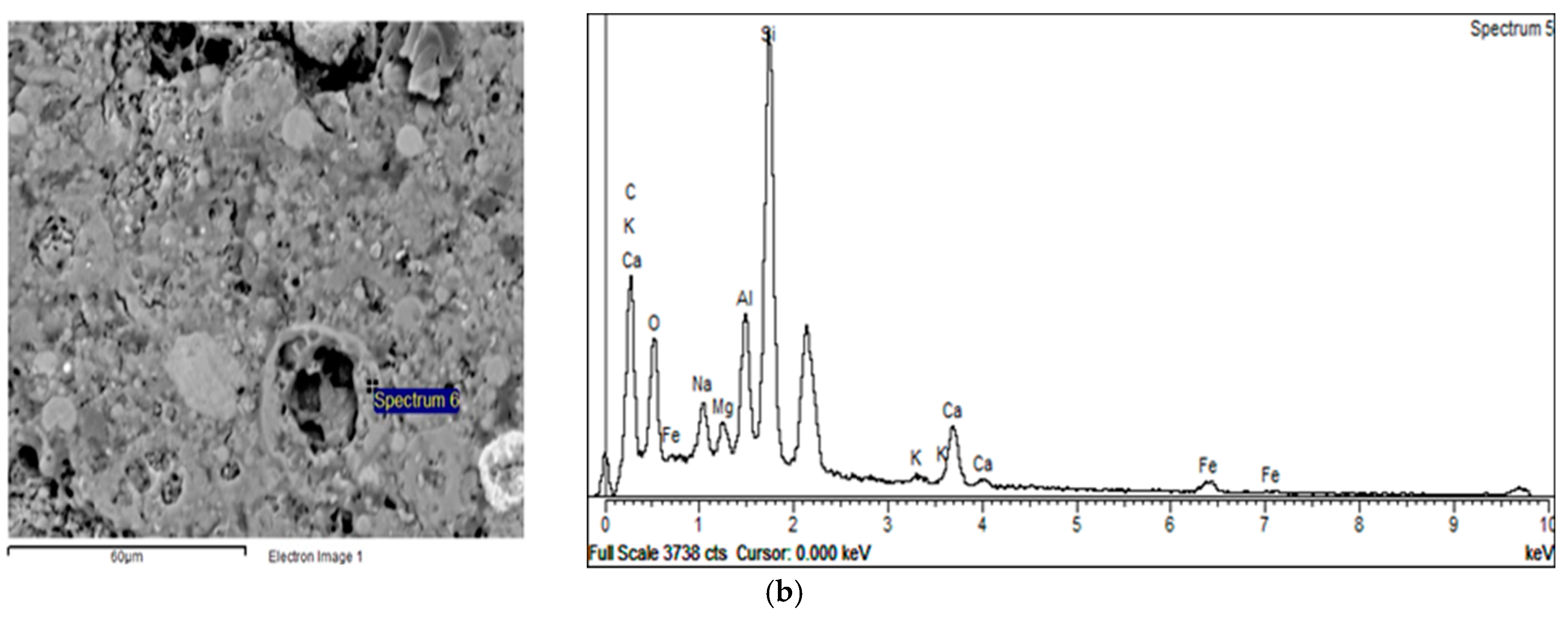
3.3. BSE-EDS Analysis
3.4. Textural Properties
4. Conclusions
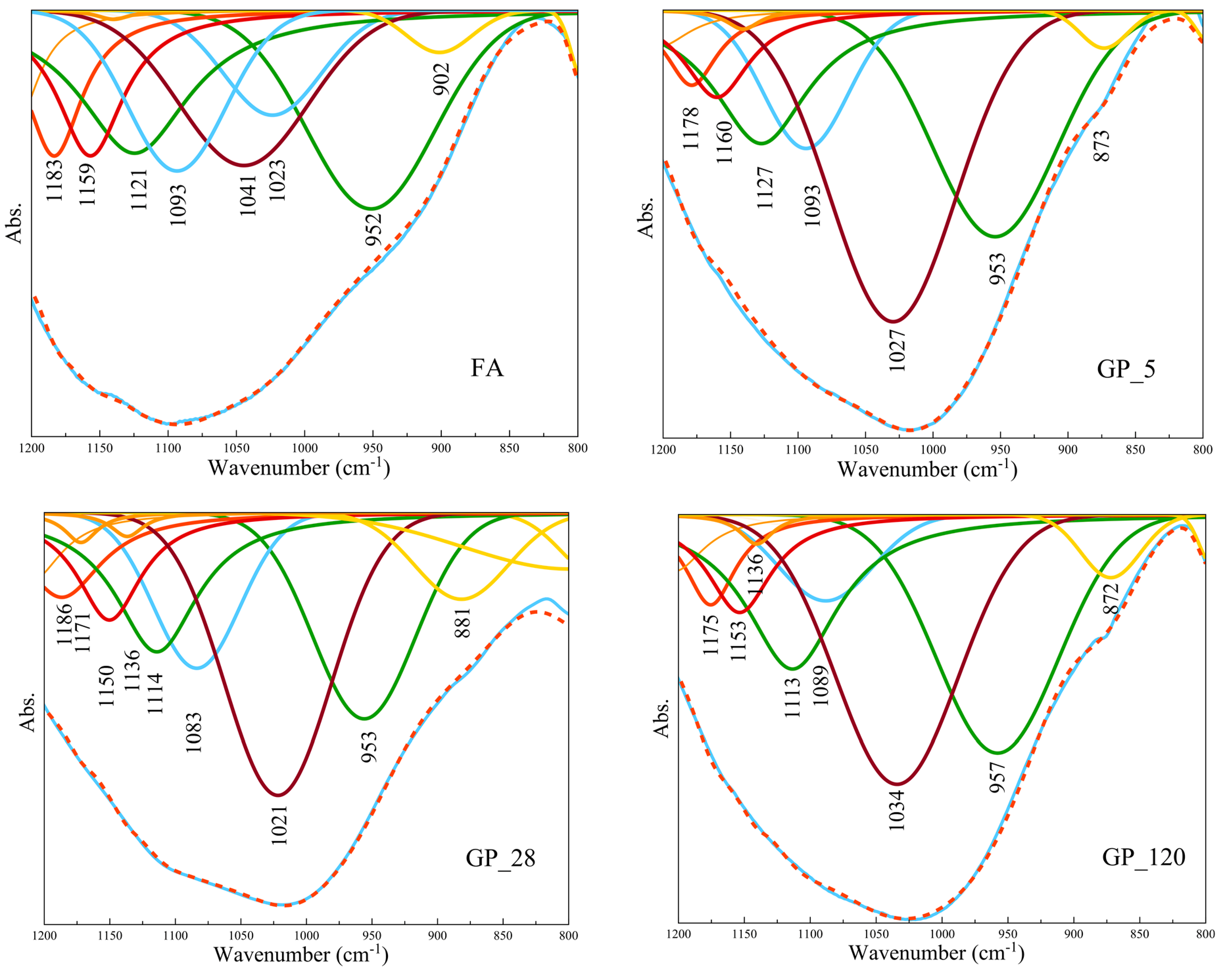
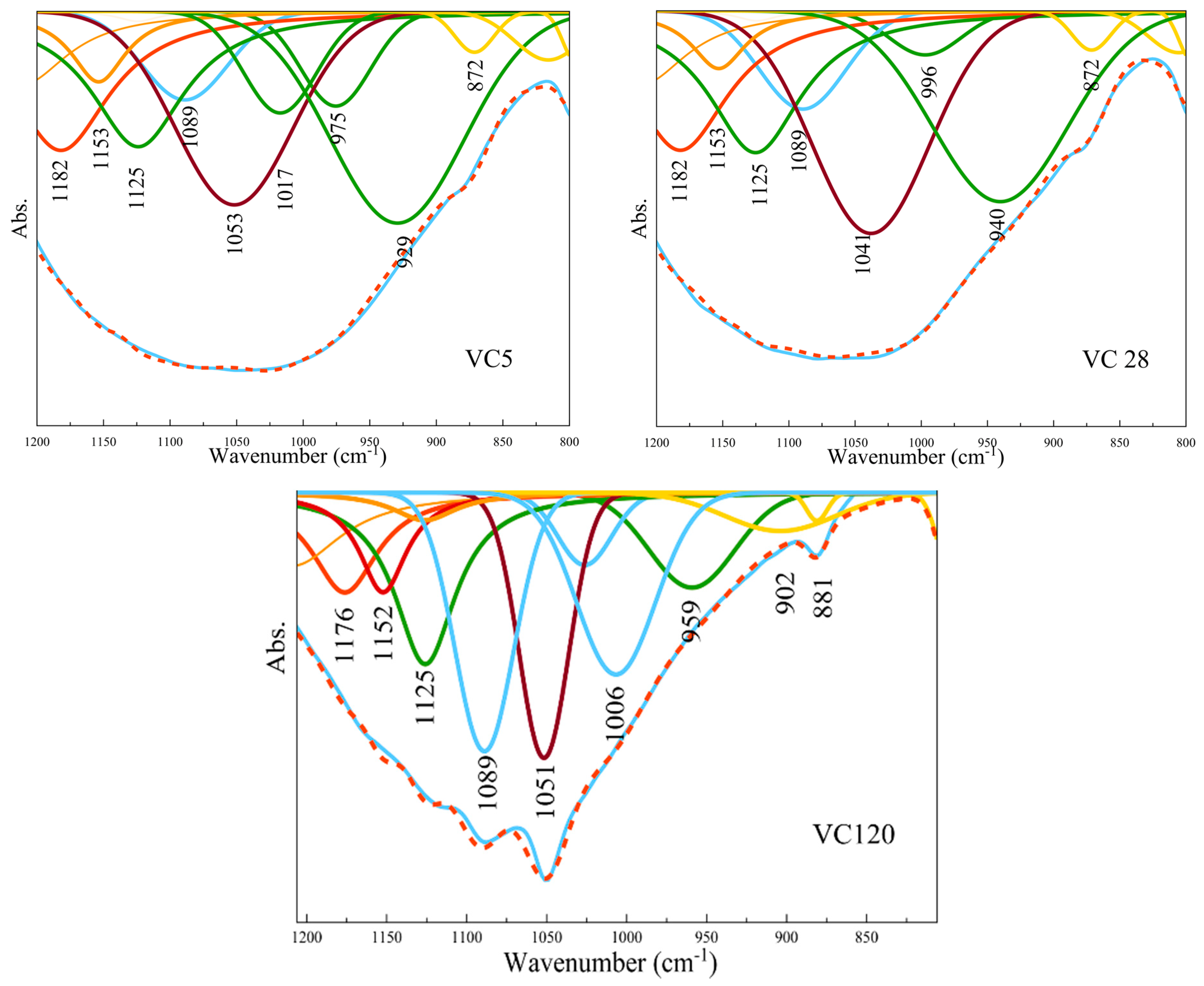
- FTIR analyses showed that the greatest changes occurred in the amorphous phase in both types of aggregate. FTIR analyses indicated a better dilution of fly ash in the aggregate obtained by crushing compared to the aggregate obtained by pelletization of what was confirmed by the changes in FTIR peak positions. Polymerization was most pronounced for the aggregates exposed to 65 °C for 5 days.
- FTIR analyses also showed that the process of carbonation occurred upon alkali activation across all investigated aggregate samples, this being more pronounced in the case of the aggregate obtained by pelletization in comparison to the aggregate obtained by the crushing method. Since the process of carbonation decreases pH value, it is presumed that the N-A-S-H structure of Gel 2 was probably formed at a low pH.
- The XRD analyses showed that neither the granulation method nor the curing conditions had a great influence on crystal phase composition. BSE-EDS analyses indicated that the structures obtained by pelletization contained phases developed during the incongruent dissolution of the parent material (silica and alumina phases), in addition to incompletely dissolved grains from the alkali-activated fly ash matrix.
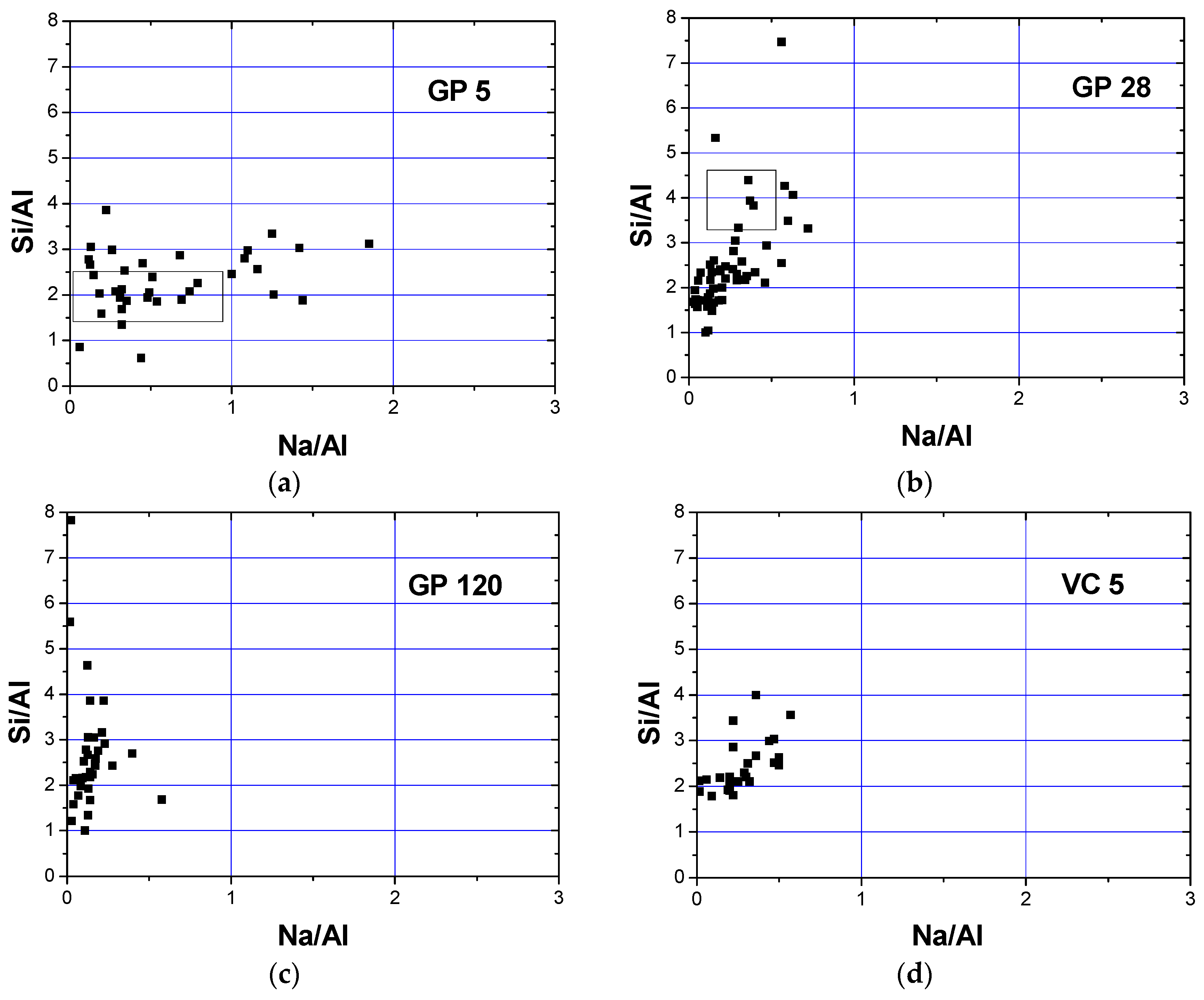
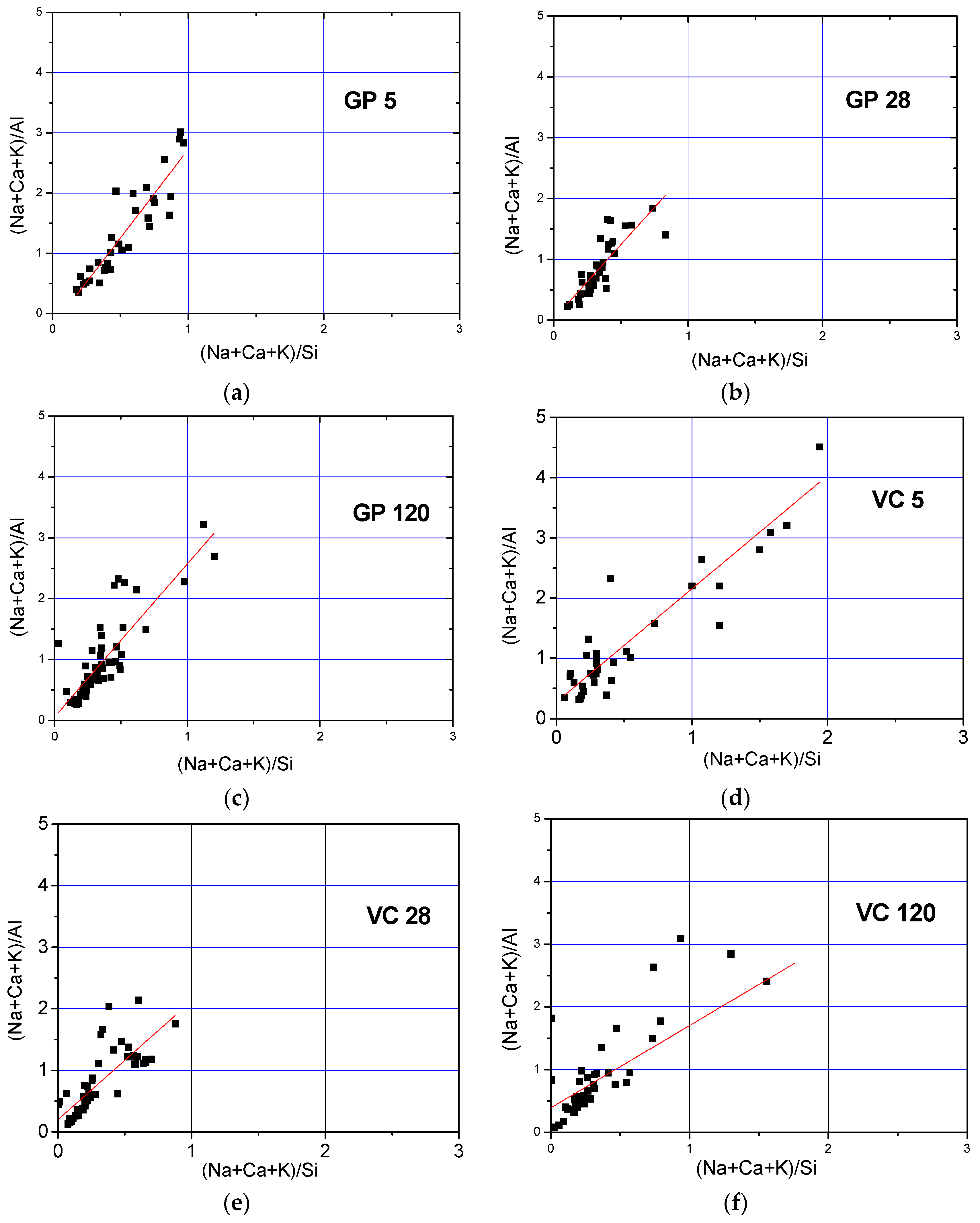
- The Si/Al atomic ratio was greater in the aggregate obtained by crushing than in that formed by pelletization. The lower Si/Al atomic ratio observed in the VC samples could be the result of two things: a higher activator/fly ash ratio value on the granule surface, indicating an increase in silicon concentration, and the decrease in pH value due to carbonation.
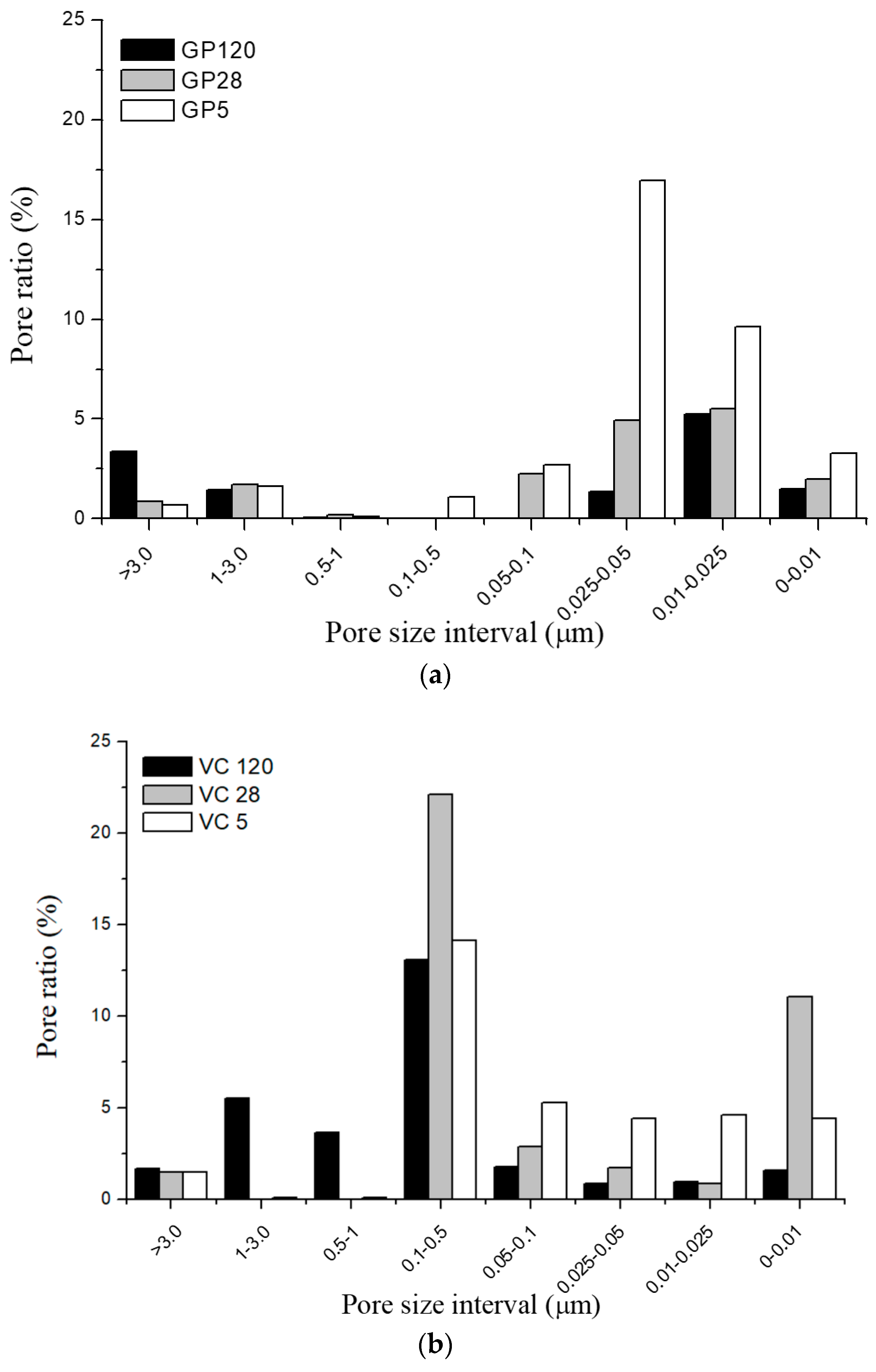
- The total porosity values were lower for the samples cured in normal conditions for 28 and 120 days in comparison to those cured at 65 °C. Pore size distribution was correlated to the amount of gel and the curing regime; exposure to an elevated temperature led to a higher ratio of pores smaller than 0.5 μm in both types of aggregates. Conversely, curing under normal conditions for 120 days led to the creation of larger pores.
Author Contributions
Funding
Conflicts of Interest
References
- Gjorv, O.E.; Sakai, K. Concrete Technology for a Sustainable Development in the 21st Century; E&FN Spon: London, UK, 2000. [Google Scholar]
- Bhardwaj, B.; Kumar, P. Waste foundry sand in concrete: A review. Constr. Build. Mater. 2017, 156, 661–674. [Google Scholar] [CrossRef]
- The Importance of Using Sustainable Aggregates. Available online: https://www.enr.com/articles/39810-the-importance-of-using-sustainable-aggregates (accessed on 16 July 2016).
- Limbachiya, M.C.; Meddah, M.S.; Ouchagour, Y. Use of recycled concrete aggregate in fly ash concrete. Constr. Build. Mater. 2012, 27, 439–449. [Google Scholar] [CrossRef]
- Zegardło, B.; Szeląg, M.; Ogrodnik, P. Concrete resistant to spalling made with recycled aggregate from sanitary ceramic wastes—The effect of moisture and porosity on destructive processes occurring in fire conditions. Constr. Build. Mater. 2018, 173, 58–68. [Google Scholar] [CrossRef]
- Cachim, B. Mechanical properties of brick aggregate concrete. Constr. Build. Mater. 2009, 23, 1292–1297. [Google Scholar] [CrossRef]
- Adaway, M.; Wang, Y. Recycled glass as a partial replacement for fine aggregate in structural concrete–Effects on compressive strength. Electron. J. Struct. Eng. 2015, 14, 116–122. Available online: https://www.researchgate.net/publication/282952856 (accessed on 17 May 2016).
- Ismail, Z.; Al-Hashmi, A. Use of waste plastic in concrete mixture as aggregate replacement. Waste Manag. 2008, 28, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, C.; Freuerborn, H.J.; Weir, A. Coal Combustion Products: A Global Perspective. In Proceedings of the World Coal Ash WOCA, Lexington, KY, USA, 22–25 April 2013. [Google Scholar]
- Ducman, V.; Radeka, M. Alkali activated green building materials—Selected case study of alkali activated aggregate. Ceram. Mod. Techn. 2019, in press. [Google Scholar] [CrossRef]
- Baykal, G.; Döven, A.G. Utilization of fly ash by pelletization process; theory, application areas and research results. Resour. Conserv. Recy. 2000, 30, 59–77. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Keyte, L.M. Fly ash glass chemistry and inorganic polymer cements. In Alkali Activated Materials-Structure, Processing, Properties and Industrial Applications, 1st ed.; Provis, J., van Deventer, J.S.J., Eds.; Woodhead Publishing Limited: Oxford/Cambridge, UK, 2009; pp. 15–36. [Google Scholar]
- Xie, J.; Kayali, O. Effect of initial water content and curing moisture conditions on the development of fly ash-based geopolymers in heat and ambient temperature. Constr. Build. Mater. 2014, 67, 20–28. [Google Scholar] [CrossRef]
- Walkley, B.; Nicolas, R.; Sani, M.; Gehman, J.; van Deventer, J.S.J.; Provis, J. Phase evolution of Na2O–Al2O3–SiO2–H2O gels in synthetic aluminosilicate binders. Dalton Trans. 2016, 45, 5521–5535. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Fernández-Jimenez, A.; Palomo, A. Alkali activation of fly ash, Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, L.; Komarneni, S.; Zhou, C.; Tong, D.; Yang, H.; Yu, W.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Provis, J.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.; Provis, J.; Walkley, B.; San Nicolas, R.; Gehman, J.; Brice, D.; Kilcullen, A.; Duxson, P.; van Deventer, J.S.J. Gel nanostructure in alkali-activated binders based on slag and fly ash and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jimenez, A.; Palomo, A. Alkali activation of fly ash, Part I: Effect of curing conditions on the carbonation of the reaction products. Fuel 2005, 84, 2048–2054. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jimenez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Harilal, B.; Thomas, J. Concrete made using cold bonded artificial aggregate. Am. J. Eng. Res. 2013, 1, 20–25. [Google Scholar]
- Snellings, R. Surface chemistry of calcium aluminosilicate glasses. J. Am. Ceram. Soc. 2015, 98, 303–314. [Google Scholar] [CrossRef]
- Snellings, R. Solution-controlled dissolution of supplementary cementitious material glasses at Ph 13: The effect of solution composition on glass dissolution rates. J. Am. Ceram. Soc. 2013, 96, 2467–2475. [Google Scholar] [CrossRef]
- Chappex, T.; Scrivener, K. The influence of aluminium on the dissolution of amorphous silica and its relation to alkali silica reaction. Cem. Concr. Res. 2012, 42, 1645–1649. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. Effect of alkali cations on aluminium incorporation in alkali activated materials gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Kobayashi, K.; Uno, Y. Influence of alkali on carbonation of concrete, Part 2-Influence of alkali in cement on rate of carbonation of concrete. Cem. Concr. Res. 1990, 20, 619–622. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiery, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef] [Green Version]
- BS EN 196-2:2015: Method of Testing Cement—Part 2: Chemical Analysis of Cement; BSI: London, UK, 2015.
- BS EN 450-1:2012: Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria; BSI: London, UK, 2012.
- BS EN 1097-3:1999: Tests for Mechanical and Physical Properties of Aggregates—Part 3: Determination of Loose Bulk Density and Voids; BSI: London, UK, 1999.
- Singh, G.B.; Subramaniam, K.V.L. Evaluation of sodium content and sodium hydroxide molarity on compressive strength of alkali activated low-calcium fly ash. Cem. Concr. Compos. 2017, 81, 122–132. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of alkali concentration on reaction kinetics of fly ash geopolymerization. J. Non-Cryst. Solids 2019, 505, 241–251. [Google Scholar] [CrossRef]
- BS EN 1097-6:2013: Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption; BSI: London, UK, 2013.
- Kovalchuk, G.; Fernández-Jiménez, A.; Palomo, A. Alkali-activated fly ash: Effect of thermal curing conditions on mechanical and microstructural development—Part II. Fuel 2005, 86, 315–322. [Google Scholar] [CrossRef]
- Clayden, N.; Esposito, S.; Aronne, A.; Pernice, P. Solid state 27 Al NMR and FTIR study of lanthanum aluminosilicate glasses. J. Non-Cryst. Solids 1999, 258, 11–19. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Reactivity and reaction products of alkali-activated, fly ash/slag paste. Constr. Build. Mater. 2015, 81, 303–312. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liegeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between alkali activated materials, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Use of infrared spectroscopy to study geopolymerization of heterogeneous amorphous aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Compos. 2019, in press. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Aspects 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Bobrowski, A.; Kmita, A.; Starowicz, M.; Stypuła, B.; Hutera, B. Effect of magnesium oxide nanoparticles on water glass structure. Arch. Found. Eng. 2012, 12, 9–12. [Google Scholar] [CrossRef]
- Gao, X.; Yuan, B.; Yu, Q.; Brouwers, H.J.H. Characterization and application of municipal solid waste incineration (MSWI) bottom ash and waste granite powder in alkali activated slag. J. Clean. Prod. 2017, 164, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Hajimohammadi, A.; Provis, J.; Deventer, J.S.J. Time-resolved and spatially resolved infrared spectroscopic observation of seeded nucleation controlling geopolymer gel formation. J. Colloid Interface Sci. 2011, 357, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Fernandez-Jimenez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 109, 180–191. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- García Loderio, I.; Fernandez-Jimenez, A.; Palomo, A.; MaCPhee, D.E. Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminum. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.; Lukey, G.; Mallicoat, S.; Kriven, W.; van Deventer, J S.J. Understanding the relationship between alkali activated material composition, microstructure and mechanical properties. Colloids Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Bernal, S.; Provis, J.L.; De Gutiérrez, R.M.; van Deventer, J.S.J. Accelerated carbonation testing of alkali-activated slag/metakaolin blended concretes: Effect of exposure conditions. Mater. Struct. 2015, 48, 653–669. [Google Scholar] [CrossRef]




| SiO2 | Al2O3 | Fe2O3 | SO3 | CaO | MgO | Na2O | K2O | Cl− | SiO2r 1 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| 56.1 | 19.7 | 5.36 | 3.1 | 6.95 | 1.83 | 0.60 | 2.05 | 0.007 | 37.34 | 4.28 |
| Aggregate | Oven Dried Particle Density, kg/m3 | Apparent Particle Density, kg/m3 | Water Absorption after 24 h, % |
|---|---|---|---|
| GP 5 | 1495 | 2585 | 28.2 |
| GP 28 | 1490 | 2526 | 27.5 |
| GP 120 | 1492 | 2530 | 26.0 |
| VC 5 | 1470 | 2400 | 26.4 |
| VC 28 | 1348 | 2570 | 35.3 |
| VC 120 | 1450 | 2350 | 26.5 |
| FA | GP 5 | GP 28 | GP 120 | VC 5 | VC 28 | VC 120 |
|---|---|---|---|---|---|---|
| Absorption peak position (cm−1) | ||||||
| 873 | 881 | 872 | 872 | 872 | 881 | |
| 902 | 902 | |||||
| 952 | 953 | 953 | 957 | 929 | 940 | 959 |
| 975 | ||||||
| 1023 | 1027 | 1021 | 1034 | 1017 | 996 | 1006 |
| 1041 | 1053 | 1041 | 1051 | |||
| 1093 | 1093 | 1083 | 1089 | 1089 | 1089 | 1089 |
| 1121 | 1127 | 1114 | 1113 | 1125 | 1125 | 1125 |
| 1136 | 1136 | |||||
| 1159 | 1160 | 1150 | 1153 | 1153 | 1153 | 1152 |
| 1171 | ||||||
| 1183 | 1178 | 1186 | 1175 | 1182 | 1182 | 1176 |
| Sample | Slope of the Linear Curve Si/Al |
|---|---|
| GP 5 | 2.94 |
| GP 28 | 2.41 |
| GP 120 | 2.51 |
| VC 5 | 1.88 |
| VC 28 | 1.93 |
| VC 120 | 1.95 |
| Sample | Total Porosity (%) |
|---|---|
| GP 5 | 36.1 |
| GP 28 | 17.4 |
| GP 120 | 12.9 |
| VC 5 | 34.6 |
| VC 28 | 40.2 |
| VC 120 | 29.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudić, O.; Ducman, V.; Malešev, M.; Radonjanin, V.; Draganić, S.; Šupić, S.; Radeka, M. Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes. Materials 2019, 12, 776. https://doi.org/10.3390/ma12050776
Rudić O, Ducman V, Malešev M, Radonjanin V, Draganić S, Šupić S, Radeka M. Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes. Materials. 2019; 12(5):776. https://doi.org/10.3390/ma12050776
Chicago/Turabian StyleRudić, Ognjen, Vilma Ducman, Mirjana Malešev, Vlastimir Radonjanin, Suzana Draganić, Slobodan Šupić, and Miroslava Radeka. 2019. "Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes" Materials 12, no. 5: 776. https://doi.org/10.3390/ma12050776
APA StyleRudić, O., Ducman, V., Malešev, M., Radonjanin, V., Draganić, S., Šupić, S., & Radeka, M. (2019). Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes. Materials, 12(5), 776. https://doi.org/10.3390/ma12050776










