Effect of Stoichiometry on Shape Memory Properties and Functional Stability of Ti–Ni–Pd Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cyclic Responce of Transformation Temperatures
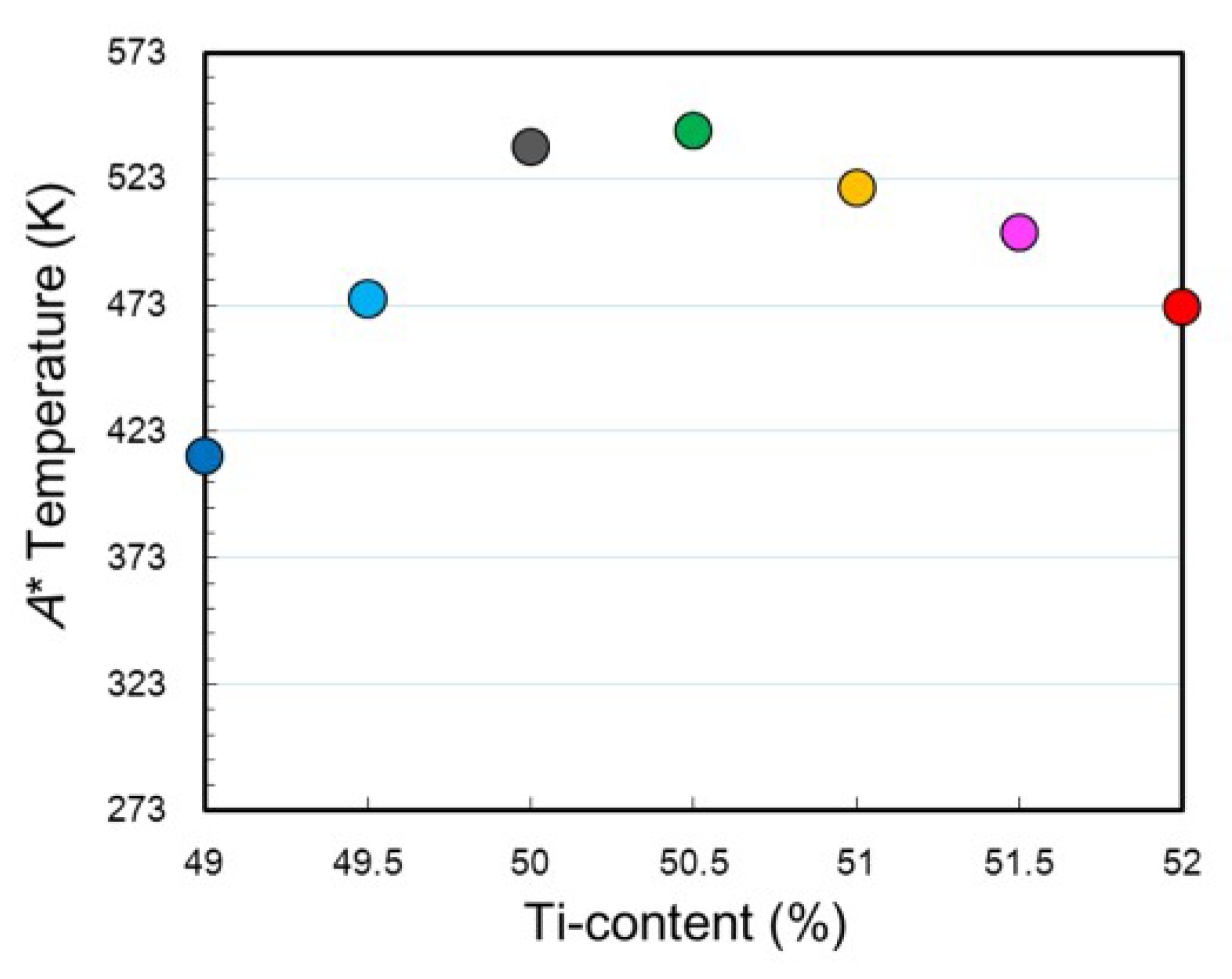
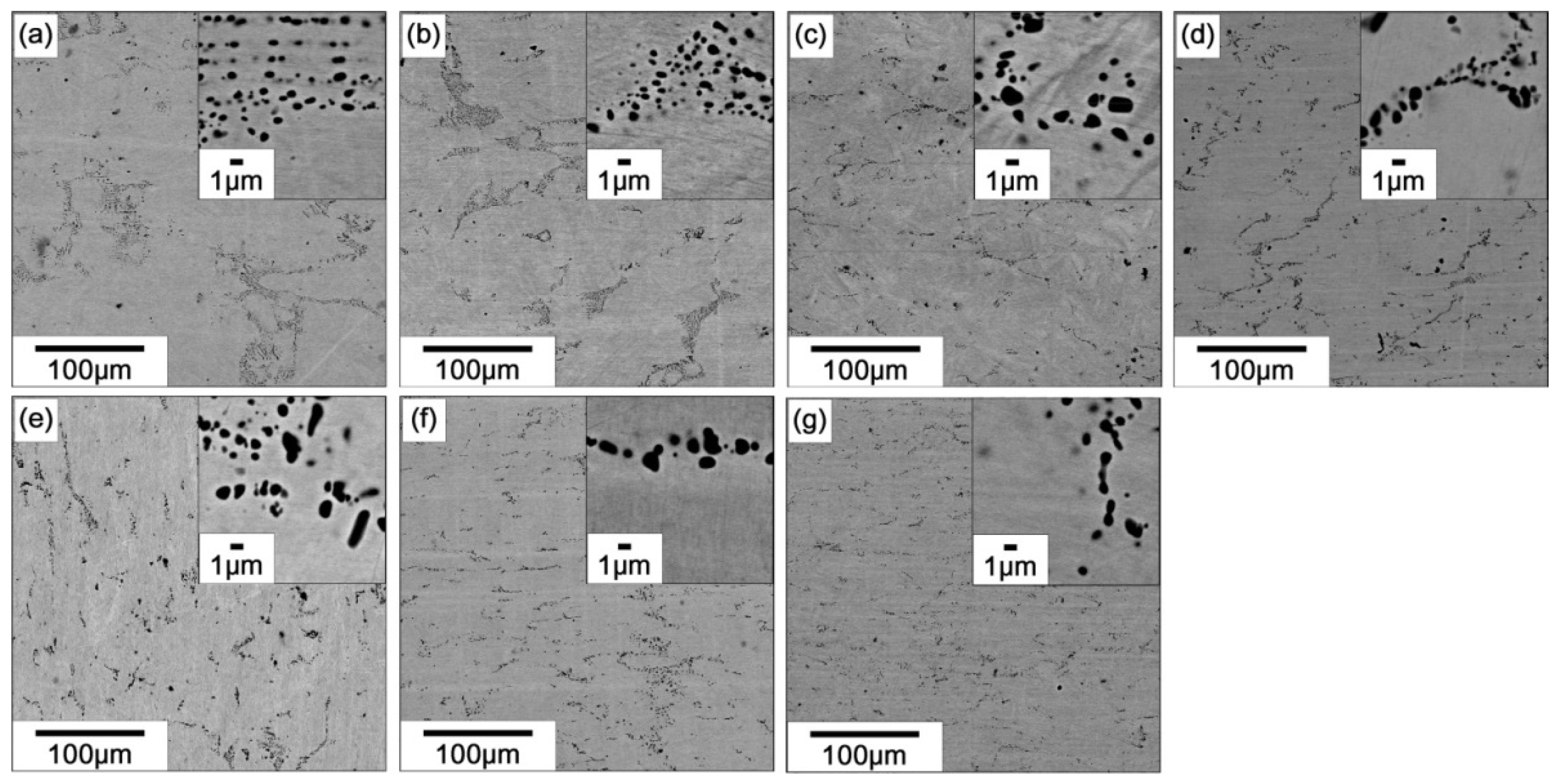
3.2. Ti Content Dependence of Transformation Temperature and Microstructure
3.3. Shape Memory Properties
4. Conclusions
- (1)
- The Ti content of the matrix region increases linearly while the Ni content of the matrix region decreases with increasing the nominal Ti content. The martensitic transformation temperature shows the highest value when the Ti content in the matrix is near the stoichiometric composition and it decreases with increasing or decreasing the Ti content as compared to the stoichiometric composition.
- (2)
- The martensitic transformation temperature decreases during thermal cycling and the degree of decrease in the transformation temperature becomes more pronounced as the composition of the alloy departs from the stoichiometric composition on both Ti-rich and Ti-lean sides. Ti2Pd and P phases are formed during thermal cycling in Ti-rich and Ti-lean alloys, respectively.
- (3)
- Both Ti-rich and Ti-lean alloys exhibit excellent dimensional stabilities, higher recovery ratio and larger work output during thermal cycling under constant stresses when compared with the alloys with near-stoichiometric composition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Humbeeck, J. Shape memory alloys: A material and a technology. Adv. Eng. Mater. 2001, 3, 837–850. [Google Scholar] [CrossRef]
- Benafan, O.; Brown, J.; Calkins, F.; Kumar, P.; Stebner, A.; Turner, T.; Vaidyanathan, R.; Webster, J.; Young, M. Shape memory alloy actuator design: Casmart collaborative best practices and case studies. Int. J. Mech. Mater. Des. 2014, 10, 1–42. [Google Scholar] [CrossRef]
- Yamauchi, K.; Ohkata, I.; Tsuchiya, K.; Miyazaki, S. Shape Memory and Superelastic Alloys: Technologies and Applications, 1st ed.; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Ma, J.; Karaman, I.; Noebe, R.D. High temperature shape memory alloys. Int. Mater. Rev. 2010, 55, 257–314. [Google Scholar] [CrossRef]
- Firstov, G.S.; Kosorukova, T.A.; Koval, Y.N.; Verhovlyuk, P.A. Directions for high-temperature shape memory alloys’ improvement: Straight way to high-entropy materials? Shape Mem. Superelasticity 2015, 1, 400–407. [Google Scholar] [CrossRef]
- Sehitoglu, H.; Patriarca, L.; Wu, Y. Shape memory strains and temperatures in the extreme. Curr. Opin. Solid State Mater. Sci. 2017, 21, 113–120. [Google Scholar] [CrossRef]
- Niendorf, T.; Krooß, P.; Somsen, C.; Eggeler, G.; Chumlyakov, Y.I.; Maier, H.J. Martensite aging–Avenue to new high temperature shape memory alloys. Acta Mater. 2015, 89, 298–304. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Takebe, T.; Shimojo, M. Phase transformation and shape memory effect of Ti–Pd–Pt–Zr high-temperature shape memory alloys. Shape Mem. Superelasticity 2017, 3, 381–391. [Google Scholar] [CrossRef]
- Canadinc, D.; Trehern, W.; Ma, J.; Karaman, I.; Sun, F.; Chaudhry, Z. Ultra-high temperature multi-component shape memory alloys. Scr. Mater. 2019, 158, 83–87. [Google Scholar] [CrossRef]
- Vermaut, P.; Declairieux, C.; Ochin, P.; Kolomytsev, V.; Pasko, A.; Monastyrsky, G.; Denquin, A.; Portier, R. Martensitic transformation and shape memory effect at very high temperatures in HfPd, and TiAu intermetallic compounds. J. Alloys Compd. 2013, 577 (Suppl. S1), S388–S392. [Google Scholar] [CrossRef]
- Hsieh, S.F.; Wu, S.K. Room-temperature phases observed in Ti53-xNi47Zrx high-temperature shape memory alloys. J. Alloys Compd. 1998, 266, 276–282. [Google Scholar] [CrossRef]
- Meng, X.L.; Cai, W.; Zheng, Y.F.; Zhao, L.C. Phase transformation and precipitation in aged Ti–Ni–Hf high-temperature shape memory alloys. Mater. Sci. Eng. A 2006, 438–440, 666–670. [Google Scholar] [CrossRef]
- Frenzel, J.; Wieczorek, A.; Opahle, I.; Maaß, B.; Drautz, R.; Eggeler, G. On the effect of alloy composition on martensite start temperatures and latent heats in Ni–Ti-based shape memory alloys. Acta Mater. 2015, 90, 213–231. [Google Scholar] [CrossRef]
- Umale, T.; Salas, D.; Tomes, B.; Arroyave, R.; Karaman, I. The effects of wide range of compositional changes on the martensitic transformation characteristics of NiTiHf shape memory alloys. Scr. Mater. 2019, 161, 78–83. [Google Scholar] [CrossRef]
- Acar, E.; Karaca, H.E.; Tobe, H.; Noebe, R.D.; Chumlyakov, Y.I. Characterization of the shape memory properties of a Ni45.3Ti39.7Hf10Pd5 alloy. J. Alloys Comp. 2013, 578, 297–302. [Google Scholar] [CrossRef]
- Karaca, H.E.; Acar, E.; Ded, G.S.; Saghaian, S.M.; Basaran, B.; Tobe, H.; Kok, M.; Maier, H.J.; Noebe, R.D.; Chumlyakov, Y.I. Microstructure and transformation related behaviors of a Ni45.3Ti29.7Hf20Cu5 high temperature shape memory alloy. Mater. Sci. Eng. A 2015, 627, 82–94. [Google Scholar] [CrossRef]
- Kockar, B.; Karaman, I.; Kim, J.I.; Chumlyakov, Y. A method to enhance cyclic reversibility of NiTiHf high temperature shape memory alloys. Scr. Mater. 2006, 54, 2203–2208. [Google Scholar] [CrossRef]
- Meng, X.L.; Zheng, Y.F.; Wang, Z.; Zhao, L.C. Shape memory properties of the Ti36Ni49Hf15 high temperature shape memory alloy. Mater. Lett. 2000, 45, 128–132. [Google Scholar] [CrossRef]
- Bigelow, G.S.; Garg, A.; Padula, S.A., II; Gaydosh, D.J.; Noebe, R.D. Load-biased shape memory and superelastic properties of a precipitation strengthened high temperature Ni50.3Ti29.7Hf20 alloy. Scr. Mater. 2011, 64, 725–728. [Google Scholar] [CrossRef]
- Karaca, H.E.; Saghaian, S.M.; Ded, G.; Tobe, H.; Basaran, B.; Maier, H.J.; Noebe, R.D.; Chumlyakov, Y.I. Effects of nanoprecipitation on the shape memory and material properties of an Ni-rich NiTiHf high temperature shape memory alloy. Acta Mater. 2013, 61, 7422–7431. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Noebe, R.D.; Santamarta, R.; Pons, J. Effect of precipitation on the microstructure and the shape memory response of the Ni50.3Ti29.7Zr20 high temperature shape memory alloy. Scr. Mater. 2013, 69, 354–357. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Santamarta, R.; Pons, J.; Hayrettin, C.; Noebe, R.D. Relationship between crystallographic compatibility and thermal hysteresis in Ni-rich NiTiHf and NiTiZr high temperature shape memory alloys. Acta Mater. 2016, 121, 374–383. [Google Scholar] [CrossRef]
- Karakoc, O.; Hayrettin, C.; Canadinc, D.; Karaman, I. Role of applied stress level on the actuation fatigue behavior of NiTiHf high temperature shape memory alloys. Acta Mater. 2018, 153, 156–168. [Google Scholar] [CrossRef]
- Xu, Y.; Shimizu, S.; Suzuki, Y.; Otsuka, K.; Ueki, T.; Mitose, K. Recovery and recrystallization processes in Ti–Pd–Ni high-temperature shape memory alloys. Acta Mater. 1997, 45, 1503–1511. [Google Scholar] [CrossRef]
- Shimizu, S.; Xu, Y.; Okunishi, E.; Tanaka, S.; Otsuka, K.; Mitose, K. Improvement of shape memory characteristics by precipitation-hardening of Ti–Pd–Ni alloys. Mater. Lett. 1998, 34, 23–29. [Google Scholar] [CrossRef]
- Bigelow, G.S.; Padula, S.A., II; Grag, A.; Gaydosh, D.; Noebe, R.D. Characterization of ternary NiTiPd high-temperature shape-memory alloys under load-biased thermal cycling. Metall. Mater. Trans. A 2010, 41, 3065–3079. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D. Work output of the two-way shape memory effect in Ti50.5Ni24.5Pd25 high-temperature shape memory alloy. Scr. Mater. 2011, 65, 903–906. [Google Scholar] [CrossRef]
- Kockar, B.; Atli, K.C.; Ma, J.; Haouaoui, M.; Karaman, I.; Nagasako, M.; Kainuma, R. Role of severe plastic deformation on the cyclic reversibility of a Ti50.3Ni33.7Pd16 high temperature shape memory alloy. Acta Mater. 2010, 58, 6411–6420. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D.; Garg, A.; Chumlyakov, Y.I.; Kireeva, I.V. Improvement in the shape memory response of Ti50.5Ni24.5Pd25 high-temperature shape memory alloy with scandium microalloying. Metall. Mater. Trans. A 2010, 41, 2485–2497. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D. Influence of tantalum additions on the microstructure and shape memory response of Ti50.5Ni24.5Pd25 high-temperature shape memory alloy. Mater. Sci. Eng. A 2014, 613, 250–258. [Google Scholar] [CrossRef]
- Khan, I.M.; Kim, H.Y.; Nam, T.H.; Miyazaki, S. Effect of Cu addition on the high temperature shape memory properties of Ti50Ni25Pd25 alloy. J. Alloys Compd. 2013, 577, S383–S387. [Google Scholar] [CrossRef]
- Khan, I.M.; Kim, H.Y.; Nam, T.H.; Miyazaki, S. Formation of nanoscaled precipitates and their effects on the high-temperature shape-memory characteristics of a Ti50Ni15Pd25Cu10 alloy. Acta Mater. 2012, 60, 5900–5913. [Google Scholar] [CrossRef]
- Sasaki, T.T.; Hornbuckle, B.C.; Noebe, R.D.; Bigelow, G.S.; Weaver, M.L.; Thompson, G.B. Effect of aging on microstructure and shape memory properties of a Ni-48Ti-25Pd (At. Pct) alloy. Metall. Mater. Trans. A 2013, 44, 1388–1400. [Google Scholar] [CrossRef]
- Coppa, A.C.; Kapoor, M.; Hornbuckle, B.C.; Weaver, M.L.; Noebe, R.D.; Thompson, G.B. Influence of dilute Hf additions on precipitation and martensitic transformation in Ni-Ti-Pd alloys. JOM 2015, 67, 2244–2250. [Google Scholar] [CrossRef]
- Benafan, O.; Garg, A.; Noebe, R.D.; Bigelow, G.S.; Padula, S.A., II; Gaydosh, D.J.; Vaidyanathan, R.; Clausen, B.; Vogel, S.C. Thermomechanical behavior and microstructural evolution of a Ni(Pd)-rich Ni24.3Ti49.7Pd26 high temperature shape memory alloy. J. Alloys Compd. 2015, 643, 275–289. [Google Scholar] [CrossRef]
- Namigata, Y.; Hattori, Y.; Khan, I.M.; Kim, H.Y.; Miyazaki, S. Enhancement of shape memory properties through precipitation hardening in a Ti-rich Ti-Ni-Pd high temperature shape memory alloy. Mater. Trans. 2016, 57, 241–249. [Google Scholar] [CrossRef]
- Atli, K.C.; Karaman, I.; Noebe, R.D.; Garg, A.; Chumlyakov, Y.I.; Kireeva, I.V. Shape memory characteristics of Ti49.5Ni25Pd25Sc0.5 high-temperature shape memory alloy after severe plastic deformation. Acta Mater. 2011, 59, 4747–4760. [Google Scholar] [CrossRef]
- Khan, I.M.; Kim, H.Y.; Namigata, Y.; Nam, T.H.; Miyazaki, S. Combined effects of work hardening and precipitation strengthening on the cyclic stability of TiNiPdCu-based high-temperature shape memory alloys. Acta Mater. 2013, 61, 4797–4810. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef] [Green Version]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.F.X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 58, 3444–3458. [Google Scholar] [CrossRef]
- Carl, M.; Smith, J.D.; Doren, B.V.; Young, M.L. Effect of Ni-content on the transformation temperatures in NiTi-20 at. % Zr high temperature shape memory alloys. Metals 2017, 7, 511. [Google Scholar] [CrossRef]
- Narayana, P.L.; Kim, S.W.; Hong, J.K.; Reddy, N.S.; Yeom, J.T. Estimation of transformation temperatures in Ti–Ni–Pd shape memory alloys. Met. Mater. Int. 2018, 24, 919–925. [Google Scholar] [CrossRef]
- Delville, R.; Schryvers, D. Transmission electron microscopy study of combined precipitation of Ti2Ni(Pd) and Ti2Pd(Ni) in a Ti50Ni30Pd20 alloy. Intermetallics 2010, 18, 2353–2360. [Google Scholar] [CrossRef]
- Kovarik, L.; Yang, F.; Garg, A.; Diercks, D.; Kaufman, M.; Noebe, R.D.; Mills, M.J. Structural analysis of a new precipitate phase in high-temperature TiNiPt shape memory alloys. Acta Mater. 2010, 58, 4660–4673. [Google Scholar] [CrossRef]
- Yang, F.; Kovarik, L.; Phillipa, P.J.; Noebe, R.D.; Mills, M.J. Characterizations of precipitate phases in a Ti–Ni–Pd alloy. Scr. Mater. 2012, 67, 145–148. [Google Scholar] [CrossRef]
- Coppa, A.C.; Kapoor, M.; Noebe, R.D.; Thompson, G.B. The compositional stability of the P-phase in Ni-Ti-Pd shape memory alloys. Intermetallics 2015, 67, 56–62. [Google Scholar] [CrossRef]
- Ramaiah, K.V.; Saikrishna, C.N.; Gouthama; Bhaumik, S.K. Microstructure and transformation behaviour of Ni75-XTiXPd25 high temperature shape memory alloys. J. Alloys Compd. 2013, 554, 319–326. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, Y.; Taguchi, T.; Kim, H.Y.; Miyazaki, S. Effect of Stoichiometry on Shape Memory Properties and Functional Stability of Ti–Ni–Pd Alloys. Materials 2019, 12, 798. https://doi.org/10.3390/ma12050798
Hattori Y, Taguchi T, Kim HY, Miyazaki S. Effect of Stoichiometry on Shape Memory Properties and Functional Stability of Ti–Ni–Pd Alloys. Materials. 2019; 12(5):798. https://doi.org/10.3390/ma12050798
Chicago/Turabian StyleHattori, Yuki, Takahiro Taguchi, Hee Young Kim, and Shuichi Miyazaki. 2019. "Effect of Stoichiometry on Shape Memory Properties and Functional Stability of Ti–Ni–Pd Alloys" Materials 12, no. 5: 798. https://doi.org/10.3390/ma12050798



