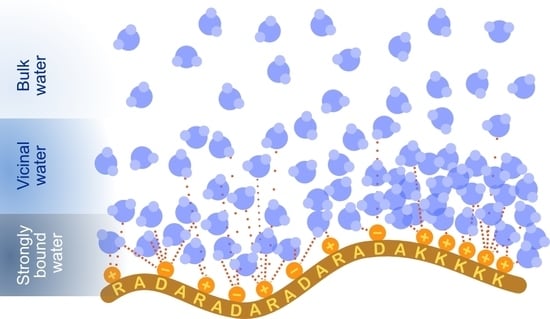
Charge and Peptide Concentration as Determinants of the Hydrogel Internal Aqueous Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Hydrogel Preparation
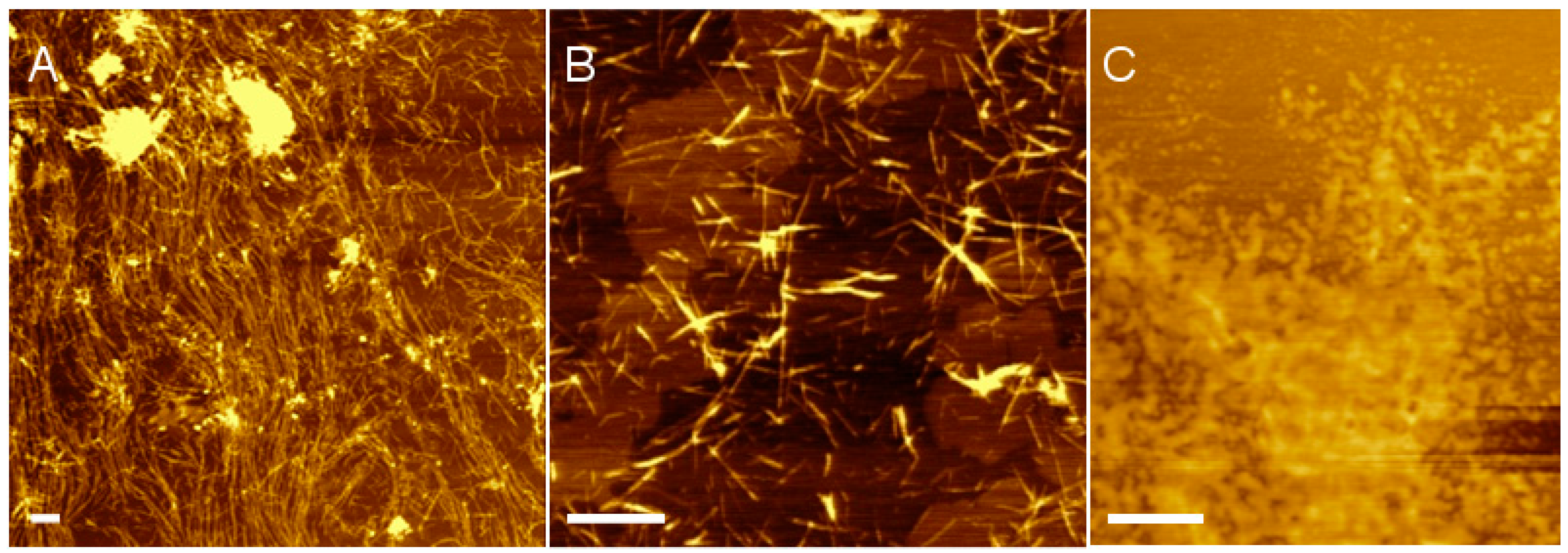
2.3. Atomic Force Microscopy
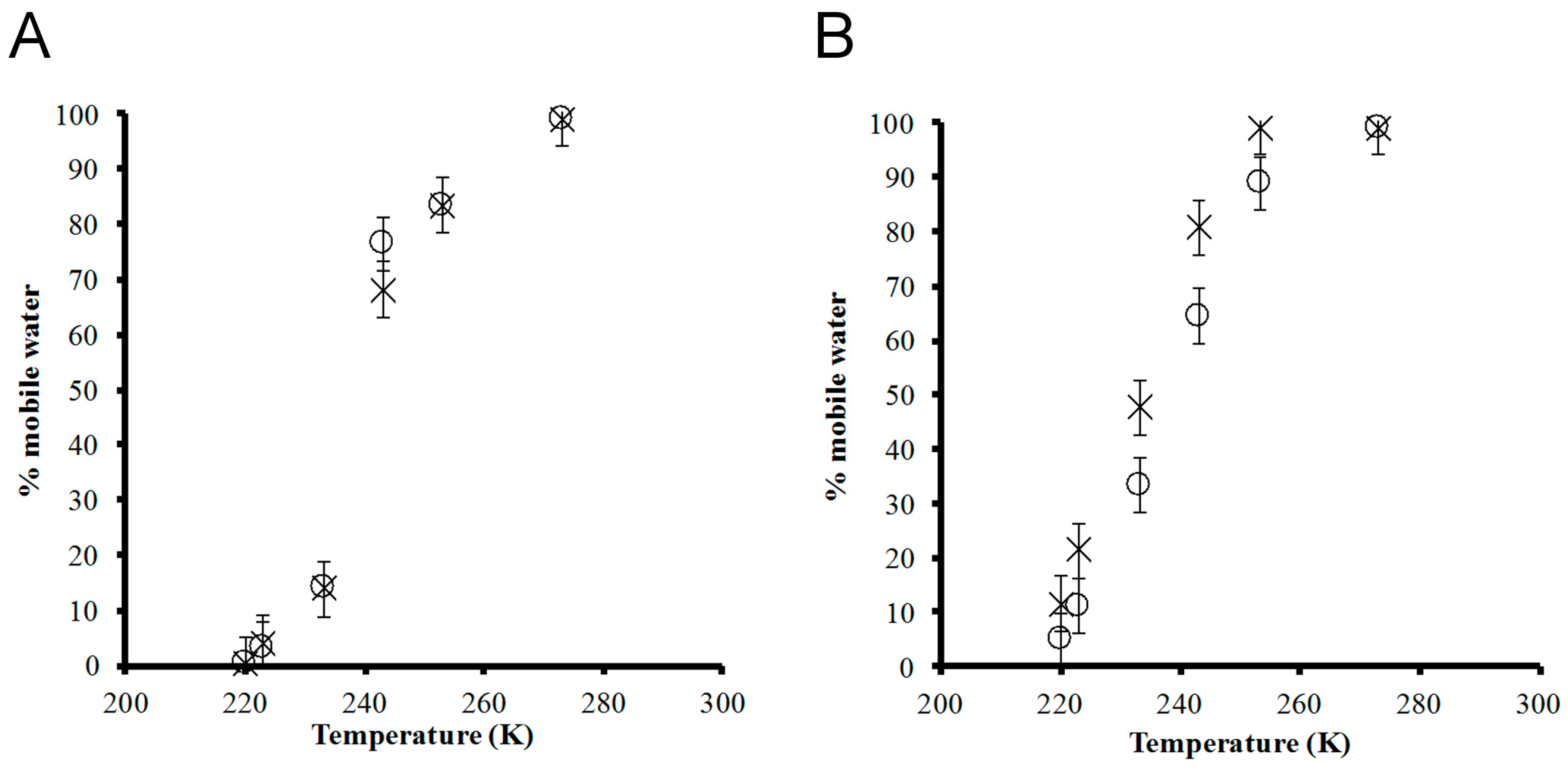
2.4. Solid–State 2H NMR
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Johnson, T.D.; Christman, K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2013, 10, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Koene, M.; Lischer, C. Intraarticular application of polyacrylamide hydrogel as a treatment of osteoarthritis in the distal interphalangeal joint: Case series with 12 horses. Pferdeheilkunde 2012, 28, 650–656. [Google Scholar]
- Zhang, S.; Holmes, S.; Lockshing, C.; Rich, A. Spontaneous Assembly of a Self-Complementary Oligopeptide to form a Stable Macroscopic Membrane. Proc. Natl. Acad. Sci. USA 1993, 90, 3334–3338. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.C.; de Lacalle, S.; Su, X.; Liu, G.; Rich, A.; Zhang, S.G. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl. Acad. Sci. USA 2000, 97, 6728–6733. [Google Scholar] [CrossRef] [PubMed]
- Schachner, M. Nervous engineering. Nature 2000, 405, 747–748. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, H.; Luo, H.; Wang, S.; Zhou, Q.; Du, X.; Tang, C.; Chen, L.; Liu, J.; Shi, Y.K.; et al. Temperature and pH effects on biophysical and morphological properties of self-assembling peptide RADA16-1. J. Pept. Sci. 2008, 14, 152–162. [Google Scholar] [CrossRef]
- Ellis-Behnke, R.G.; Liang, Y.X.; Tay, D.K.C.; Kau, P.W.F.; Schneider, G.E.; Zhang, S.; Wu, W.; So, K.F. Nano hemostat solution: Immediate hemostasis at the nanoscale. Nanomedicine 2006, 2, 207–215. [Google Scholar] [CrossRef]
- Zou, Z.; Zheng, Q.; Wu, Y.; Guo, X.; Yang, S.; Li, J.; Pan, H. Biocompatibility and bioactivity of designer self-assembling nanofiber scaffold containing FGL motif for rat dorsal root ganglion neurons. J. Biomed. Mater. Res. A 2010, 95, 1125–1131. [Google Scholar] [CrossRef]
- Koss, K.; Tsui, C.; Unsworth, L.D. Induced Neural Differentiation of MMP-2 Cleaved (RADA)4 Drug Delivery Systems. J. Control. Release 2016, 243, 204–213. [Google Scholar] [CrossRef]
- Saini, A. (RADA)4 Self-Assembling Peptide Based Hydrogels: Design, Characterization and In-Vitro Biological Evaluation. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2016. [Google Scholar]
- Saini, A.; Serrano, K.; Koss, K.; Unsworth, L.D. Evaluation of the hemocompatibility and rapid hemostasis of (RADA)4 peptide-based hydrogels. Acta Biomater. 2016, 31, 71–79. [Google Scholar] [CrossRef]
- Zhang, S.; Lockshin, C.; Cook, R.; Rich, A. Unusually stable beta-sheet formation in an ionic self-complementary oligopeptide. Biopolymers 1994, 34, 663–672. [Google Scholar] [CrossRef]
- Ellis-Behnke, R.G.; Liang, Y.X.; You, S.W.; Tay, D.K.C.; Zhang, S.; So, K.F.; Schneider, G.E. Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. USA 2006, 103, 5054–5059. [Google Scholar] [CrossRef]
- Segers, V.F.M.; Lee, R.T. Local delivery of proteins and the use of self-assembling peptides. Drug Discov. Today 2007, 12, 561–568. [Google Scholar] [CrossRef]
- Guo, J.; Leung, K.K.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.H.; Zhang, W.; Pu, J.K.; Ng, G.K.; Wong, W.M.; et al. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine 2009, 5, 345–351. [Google Scholar] [CrossRef]
- Gun’ko, M.V.; Savina, I.N.; Mikhalovsky, S.V. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Mueller-Plathe, F. Different states of water in hydrogels? Macromolecules 1998, 31, 6721–6723. [Google Scholar] [CrossRef]
- Kim, Y.S.; Dong, L.M.; Hickner, M.A.; Glass, T.E.; Webb, V.; McGrath, J.E. State of water in disulfonated poly(arylene ether sulfone) copolymers and a perfluorosulfonic acid copolymer (nafion) and its effect on physical and electrochemical properties. Macromolecules 2003, 36, 6281–6285. [Google Scholar] [CrossRef]
- Mequanint, K.; Sheardown, H. 2-methacryloyloxyethyl N-butylcarbamate: A new co-monomer for synthesis of polyurethane hydrogels with improved mechanical properties for biomedical applications. J. Biomater. Sci. Polym. Ed. 2005, 16, 1303–1318. [Google Scholar] [CrossRef]
- Mequanint, K.; Patel, A.; Bezuidenhout, D. Synthesis, swelling behavior, and biocompatibility of novel physically cross-linked polyurethane-block-poly(glycerol methacrylate) hydrogels. Biomacromolecules 2006, 7, 883–891. [Google Scholar] [CrossRef]
- Miwa, Y.; Ishida, H.; Tanaka, M.; Mochizuki, A. 2H-NMR and 13-C NMR Study of the Hydration Behavior of Poly(2-methoxyethyl acrylate), Poly(2-hydroxyethyl methacrylate) and Poly(tetrahydrofurfuryl acrylate) in Relation to Their Blood Compatibility as Biomaterials. J. Biomater. Sci. 2010, 21, 1911–1924. [Google Scholar] [CrossRef]
- Tanaka, M.; Mochizuki, A. Clarification of the Blood Compatibility Mechanism by Controlling the Water Structure at the Blood-Poly(meth)acrylate Interface. J. Biomater. Sci. Polym. Ed. 2010, 21, 1849–1863. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Saini, A.; Koss, K.; Unsworth, L.D. Effect of Peptide Concentration on Water Structure, Morphology, and Thermal Stability of Self-Assembling (RADA)4 Peptide Matrices. J. Biomater. Tissue Eng. 2014, 4, 895–905. [Google Scholar] [CrossRef]
- Boyle, N.G.; Coey, J.M.D.; McBrierty, V.J. Low-temperature behaviour of water in nafion membranes. Chem. Phys. Lett. 1982, 86, 16–19. [Google Scholar] [CrossRef]
- Xing, J.Z.; Lu, L.; Unsworth, L.D.; Major, P.W.; Doschak, M.R.; Kaipatur, N.R. RANKL Release from Self-Assembling Nanofiber Hydrogels for Inducing Osteoclastogenesis In Vitro. Acta Biomater. 2017, 49, 306–315. [Google Scholar] [CrossRef]
- Bernard, G.M.; Goyal, A.; Miskolzie, M.; McKay, R.; Wu, Q.; Wasylishen, R.E.; Michaelis, V.K. Methylammonium Lead Chloride: A Sensitive Sample for an Accurate NMR Thermometer. J. Magn. Reson. Imaging 2017, 283, 14–21. [Google Scholar] [CrossRef]
- Eichele, K. WSolids1—Solid State NMR Simulations; Universität Tübingen: Tübingen, Germany, 2015. [Google Scholar]
- Kuntz, I.D. Hydration of macromolecules. III. Hydration of polypeptides. J. Am. Chem. Soc. 1971, 93, 514–516. [Google Scholar] [CrossRef]
- Wolfenden, R.; Andersson, L.; Cullis, P.M.; Southgate, C.C.B. Affinities of amino acid side chains for solvent water. Biochemistry 1981, 20, 849–855. [Google Scholar] [CrossRef]
- Gao, B.; Wyttenbach, T.; Bowers, M.T. Protonated arginine and protonated lysine: Hydration and its effect on the stability of salt-bridge structures. J. Phys. Chem. B 2009, 113, 9995–10000. [Google Scholar] [CrossRef]
- Kabiri, M.; Bushnak, I.; McDermot, M.T.; Unsworth, L.D. Toward a Mechanistic Understanding of Ionic Self-Complementary Peptide Self-Assembly: Role of Water Molecules and Ions. Biomacromolecules 2013, 14, 3943–3950. [Google Scholar] [CrossRef]
- Nagai, Y.; Unsworth, L.D.; Koutsopoulos, S.; Zhang, S. Slow release of molecules in self-assembling peptide nanofiber scaffold. J. Control. Release 2006, 115, 18–25. [Google Scholar] [CrossRef] [PubMed]
- McBrierty, V.J.; Martin, S.J.; Karasz, F.E. Understanding Hydrated Polymers: The Perspective of NMR. J. Mol. Liq. 1999, 80, 179–205. [Google Scholar] [CrossRef]
- Carles, J.E.; Scallan, A.M. The determination of the amount of bound water within cellulosic gels by NMR spectroscopy. J. Appl. Polym. Sci. 1973, 17, 1855–1865. [Google Scholar] [CrossRef]
- Radloff, D.; Boeffel, C.; Speiss, H.W. Cellulose and cellulose/poly(vinyl alcohol) blends. 2. Water organisation revealed by solid state nmr spectroscopy. Macromolecules 1996, 29, 1528–1534. [Google Scholar] [CrossRef]
- Long, J.R.; Ebelhäuser, R.; Griffin, R.G. 2H NMR Line Shapes and Spin–Lattice Relaxation in Ba(ClO3)2·2H2O. J. Phys. Chem. A 1997, 101, 988–994. [Google Scholar] [CrossRef]
- Murase, N.; Gonda, K.; Watanabe, T. Unfrozen compartmentalised water in gels and its amorphous crystallisation during warming. J. Phys. Chem. 1986, 90, 5420–5426. [Google Scholar] [CrossRef]
- Li, S.; Dickinson, L.C.; Chinachoti, P. Mobility of “Unfreezable” and “Freezable” Water in Waxy Corn Starch by 2H and 1H NMR. J. Agric. Food Chem. 1998, 46, 62–71. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, R.; Chen, T.; Sun, P. 2H Solid-State NMR Analysis of the Dynamics and Organization of Water in Hydrated Chitosan. Polymers 2016, 8, 149. [Google Scholar] [CrossRef]
- Pake, G.E. Nuclear resonance absorption in hydrated crystals: Fine structure of the proton line. J. Chem. Phys. 1948, 16, 327–336. [Google Scholar] [CrossRef]
- Taylor, H.S.; Selwood, P.W. Some Properties of Heavy Water. J. Am. Chem. Soc. 1934, 56, 998–999. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgersma, S.V.; Ha, M.; Yang, J.-L.J.; Michaelis, V.K.; Unsworth, L.D. Charge and Peptide Concentration as Determinants of the Hydrogel Internal Aqueous Environment. Materials 2019, 12, 832. https://doi.org/10.3390/ma12050832
Elgersma SV, Ha M, Yang J-LJ, Michaelis VK, Unsworth LD. Charge and Peptide Concentration as Determinants of the Hydrogel Internal Aqueous Environment. Materials. 2019; 12(5):832. https://doi.org/10.3390/ma12050832
Chicago/Turabian StyleElgersma, Scott V., Michelle Ha, Jung-Lynn Jonathan Yang, Vladimir K. Michaelis, and Larry D. Unsworth. 2019. "Charge and Peptide Concentration as Determinants of the Hydrogel Internal Aqueous Environment" Materials 12, no. 5: 832. https://doi.org/10.3390/ma12050832
APA StyleElgersma, S. V., Ha, M., Yang, J.-L. J., Michaelis, V. K., & Unsworth, L. D. (2019). Charge and Peptide Concentration as Determinants of the Hydrogel Internal Aqueous Environment. Materials, 12(5), 832. https://doi.org/10.3390/ma12050832