Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Characterization Analysis
3.2. Removal Torque Test
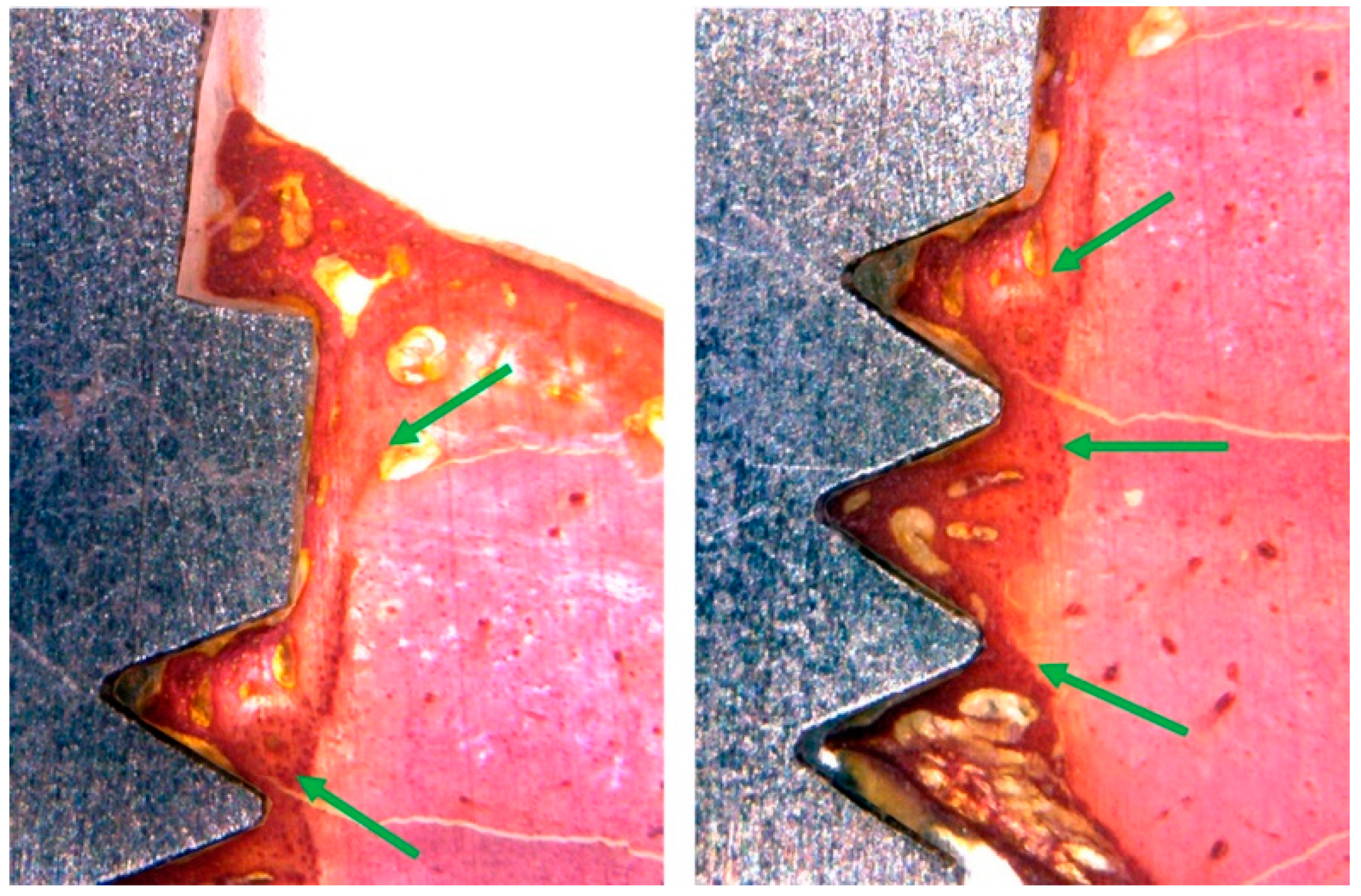
3.3. Histomorphological Analysis
3.4. Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kammermeier, A.; Rosentritt, M.; Behr, M.; Schneider-Feyrer, S.; Preis, V. In vitro performance of one- and two-piece zirconia implant systems for an anterior application. J. Dent. 2016, 53, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hong, G.; Lin, H.; Shimizu, Y.; Wu, Y.; Zheng, G.; Zhang, H.; Sasaki, K. Biomechanical and histological evaluation of the osseointegration capacity of two types of zirconia implant. Int. J. Nanomed. 2016, 11, 6507–6516. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devi. 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Hagemann, A.; Hahnel, S.; Behr, M.; Preis, V. In vitro performance of zirconia and titanium implant/abutment systems for an anterior application. J. Dent. 2014, 42, 1019–1026. [Google Scholar] [CrossRef]
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar]
- Vechiato-Filho, A.J.; Pesqueira, A.A.; De Souza, G.M.; dos Santos, D.M.; Pellizzer, E.P.; Goiato, M.C. Are zirconia implant abutments safe and predictable in posterior regions? A systematic review and meta-analysis. Int. J. Prosthodont. 2016, 29, 233–244. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Yildirim, M.; Fischer, H.; Marx, R.; Edelhoff, D. In vivo fracture resistance of implant-supported all-ceramic restorations. J. Prosthet. Dent. 2003, 90, 325–331. [Google Scholar] [CrossRef]
- Bankoğlu Güngör, M.; Aydın, C.; Yılmaz, H.; Gül, E.B. An overview of zirconia dental implants: Basic properties and clinical application of three cases. J. Oral Implantol. 2014, 40, 485–494. [Google Scholar] [CrossRef]
- Nkamgeu, E.M.; Adnet, J.J.; Bernard, J.; Zierold, K.; Kilian, L.; Jallot, E.; Benhayoune, H.; Bonhomme, P. In vitro effects of zirconia and alumina particles on human blood monocyte-derived macrophages: X-ray microanalysis and flow cytometric studies. J. Biomed. Mater. Res. 2000, 52, 587–594. [Google Scholar] [CrossRef]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implant. 2002, 17, 793–798. [Google Scholar]
- Kohal, R.J.; Wolkewitz, M.; Tsakona, A. The effects of cyclic loading and preparation on the fracture strength of zirconium dioxide implants: An in vitro investigation. Clin. Oral Implant. Res. 2011, 22, 808–814. [Google Scholar] [CrossRef]
- Kohal, R.J.; Wolkewitz, M.; Hinze, M.; Han, J.S.; Bächle, M.; Butz, F. Biomechanical and histological behavior of zirconia implants: An experiment in the rat. Clin. Oral Implant. Res. 2009, 20, 333–339. [Google Scholar] [CrossRef]
- Thoma, D.S.; Benic, G.I.; Muñoz, F.; Kohal, R.; Sanz Martin, I.; Cantalapiedra, A.G.; Hämmerle, C.H.; Jung, R.E. Histological analysis of loaded zirconia and titanium dental implants: An experimental study in the dog mandible. J. Clin. Periodontol. 2015, 42, 967–975. [Google Scholar] [CrossRef]
- Gahlert, M.; Röhling, S.; Wieland, M.; Sprecher, C.M.; Kniha, H.; Milz, S. Osseointegration of zirconia and titanium dental implants: A histological and histomorphometrical study in the maxilla of pigs. Clin. Oral Implant. Res. 2009, 20, 1247–1253. [Google Scholar] [CrossRef]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Guazzato, M.; Albakry, M.; Ringer, S.P.; Swain, M.V. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent. Mater. 2004, 20, 449–456. [Google Scholar] [CrossRef]
- Kim, J.W.; Covel, N.S.; Guess, P.C.; Rekow, E.D.; Zhang, Y. Concerns of hydrothermal degradation in CAD/CAM zirconia. J. Dent. Res. 2010, 89, 91–95. [Google Scholar] [CrossRef]
- Wittneben, J.G.; Gavric, J.; Belser, U.C.; Bornstein, M.M.; Joda, T.; Chappuis, V.; Sailer, I.; Brägger, U. Esthetic and clinical performance of implant-supported all-ceramic crowns made with prefabricated or CAD/CAM zirconia abutments. J. Dent. Res. 2017, 96, 163–170. [Google Scholar] [CrossRef]
- Gehrke, P.; Johannson, D.; Fischer, C.; Stawarczyk, B.; Beuer, F. In vitro fatigue and fracture resistance of one- and two-piece CAD/CAM zirconia implant abutments. Int. J. Oral Maxillofac. Implant. 2015, 30, 546–554. [Google Scholar] [CrossRef]
- Att, W.; Kurun, S.; Gerds, T.; Strub, J.R. Fracture resistance of single-tooth implant-supported all-ceramic restorations after exposure to the artificial mouth. J. Oral Rehabil. 2006, 33, 380–386. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical outcomes of zirconia dental implants: A systematic review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef]
- Morita, K.; Doi, K.; Oue, H.; Kajihara, S.; Hayashi, K.; Akagawa, Y. Influence of formalin fixation on the implant stability quotient and mechanical characteristics of bone. Br. J. Oral Maxillofac. Surg. 2013, 51, 550–554. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Marin, G.W. Biomechanical evaluation of dental implants with three different designs: Removal torque and resonance frequency analysis in rabbits. Ann. Anat. 2015, 199, 30–35. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Walboomers, X.F.; Jansen, J.A. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin. Oral Implant. Res. 2014, 25, 487–492. [Google Scholar] [CrossRef]
- Schouten, C.; Meijer, G.J.; van den Beucken, J.J.; Spauwen, P.H.; Jansen, J.A. The quantitative assessment of peri-implant bone responses using histomorphometry and micro-computed tomography. Biomaterials 2009, 30, 4539–4549. [Google Scholar] [CrossRef]
- Rocchietta, I.; Fontana, F.; Addis, A.; Schupbach, P.; Simion, M. Surface-modified zirconia implants: Tissue response in rabbits. Clin. Oral Implant. Res. 2009, 20, 844–850. [Google Scholar] [CrossRef]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujoks, C.; Handschel, J.; Wiesmann, H.P.; Kübler, N.R.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008, 4, 29. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Pérez-Albacete Martínez, C.; Piattelli, A.; Shibli, J.A.; Markovic, A.; Calvo Guirado, J.L. The influence of three different apical implant designs at stability and osseointegration process: An experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, 355–361. [Google Scholar] [CrossRef]
- Möller, B.; Terheyden, H.; Açil, Y.; Purcz, N.M.; Hertrampf, K.; Tabakov, A.; Behrens, E.; Wiltfang, J. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: An in vivo and in vitro study. Int. J. Oral Maxillofac. Surg. 2012, 41, 638–645. [Google Scholar] [CrossRef]
- Ivanoff, C.J.; Sennerby, L.; Lekholm, U. Influence of mono- and bicortical anchorage on the integration of titanium implants. A study in the rabbit tibia. Int. J. Oral Maxillofac. Surg. 1996, 25, 229–235. [Google Scholar] [CrossRef]
- Steigenga, J.; Al-Shammari, K.; Misch, C.; Nociti, F.H., Jr.; Wang, H.L. Effects of implant thread geometry on the percentage of osseointegration and resistance to reverse torque in the tibia of rabbits. J. Periodontol. 2004, 75, 1233–1241. [Google Scholar] [CrossRef]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implant. Res. 2007, 18, 662–668. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Johnson, P.; Dadgostari, S.; Caputo, A.; Davies, J.E.; Nishimura, R.D. Early endosseous integration enhanced by dual acid etching of titanium: A torque removal study in the rabbit. Clin. Oral Implant. Res. 2001, 12, 350–357. [Google Scholar] [CrossRef]
- Sul, Y.T.; Byon, E.S.; Jeong, Y. Biomechanical measurements of calcium-incorporated oxidized implants in rabbit bone: Effect of calcium surface chemistry of a novel implant. Clin. Implant. Dent. Relat. Res. 2004, 6, 101–110. [Google Scholar] [CrossRef]
- Slaets, E.; Carmeliet, G.; Naert, I.; Duyck, J. Early cellular responses in cortical bone healing around unloaded titanium implants: An animal study. J. Periodontol. 2006, 77, 1015–1024. [Google Scholar] [CrossRef]
- Halldin, A.; Jimbo, R.; Johansson, C.B.; Wennerberg, A.; Jacobsson, M.; Albrektsson, T.; Hansson, S. The effect of static bone strain on implant stability and bone remodeling. Bone 2011, 49, 783–789. [Google Scholar] [CrossRef]
- Halldin, A.; Jimbo, R.; Johansson, C.B.; Wennerberg, A.; Jacobsson, M.; Albrektsson, T.; Hansson, S. Implant stability and bone remodeling after 3 and 13 days of implantation with an initial static strain. Clin. Implant. Dent. Relat. Res. 2014, 16, 383–393. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef]
- Hoffmann, O.; Angelov, N.; Gallez, F.; Jung, R.E.; Weber, F.E. The zirconia implant-bone interface: A preliminary histologic evaluation in rabbits. Int. J. Oral Maxillofac. Implant. 2008, 23, 691–695. [Google Scholar]
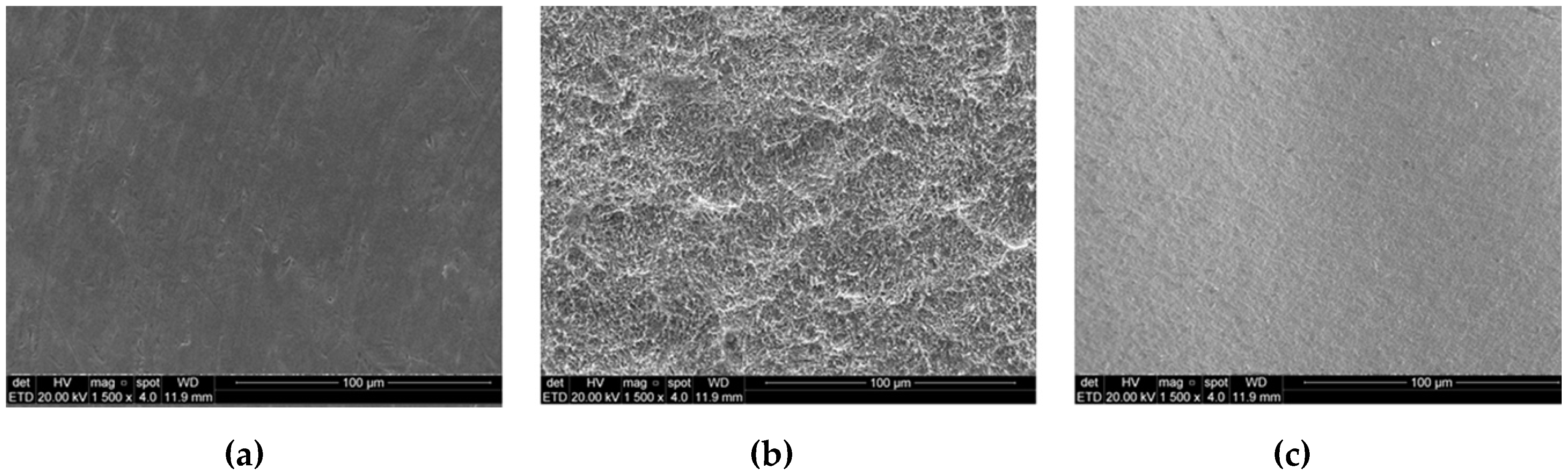








| Parameters | TiM Group | TiT Group | Zr Group |
|---|---|---|---|
| Sa | 0.18 (± 0.2) | 0.77 (± 0.2) | 0.17 (± 0.1) |
| Ra | 0.17 (± 0.1) | 0.66 (± 0.3) | 0.14 (± 0.2) |
| Z | 0.92 (± 0.8) | 2.61 (± 0.8) | 0.77 (± 2.0) |
| Group | Mean | SD | Median | Min | Max | n |
|---|---|---|---|---|---|---|
| TiM | 15.9 | 4.18 | 16.1 | 8.4 | 22.1 | 10 |
| TiT | 27.9 | 5.15 | 27.9 | 15.1 | 35.3 | 10 |
| Zr | 11.5 | 2.92 | 11.1 | 7.3 | 16.0 | 10 |
| Group | Mean | SD | Median | Min | Max | N |
|---|---|---|---|---|---|---|
| TiM | 35.4 | 4.54 | 37.1 | 28.9 | 41.3 | 10 |
| TiT | 37.8 | 4.84 | 39.9 | 29.8 | 46.1 | 10 |
| Zr | 34.0 | 6.82 | 36.8 | 23.6 | 44.0 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehrke, S.A.; Prados-Frutos, J.C.; Prados-Privado, M.; Calvo-Guirado, J.L.; Aramburú Júnior, J.; Pérez-Díaz, L.; Mazón, P.; Aragoneses, J.M.; De Aza, P.N. Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study. Materials 2019, 12, 856. https://doi.org/10.3390/ma12060856
Gehrke SA, Prados-Frutos JC, Prados-Privado M, Calvo-Guirado JL, Aramburú Júnior J, Pérez-Díaz L, Mazón P, Aragoneses JM, De Aza PN. Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study. Materials. 2019; 12(6):856. https://doi.org/10.3390/ma12060856
Chicago/Turabian StyleGehrke, Sergio Alexandre, Juan Carlos Prados-Frutos, María Prados-Privado, José Luis Calvo-Guirado, Jaime Aramburú Júnior, Leticia Pérez-Díaz, Patricia Mazón, Juan Manuel Aragoneses, and Piedad N. De Aza. 2019. "Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study" Materials 12, no. 6: 856. https://doi.org/10.3390/ma12060856
APA StyleGehrke, S. A., Prados-Frutos, J. C., Prados-Privado, M., Calvo-Guirado, J. L., Aramburú Júnior, J., Pérez-Díaz, L., Mazón, P., Aragoneses, J. M., & De Aza, P. N. (2019). Biomechanical and Histological Analysis of Titanium (Machined and Treated Surface) Versus Zirconia Implant Materials: An In Vivo Animal Study. Materials, 12(6), 856. https://doi.org/10.3390/ma12060856











