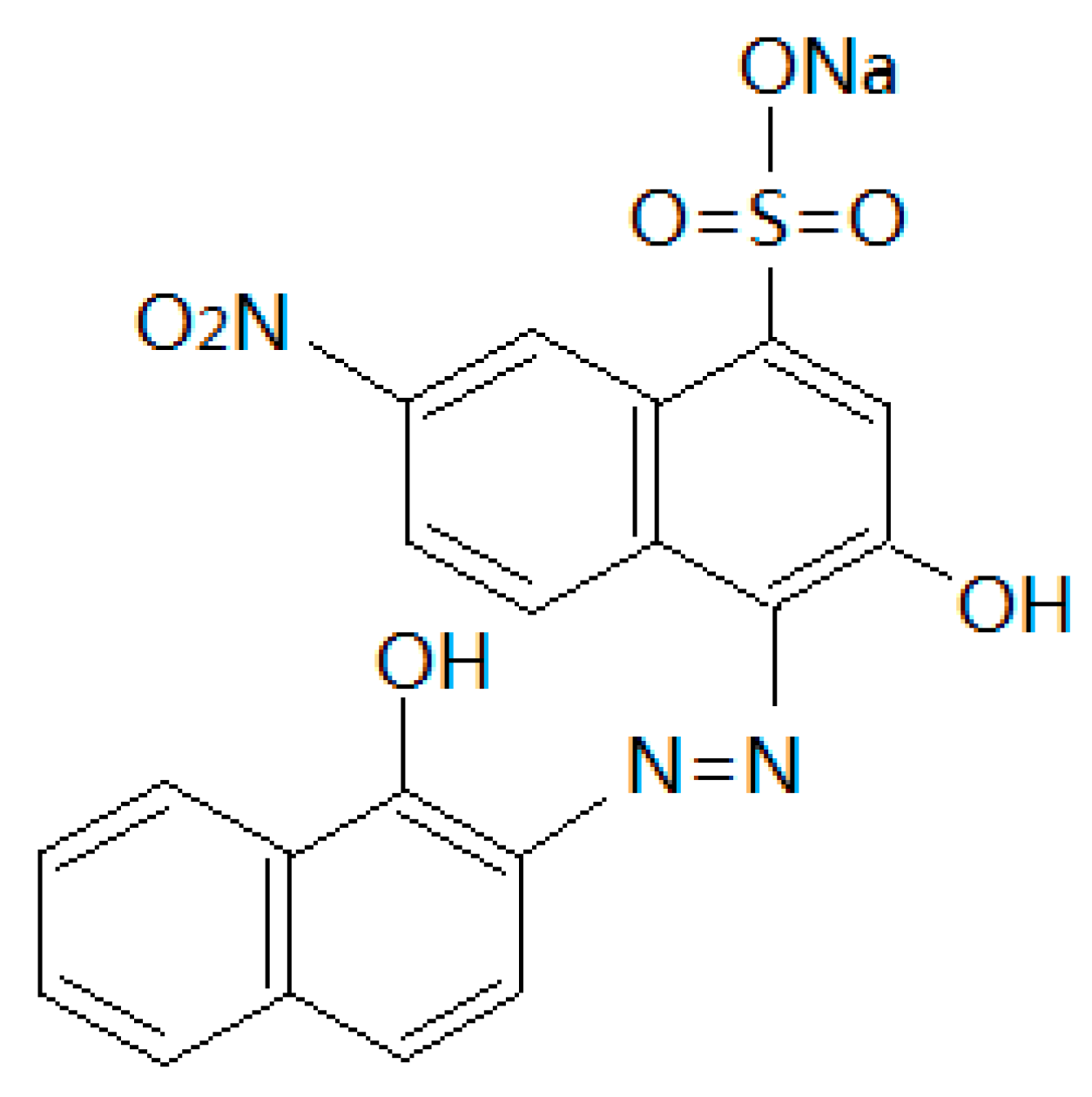
Preparation of Sludge-Derived Activated Carbon by Fenton Activation and the Adsorption of Eriochrome Black T
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
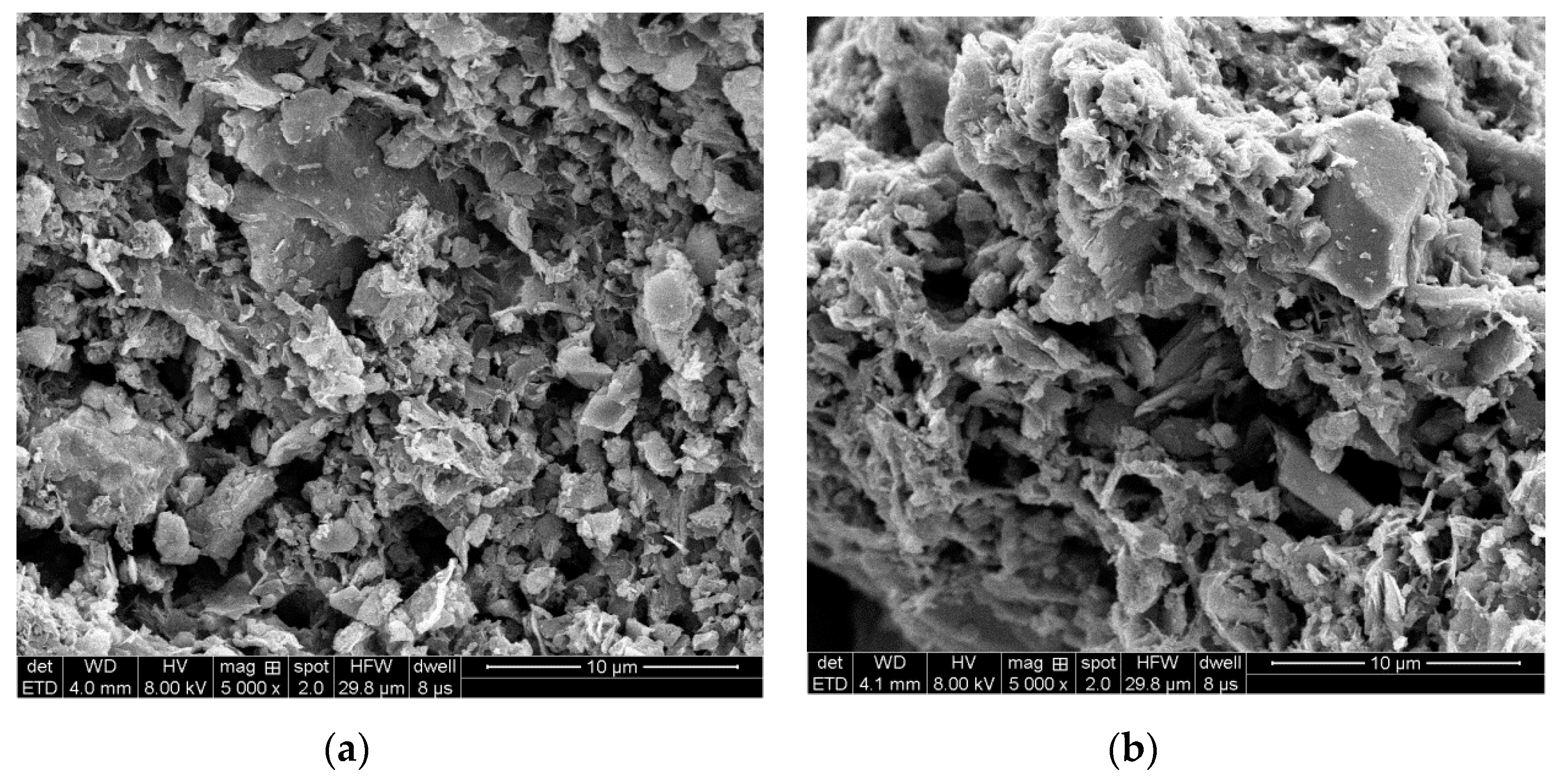
2.2. Preparation and Characterization of the SAC
2.3. Adsorption of EBT by the SAC
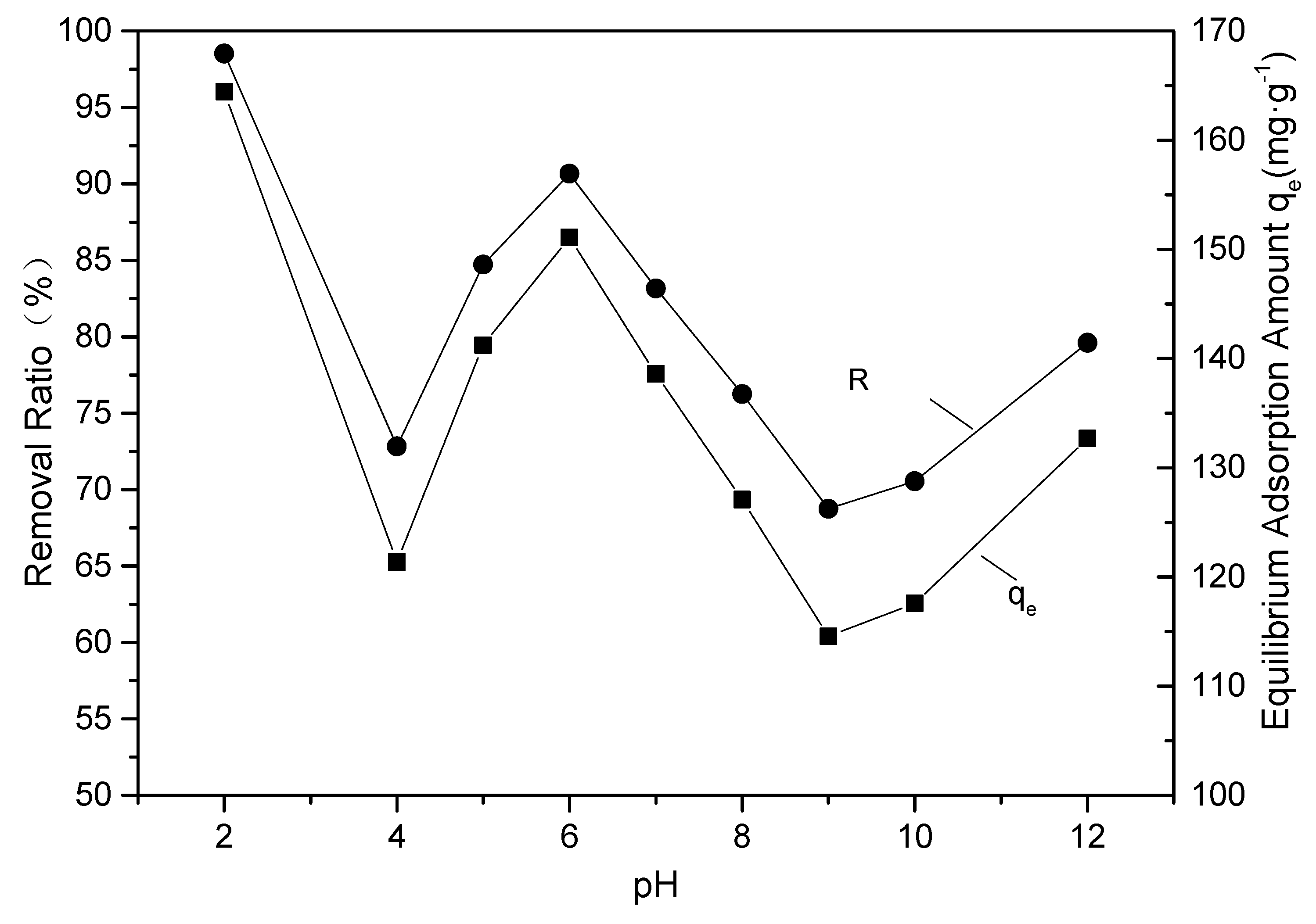
2.3.1. Effect of pH on the Adsorption of EBT by the SAC
2.3.2. Effect of the Initial EBT Concentration on the Adsorption of EBT by the SAC
2.3.3. Adsorption Kinetics Studies
2.3.4. Adsorption Isotherm Studies
3. Results and Discussion
3.1. Characterization of the SAC
3.2. Adsorption of EBT by the SAC
3.2.1. Effect of pH on Adsorption
3.2.2. Effect of the Initial EBT Concentration on the Adsorption and Adsorption Isotherm
3.2.3. Adsorption Kinetic Studies
3.2.4. Thermodynamic Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aly-Eldeen, M.A.; El-Sayed, A.A.; Salem, D.M.; El Zokm, G.M. The uptake of Eriochrome Black T dye from aqueous solutions utilizing waste activated sludge: Adsorption process optimization using factorial design. Egypt. J. Aquat. Res. 2018, 44, 179–186. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Flores, E.D.; Genuino, D.A.D.; Futalan, C.M.; Wan, M.W. Adsorption of Eriochrome Black T (EBT) dye using activated carbon prepared from waste rice hulls—Optimization, isotherm and kinetic studies. J. Taiwan Inst. Chem. Eng. 2013, 44, 646–653. [Google Scholar] [CrossRef]
- Yang, B.; Xu, H.; Yang, S.; Bi, S.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Liu, J.; Song, X.; et al. Treatment of industrial dyeing wastewater with a pilot-scale strengthened circulation anaerobic reactor. Bioresour. Technol. 2018, 264, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.A.; Hameed, B.H. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Paixão, K.; Abreu, E.; Samanamud, G.R.L.; França, A.B.; Loures, C.C.A.; Baston, E.P.; Naves, L.L.R.; Bosch, J.C.; Naves, F.L. Normal boundary intersection applied in the scale-up for the treatment process of Eriochrome Black T through the UV/TiO2/O3 system. J. Environ. Chem. Eng. 2019, 7, 102801. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 2019, 358, 992–1001. [Google Scholar] [CrossRef]
- El Hassani, K.; Kalnina, D.; Turks, M.; Beakou, B.H.; Anouar, A. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst. Sep. Purif. Technol. 2019, 210, 764–774. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dyes Pigments 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Chakraborty, S.; De, S.; DasGupta, S.; Basu, J.K. Adsorption study for the removal of a basic dye: Experimental and modeling. Chemosphere 2005, 58, 1079–1086. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2018, 276, 728–747. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Xu, Z.; Luo, M.; Zhu, C.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef]
- Kacan, E. Optimum BET surface areas for activated carbon produced from textile sewage sludges and its application as dye removal. J. Environ. Manag. 2016, 166, 116–123. [Google Scholar] [CrossRef]
- Ding, N.; Peng, C.; Ren, Y.; Liu, Y.; Wang, P.; Dong, L.; Liu, H.; Wang, D. Improving the dewaterability of citric acid wastewater sludge by Fenton treatment. J. Clean. Prod. 2018, 196, 739–746. [Google Scholar] [CrossRef]
- Masihi, H.; Gholikandi, G.B. Employing Electrochemical-Fenton process for conditioning and dewatering of anaerobically digested sludge: A novel approach. Water Res. 2018, 144, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Mousavi-Tashar, A.; Masoumi, Z.; Azarian, G. Integrated advanced oxidation process, sono-Fenton treatment, for mineralization and volume reduction of activated sludge. Ecotoxicol. Environ. Saf. 2019, 168, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Lou, Z.; Zhu, N.; Yuan, H. Preparation of the sludge based activated carbon with Fenton oxidation pretreatment and its property. Environ. Pollut. Control 2014, 36, 43–48. [Google Scholar]
- Huang, Y.; Zhang, D.; Li, Y.; Xu, Z.; Yuan, S.; Wang, L. Heavy Metals Sorption on River Sediments: Kinetics, Equilibria and Relative Selectivities. Fresenius Environ. Bull. 2015, 24, 2792–2799. [Google Scholar]
- Jiang, W.; Xing, X.; Li, S.; Zhang, X.; Wang, W. Synthesis, characterization and machine learning based performance prediction of straw activated carbon. J. Clean. Prod. 2019, 212, 1210–1223. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Mo, R.; Huang, S.; Dai, W.; Liang, J.; Sun, S. A rapid Fenton treatment technique for sewage sludge dewatering. Chem. Eng. J. 2015, 269, 391–398. [Google Scholar] [CrossRef]
- Yu, W.; Yang, J.; Shi, Y.; Song, J.; Shi, Y.; Xiao, J.; Li, C.; Xu, X.; He, S.; Liang, S.; et al. Roles of iron species and pH optimization on sewage sludge conditioning with Fenton’s reagent and lime. Water Res. 2016, 95, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Haifeng, W.; Gu, L.; Yu, H.; Qiao, X.; Zhang, D.; Ye, J. Radical assisted iron impregnation on preparing sewage sludge derived Fe/carbon as highly stable catalyst for heterogeneous Fenton reaction. Chem. Eng. J. 2018, 352, 837–846. [Google Scholar]
- Virginie, M.; Adánez, J.; Courson, C.; De Diego, L.F.; García-Labiano, F.; Niznansky, D.; Kiennemann, A.; Gayán, P.; Abad, A.; et al. Effect of Fe–olivine on the tar content during biomass gasification in a dual fluidized bed. Appl. Catal. B Environ. 2012, 121–122, 214–222. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; Makhfouk, M.E.L. Removal of Methylene Blue and Eriochrome Black T from aqueous solutions by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320–326. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Ghorbani, F.; Younesi, H.; Ghasempouri, S.M.; Zinatizadeh, A.A.; Amini, M.; Daneshi, A. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chem. Eng. J. 2008, 145, 267–275. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Adsorption of Reactive Black 5 from an aqueous solution: Equilibrium and kinetic studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: Effect of temperature. Process Biochem. 2000, 36, 431–439. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Zhang, F.; Zhou, J.; Wu, J.; Alsaedi, A.; Hayat, T.; Li, J. Insight into the mechanism of adsorption of phenol and resorcinol on activated carbons with different oxidation degrees. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 22–30. [Google Scholar] [CrossRef]
- Duan, S.; Wang, Y.; Liu, X.; Shao, D.; Hayat, T.; Alsaedi, A.; Li, J. Removal of U(VI) from Aqueous Solution by Amino Functionalized Flake Graphite Prepared by Plasma Treatment. ACS Sustain. Chem. Eng. 2017, 5, 4073–4085. [Google Scholar] [CrossRef]
- Khenniche, L.; Benissad-Aissani, F. Adsorptive Removal of Phenol by Coffee Residue Activated Carbon and Commercial Activated Carbon: Equilibrium, Kinetics, and Thermodynamics. J. Chem. Eng. Data 2010, 55, 4677–4686. [Google Scholar] [CrossRef]





| Item | Analysis Results |
|---|---|
| Sewage sludge | |
| pH | 6.8–8.1 |
| Water content (%) | 98.6–99.4% |
| Total solid (g·L−1) | 17.63 |
| Volatile solid (g·L−1) | 14.01 |
| Element composition of dried sewage sludge | |
| C (%) | 36.01 |
| H (%) | 5.09 |
| N (%) | 6.22 |
| S (%) | 1.13 |
| Properties | F-SAC | P-SAC |
|---|---|---|
| Brunauer-Emmett-Teller (BET) surface area (m2·g−1) | 172.8 | 92.6 |
| Pore volume (cm3·g−1) | 0.196 | 0.099 |
| Smicro (m2·g−1) | 10.0 | 35.13 |
| Vmicro (cm3·g−1) | 4.33 × 10−3 | 1.11 × 10−2 |
| Average pore diameter (nm) | 4.54 | 4.28 |
| Models | F-SAC | P-SAC | |
|---|---|---|---|
| Freundlich | equation | y = 0.2234x + 1.8327 | y = 0.0379x + 1.8497 |
| KF | 68.03 | 70.75 | |
| 1/n | 0.2234 | 0.0379 | |
| R2 | 0.9483 | 0.7717 | |
| Langmuir | equation | y = 4.579 × 10−3x + 0.0367 | y = 0.0101x + 0.2233 |
| KL | 0.1247 | 0.04523 | |
| qm | 218.4 | 99.01 | |
| R2 | 0.9196 | 0.9883 | |
| Lagrangian First-Order Model | |||||
| T (K) | equation | k1 (min−1) | qe (mg·g−1) | R2 | Experimental qe (mg·g−1) |
| 288 | y = −1.620 × 10−3x + 4.290 | 1.620 × 10−3 | 72.95 | 0.9838 | 96.9 |
| 298 | y = −1.645 × 10−3x + 4.727 | 1.645 × 10−3 | 112.9 | 0.9827 | 153.4 |
| 308 | y = −2.115 × 10−3x + 4.880 | 2.115 × 10−3 | 131.6 | 0.9932 | 165.1 |
| 318 | y = −2.866 × 10−3x + 4.797 | 2.370 × 10−3 | 121.2 | 0.9329 | 178.2 |
| Pseudo-Second-Order Model | |||||
| T (K) | equation | k2 (g·mg−1·min−1) | qe (mg·g−1) | R2 | Experimental qe (mg·g−1) |
| 288 | y = 9.151 × 10−3x + 3.063 | 1.734 × 10−5 | 109.3 | 0.9979 | 96.90 |
| 298 | y = 5.827 × 10−3x + 1.825 | 1.861 × 10−5 | 171.6 | 0.9976 | 153.4 |
| 308 | y = 5.494 × 10−3x + 1.370 | 2.203 × 10−5 | 182.0 | 0.9988 | 165.1 |
| 318 | y = 5.653 × 10−3x + 0.8385 | 3.811 × 10−5 | 176.9 | 0.9988 | 178.2 |
| ΔH (kJ·mol−1) | ΔS (J·mol−1·K−1) | ΔG (kJ·mol−1) | |||
|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | 318 K | ||
| 143.4 | 492.4 | −1.586 | −3.338 | −8.262 | −13.186 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Zhang, D.; Gu, L.; Yu, H.; Pan, M.; Huang, Y. Preparation of Sludge-Derived Activated Carbon by Fenton Activation and the Adsorption of Eriochrome Black T. Materials 2019, 12, 882. https://doi.org/10.3390/ma12060882
Wen H, Zhang D, Gu L, Yu H, Pan M, Huang Y. Preparation of Sludge-Derived Activated Carbon by Fenton Activation and the Adsorption of Eriochrome Black T. Materials. 2019; 12(6):882. https://doi.org/10.3390/ma12060882
Chicago/Turabian StyleWen, Haifeng, Daofang Zhang, Lin Gu, Haixiang Yu, Minmin Pan, and Yuanxing Huang. 2019. "Preparation of Sludge-Derived Activated Carbon by Fenton Activation and the Adsorption of Eriochrome Black T" Materials 12, no. 6: 882. https://doi.org/10.3390/ma12060882




