Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition
Abstract
:1. Introduction
- (i)
- the lower manufacturing cost of prototypes;
- (ii)
- decreased manufacturing time by elimination of production steps;
- (iii)
- the possibility of manufacturing parts with composition gradients;
- (iv)
- design of parts with complex geometries, hard to obtain by other techniques;
- (v)
- decreased waste material amounts during parts production;
- (vi)
- costs reduction for equipment necessary for parts manufacturing.
2. Materials and Methods
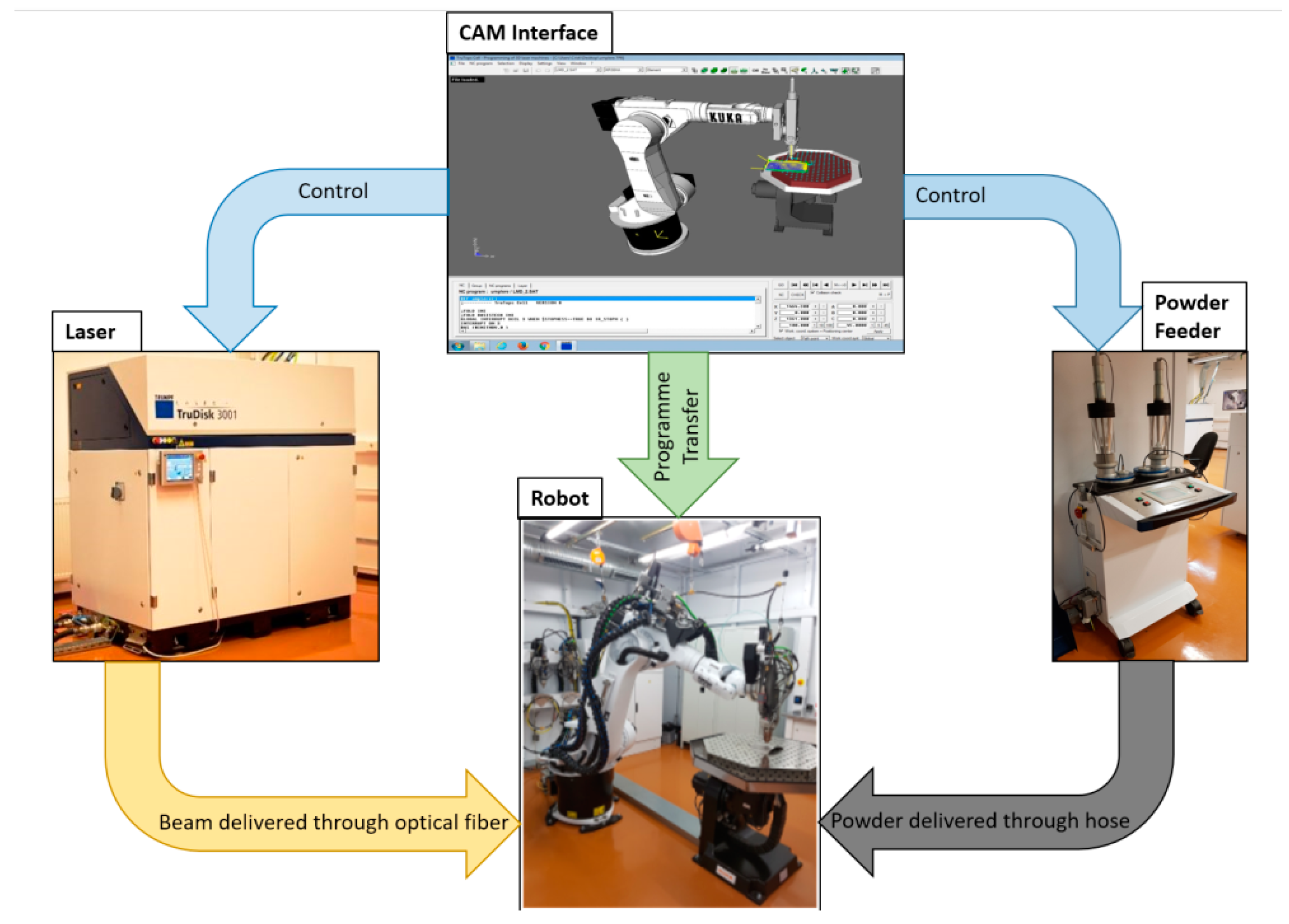
2.1. 3D Printing of Bone Plates
2.2. Machining Steps for the Manufacturing of the Final Shape
2.3. Physicochemical Characterization
2.4. In Vitro Testing
2.4.1. Cell Culture
2.4.2. MTS Assay
2.4.3. Immunofluorescence Microscopy
2.4.4. SaOs2 Cell Morphology
3. Results
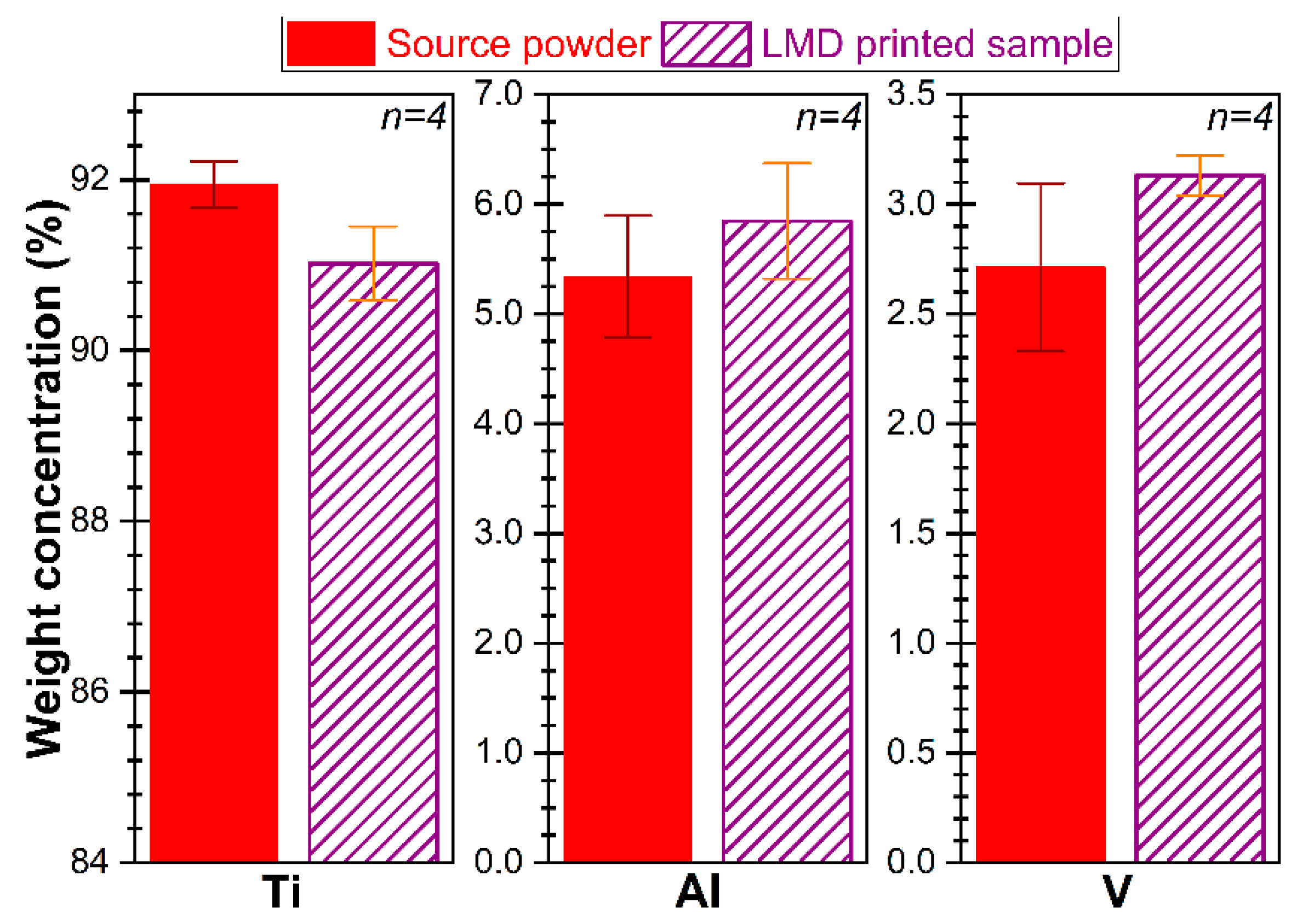
3.1. Powder Characterization
3.2. LMD Optimizations
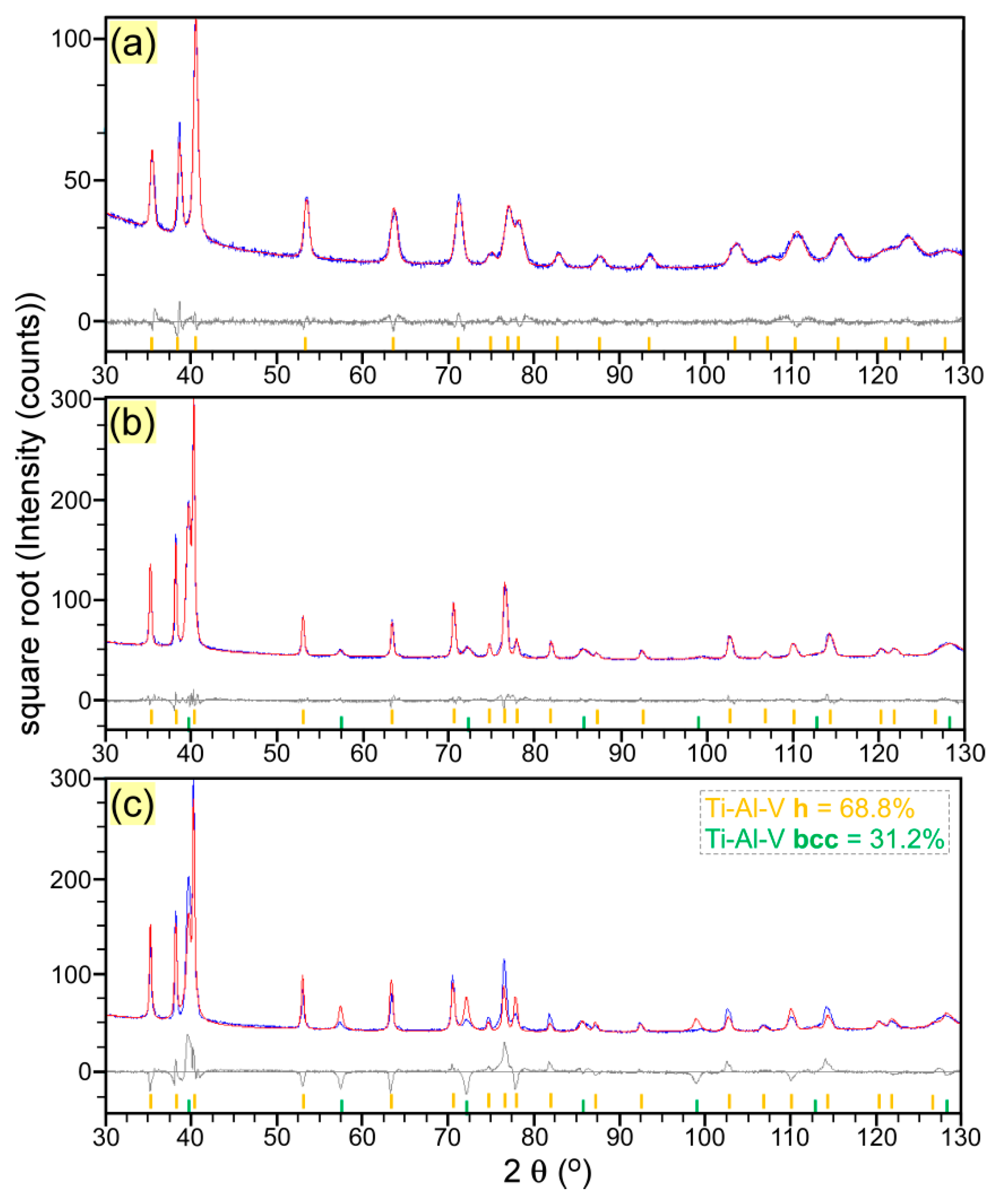
3.3. Morphological and Structural Characterizations of LMD Grown Structures
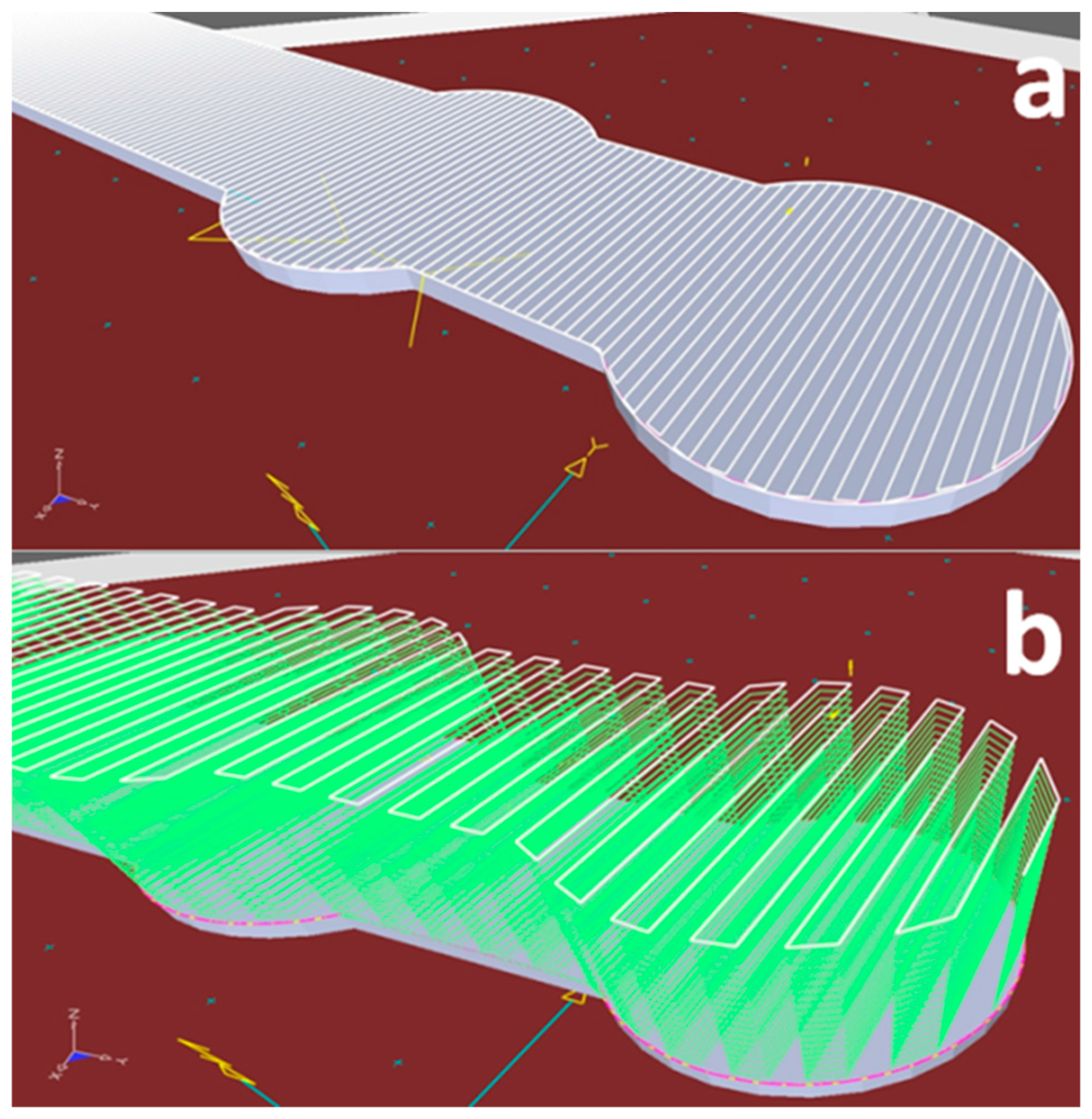
3.4. Bone Plates Manufacturing
3.5. In Vitro Tests in Cell Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Ford, S.; Despeisse, M. Additive manufacturing and sustainability: An exploratory study of the advantages and challenges. J. Clean. Prod. 2016, 137, 1573–1587. [Google Scholar] [CrossRef]
- ASTM International. A.S.T.M. F2792-12a, Standard Terminology for Additive Manufacturing Technologies, West Conshohocken, PA, 2012. Available online: http://web.mit.edu/2.810/www/files/readings/AdditiveManufacturingTerminology.pdf (accessed on 9 September 2018).
- Liu, Q.; Wang, Y.; Zheng, H.; Tang, K.; Ding, L.; Li, H.; Gong, S. Microstructure and mechanical properties of LMD–SLM hybrid forming Ti6Al4V alloy. Mater. Sci. Eng. A Struct. 2016, 660, 24–33. [Google Scholar] [CrossRef]
- Wang, J.D.; Li, L.Q.; Tao, W. Crack initiation and propagation behavior of WC particles reinforced Fe-based metal matrix composite produced by laser melting deposition. Opt. Laser Technol. 2016, 82, 170–182. [Google Scholar] [CrossRef]
- Bambach, M.; Sizova, I.; Silze, F.; Schnick, M. Hot workability and microstructure evolution of the nickel-based superalloy Inconel 718 produced by laser metal deposition. J. Alloys Compd. 2018, 740, 278–287. [Google Scholar] [CrossRef]
- Ruiz, J.E.; Cortina, M.; Arrizubieta, J.I.; Lamikiz, A. Study of the Influence of Shielding Gases on Laser Metal Deposition of Inconel 718 Superalloy. Materials 2018, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Huebner, J.; Kata, D.; Rutkowski, P.; Petrzak, P.; Kusiński, J. Grain-Boundary Interaction between Inconel 625 and WC during Laser Metal Deposition. Materials 2018, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Tian, X.; Peng, G.; Cao, Y.; Li, D. Hierarchically porous materials prepared by selective laser sintering. Mater. Des. 2017, 135, 62–68. [Google Scholar] [CrossRef]
- Dong, L.; Correia JP, M.; Barth, N.; Ahzi, S. Finite element simulations of temperature distribution and of densification of a titanium powder during metal laser sintering. Addit. Manuf. 2017, 13, 37–48. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Z.; Zeng, X. A comparison on metallurgical behaviors of 316L stainless steel by selective laser melting and laser cladding deposition. Mater. Sci. Eng. A-Struct. 2017, 685, 265–273. [Google Scholar] [CrossRef]
- Rauch, E.; Unterhofer, M.; Dallasega, P. Industry sector analysis for the application of additive manufacturing in smart and distributed manufacturing systems. Manuf. Lett. 2018, 15, 126–131. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S. Biomedical applications of additive manufacturing: Present and future. Curr. Opin. Biomed. Eng. 2017, 2, 105–115. [Google Scholar] [CrossRef]
- Fukuda, H. Additive Manufacturing Technology for Orthopedic Implants. In Advances in Metallic Biomaterials, Springer Series in Biomaterials Science and Engineering; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 4, pp. 3–26. [Google Scholar]
- Emelogua, A.; Marufuzzamana, M.; Thompson, S.M.; Shamsaei, N.; Bian, L. Additive manufacturing of biomedical implants: A feasibility assessment via supply-chain cost analysis. Addit. Manuf. 2016, 11, 97–113. [Google Scholar] [CrossRef]
- Leyenes, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Liu, X.; Man, H.C. Laser fabrication of Ag-HA nanocomposites on Ti6Al4V implant for enhancing bioactivity and antibacterial capability. Mater. Sci. Eng. C 2017, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, S.; Bertolini, R.; Medeossi, F.; Ghiotti, A.; Savio, E.; Shivpuri, R. Case study: The application of machining-conditioning to improve the wear resistance of Ti6Al4V surfaces for human hip implants. Wear 2018, 394–395, 134–142. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Tan, C.; Liu, H.; Lin, P. Microstructure and tensile properties of TiCp/Ti6Al4V titanium matrix composites manufactured by laser melting deposition. J. Mater. Process. Tech. 2018, 252, 524–536. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Lin, P.; Liu, H. Microstructure and mechanical properties of functionally graded TiCp/ Ti6Al4V composite fabricated by laser melting deposition. Ceram. Int. 2017, 43, 16638–16651. [Google Scholar] [CrossRef]
- Paydas, H.; Mertens, A.; Carrus, R.; Lecomte-Beckers, J.; Tchuindjang, J.T. Laser cladding as repair technology for Ti–6Al–4V alloy: Influence of building strategy on microstructure and hardness. Mater. Des. 2015, 85, 497–510. [Google Scholar] [CrossRef]
- Baloyi, N.M.; Popoola AP, I.; Pityana, S.L. Microstructure, hardness and corrosion properties of laser processed Ti6Al4V-based composites. Trans. Nonferr. Met. Soc. China 2015, 25, 2912–2923. [Google Scholar] [CrossRef]
- Raju, R.; Duraiselvam, M.; Petley, V.; Verma, S.; Rajendran, R. Microstructural and mechanical characterization of Ti6Al4V refurbished parts obtained by laser metal deposition. Mater. Sci. Eng. A 2015, 643, 64–71. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Fischer, M.; Laheurte, P.; Acquier, P.; Joguet, D.; Peltier, L.; Petithory, T.; Anselme, K.; Mille, P. Synthesis and characterization of Ti-27.5Nb alloy made by CLAD® additive manufacturing process for biomedical applications. Mater. Sci. Eng. C 2017, 75, 341–348. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V.; Subbotin, E.P.; Nikitin, A.I.; Nadaraia, K.V.; Kulchin, Y.N. Direct laser deposition as a method of biodegradable magnesium implant manufacturing. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 1092, 012044. [Google Scholar] [CrossRef]
- Ahsan, M.N.; Paul, C.P.; Kukreja, L.M.; Pinkerton, A.J. Porous structures fabrication by continuous and pulsed laser metal deposition for biomedical applications: Modelling and experimental investigation. J. Mater. Process. Technol. 2011, 211, 602–609. [Google Scholar] [CrossRef]
- Balla, V.K.; Xue, W.; Bose, S.; Bandyopadhyay, A. Laser-assisted Zr/ZrO(2) coating on Ti for load-bearing implants. Acta Biomater. 2009, 5, 2800–2809. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Cryst. 1981, 14, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Dinnebier, R.E.; Billinge, S.J.L. Powder Diffraction Theory and Practice; The Royal Society of Chemistry: London, UK, 2008; ISBN 978-0-85404-231-9. [Google Scholar]
- Barriobero-Vila, P.; Gussone, J.; Haubrich, J.; Sandlöbes, S.; Da Silva, J.C.; Cloetens, P.; Schell, N.; Requena, G. Inducing Stable α + β Microstructures during Selective Laser Melting of Ti-6Al-4V Using Intensified Intrinsic Heat Treatments. Materials 2017, 10, 268. [Google Scholar] [CrossRef]
- Song, L.; Xiao, H.; Ye, J.; Li, S. Direct laser cladding of layer-band-free ultrafine Ti6Al4V alloy. Surf. Coat. Tech. 2016, 307, 761–771. [Google Scholar] [CrossRef]
- Tzeng, S.J.; Lin, J.J.; Yao, Y.D.; Chen, Y.Y. Electrical Resistivity of Ti0.862Al0.102V0.036 Alloy between 4 and 1000 K. J. Phys. Soc. Jpn. 1992, 61, 674. [Google Scholar] [CrossRef]
- Farrar, P.A.; Margolin, H. The titanium rich region of the titanium-aluminum-vanadium system. Trans. Met. Soc. AIME 1961, 221, 1214. [Google Scholar] [CrossRef]
- Swarnakar, A.K.; Van der Biest, O.; Baufeld, B.J. Thermal expansion and lattice parameters of shaped metal deposited Ti-6Al-4V. J. Alloys Compd. 2011, 509, 2723. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. The formation of α + β microstructure in as-fabricated selective laser melting of Ti–6Al–4V. J. Mater. Res. 2014, 29, 2028–2035. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.-L.; Li, S.-J.; Yang, R. Biomedical titanium alloys and their additive manufacturing. Rare Met. 2016, 35, 661–671. [Google Scholar] [CrossRef]
- Galarraga, H.; Lados, D.A.; Dehoff, R.R.; Kirka, M.M.; Nandwana, P. Effects of the microstructure and porosity on properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM). Addit. Manuf. 2016, 10, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Baufeld, B.; Brandl, E.; Van der Biest, O. Wire based additive layer manufacturing: Comparison of microstructure and mechanical properties of Ti6Al4V components fabricated by laser-beam deposition and shaped metal deposition. J. Mater. Process. Technol. 2011, 211, 1146–1158. [Google Scholar] [CrossRef]
- Mercelis, P.; Kruth, J.P. Residual stresses in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2006, 12, 254–265. [Google Scholar] [CrossRef]
- Davis, J.R. Metals Handbook Desk Edition, 2nd ed.; ASM International: Materials Park, OH, USA, 1998; Volume 464. [Google Scholar]











| Feature | Laser Melting Deposition | Selective Laser Melting |
|---|---|---|
| Raw materials | Powder, wire | Powder |
| Heat source | Laser | Laser, electron beam |
| Technology | Powder is sprayed through a nozzle and melted by a laser beam | Beam transfers heat that melts a powder bed |
| Typical materials | Metals, ceramics | Metals, ceramics, polymers |
| Limitations by direction/axis | No | Yes |
| Resolution | Low | High |
| Versatility | High: used for coating, parts manufacturing, and in situ alloying | Low: limited to parts production |
| Parts size | Usually large scale objects | Usually small scale objects |
| The possibility of parts repair | Yes | No |
| Structural and compositional in situ modifications | Yes: easy to produce multi-structures and parts with compositional gradient, allows for in situ alloying | No: limited to one type of powder/cycle |
| Mesh structures | No | Yes |
| Post-processing requirements | Yes | Yes |
| Costs | High: high power laser sources and robots required | Lower: more compact machines, easy to implement in industry |
| Application in the biomedical field | Currently very low | Quite advanced: e.g., implemented in dental cabinets for the manufacturing of personalized dental prostheses |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioibasu, D.; Achim, A.; Popescu, C.; Stan, G.E.; Pasuk, I.; Enculescu, M.; Iosub, S.; Duta, L.; Popescu, A. Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition. Materials 2019, 12, 906. https://doi.org/10.3390/ma12060906
Chioibasu D, Achim A, Popescu C, Stan GE, Pasuk I, Enculescu M, Iosub S, Duta L, Popescu A. Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition. Materials. 2019; 12(6):906. https://doi.org/10.3390/ma12060906
Chicago/Turabian StyleChioibasu, Diana, Alexandru Achim, Camelia Popescu, George E. Stan, Iuliana Pasuk, Monica Enculescu, Stefana Iosub, Liviu Duta, and Andrei Popescu. 2019. "Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition" Materials 12, no. 6: 906. https://doi.org/10.3390/ma12060906
APA StyleChioibasu, D., Achim, A., Popescu, C., Stan, G. E., Pasuk, I., Enculescu, M., Iosub, S., Duta, L., & Popescu, A. (2019). Prototype Orthopedic Bone Plates 3D Printed by Laser Melting Deposition. Materials, 12(6), 906. https://doi.org/10.3390/ma12060906









