Evaluation and Selection of De-Icing Salt Based on Multi-Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Melting Test of De-Icing Salts
2.2.2. Salt Freezing Corrosion Test of Concrete under the Action of De-Icing Salt
2.2.3. Effect of De-Icing Salt on Plant Growth
3. Results and Analysis
3.1. De-Icing Ability
3.1.1. De-Icing Ability Test under 18% Concentration of De-Icing Salt
3.1.2. De-Icing Ability Test under 29% Concentration of De-Icing Salt
3.2. Salt Freezing Corrosion of Concrete under the Action of De-Icing Salt
- (1)
- Under 3% concentration of de-icing salt, the peeling amount of concrete surface increases with the increase in the number of cycles. Under the action of five de-icing salts, namely, sodium chloride, ethylene glycol, urea, sodium formate, and calcium chloride, the peeling amount of concrete is high. Among the five de-icing salts, ethylene glycol, sodium formate, and urea have higher salt freezing corrosion on concrete than sodium chloride. By contrast, magnesium chloride and calcium magnesium acetate have less salt corrosion damage to concrete.
- (2)
- Under 10% concentration of de-icing salt, the peeling amount of concrete surface increases with the increase in the number of freeze-thaw cycles. Among the eight de-icing salts, the peeling amount of concrete with sodium chloride, ethylene glycol, urea, and sodium formate is more serious. Ethylene glycol and sodium formate are more corrosive to concrete than sodium chloride. On the contrary, calcium magnesium acetate, calcium chloride, and magnesium chloride have less salt corrosion damage to concrete.
- (3)
- Under 20% concentration of de-icing salt, the peeling amount of concrete surface is extremely small. In particular, urea, calcium magnesium acetate, and magnesium chloride have not peeled off the surface of concrete after 30 freeze-thaw cycles.
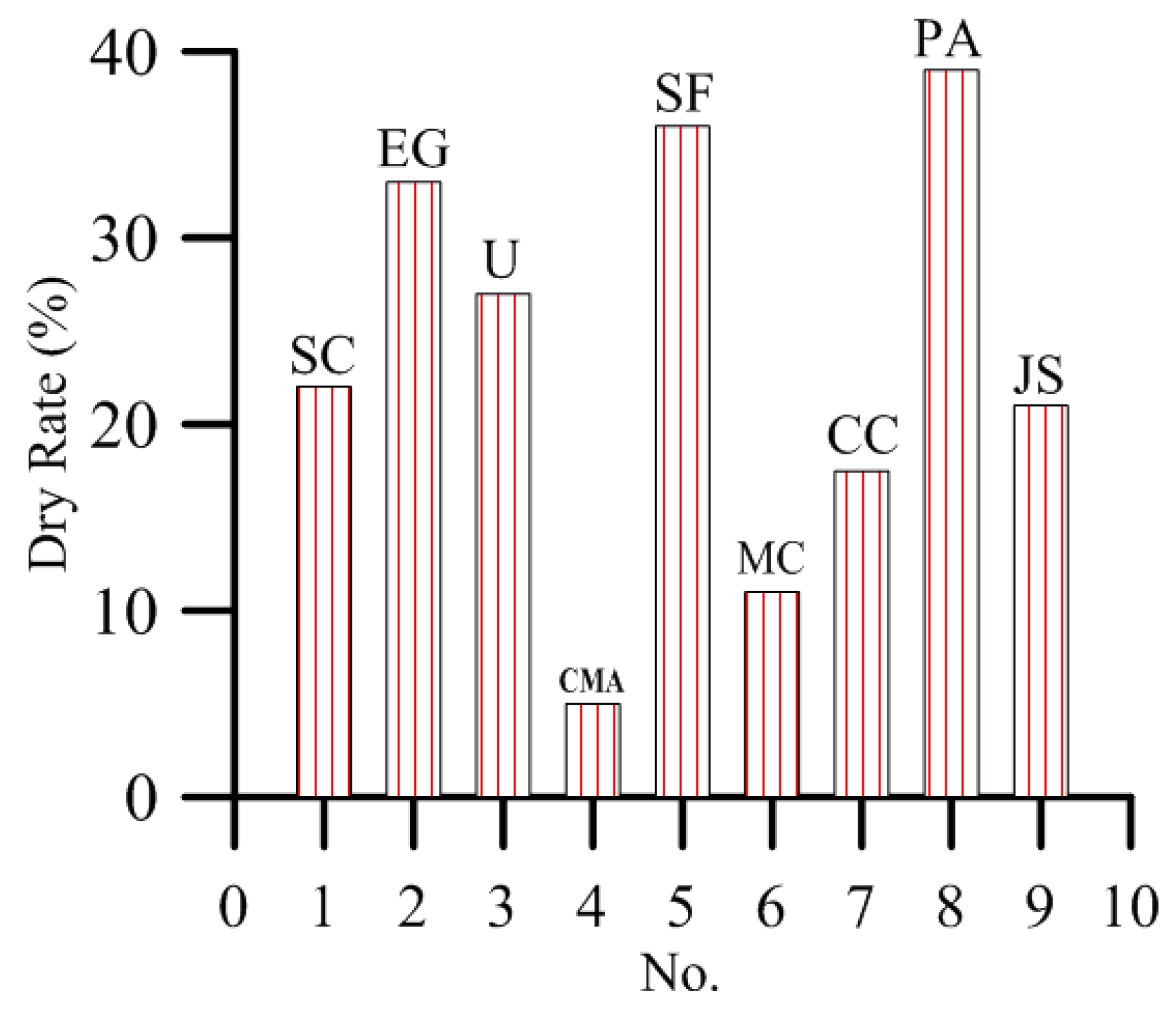
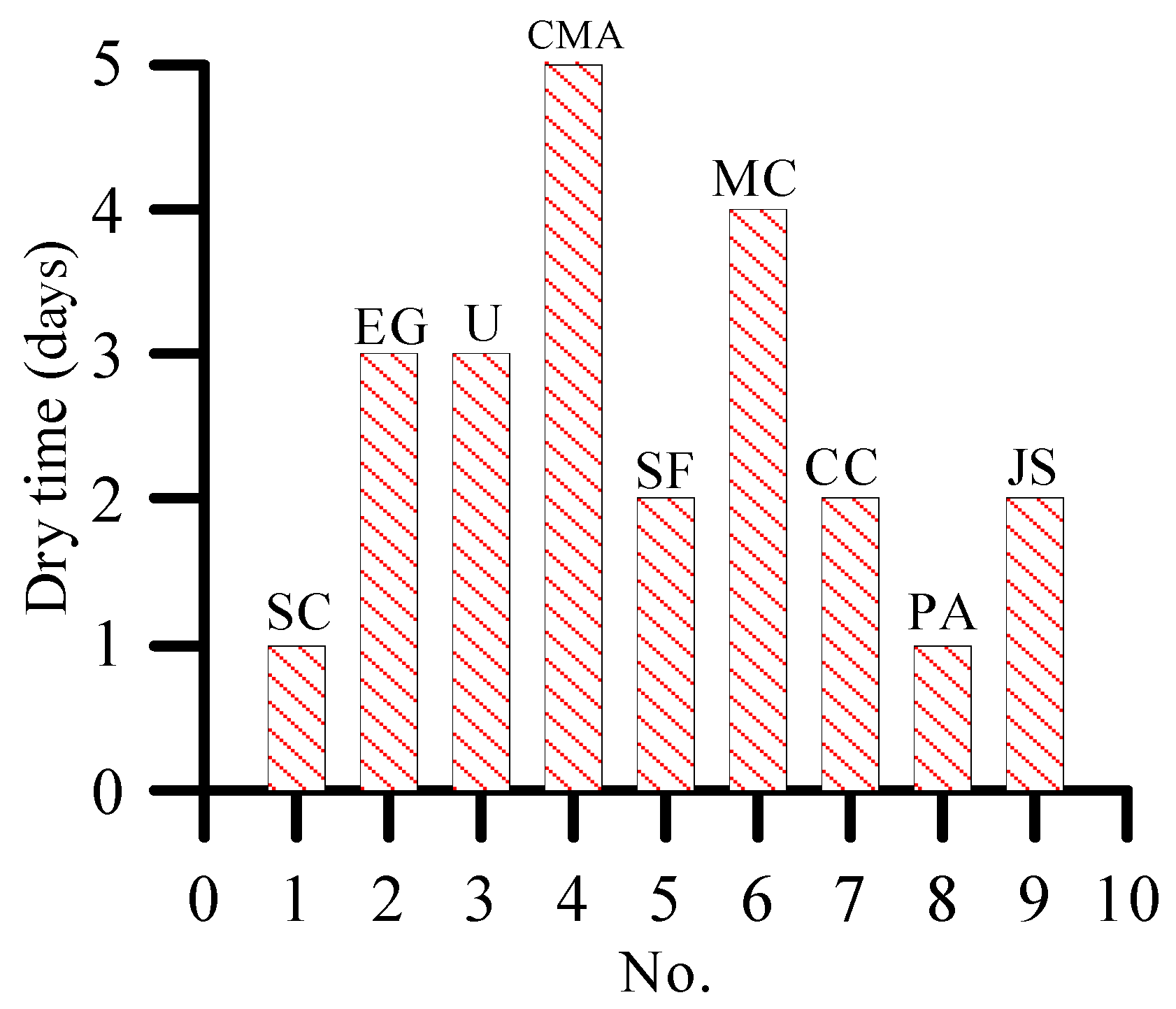
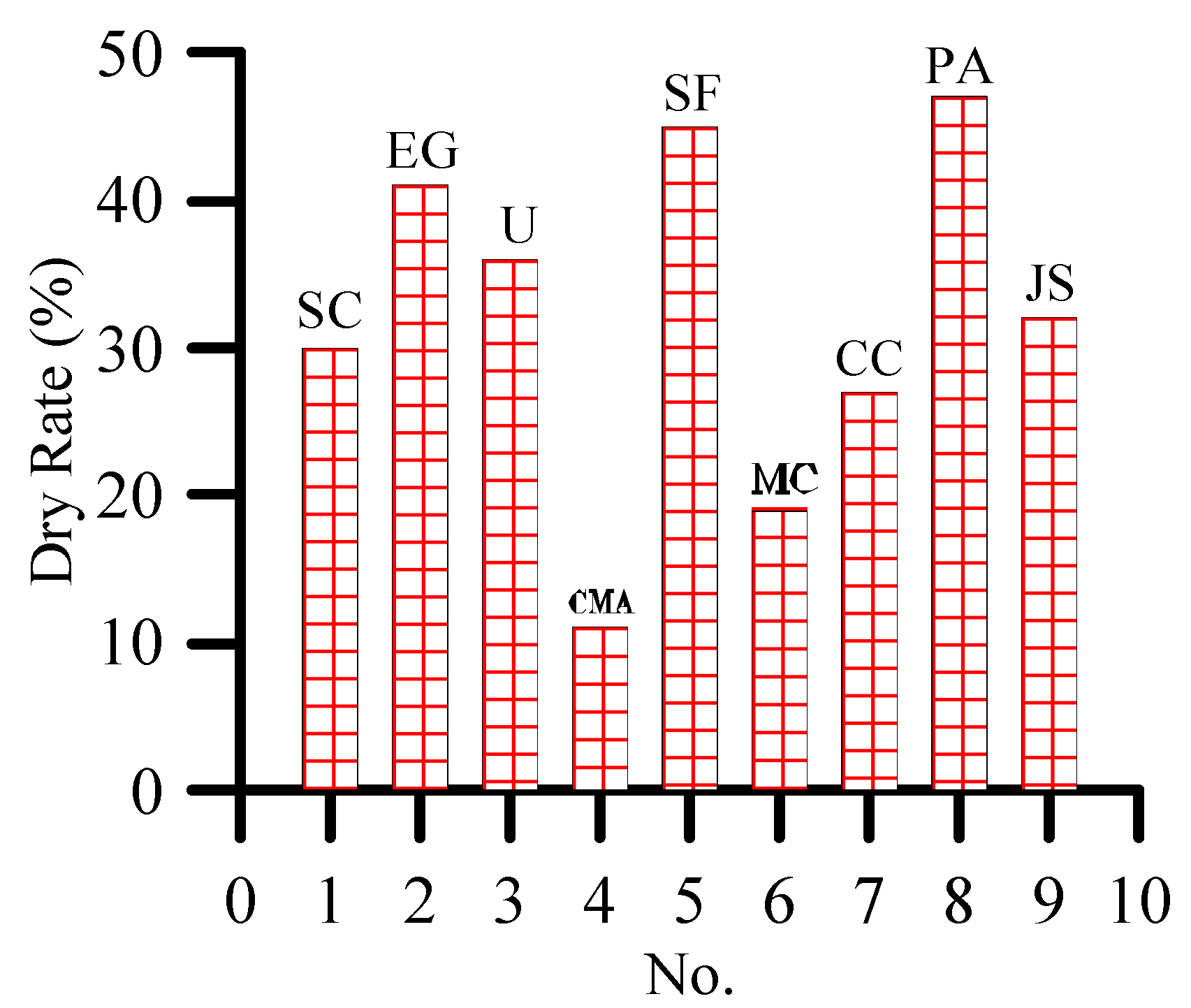
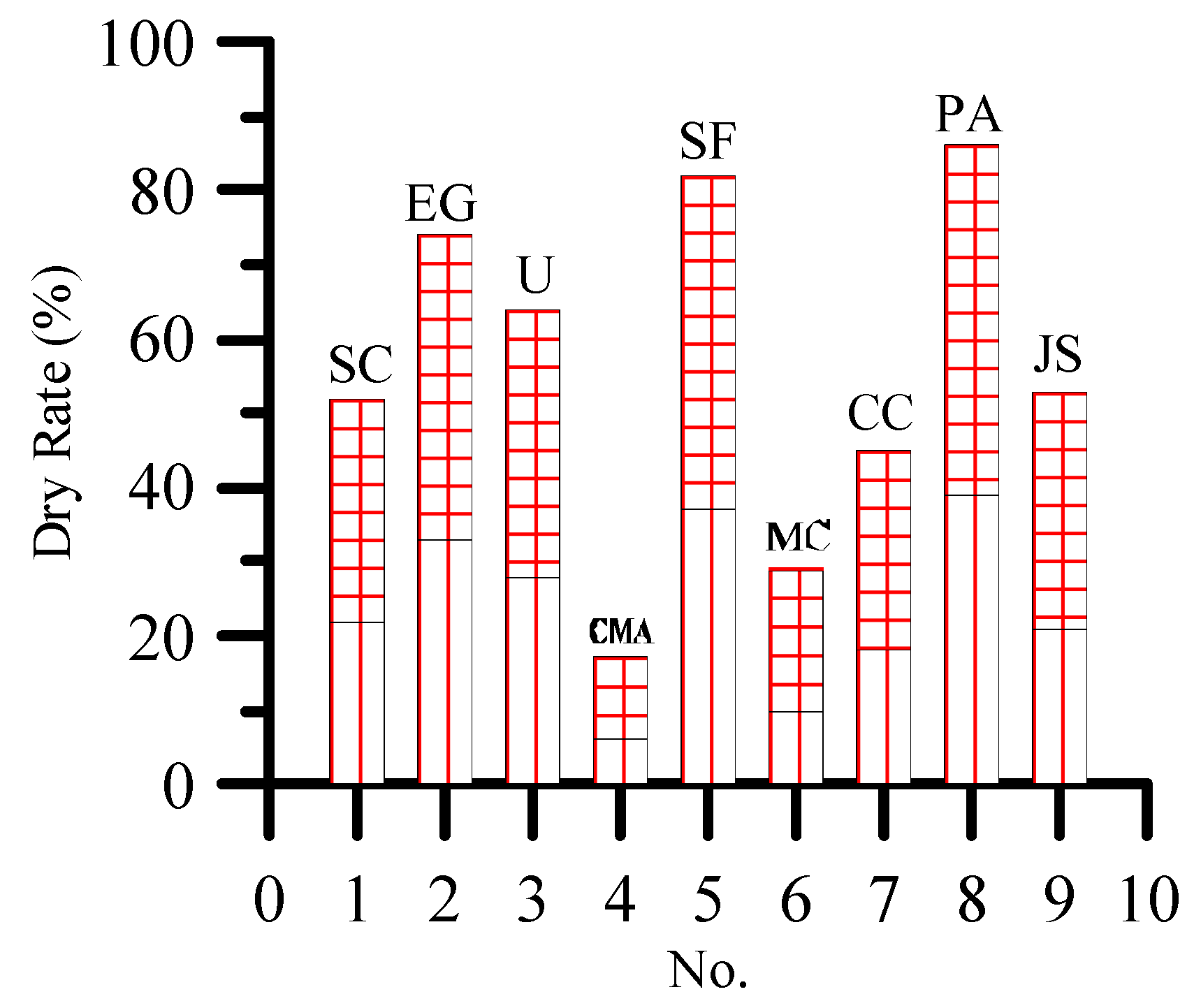
3.3. Effect of De-Icing Salt on Plant Growth
4. Comprehensive Evaluation of De-Icing Salt by Analytic Hierarchy Process
4.1. Establishment of a Hierarchical Analysis Structure
4.2. Establishment of Judgment Matrixes
4.3. Consistency Check and Hierarchical Ordering
4.4. Hierarchical Total Ordering
4.5. Decision Making
5. Conclusions
- (1)
- At 18% concentration of de-icing salt, the de-icing accumulation of all de-icing salts increases as de-icing time progresses. The maximum value is obtained at 120 min, and then the amount of de-icing decreases. At 29% concentration, the amount of de-icing reaches the maximum at 60 min and decreases at 90 min.
- (2)
- When the concentration of de-icing salt is 3%, the surface of concrete is highly exfoliated. When the concentration of de-icing salt is 20%, the surface of concrete is barely peeled off after 30 freeze-thaw cycles.
- (3)
- At 10% concentration, the dry rate of plants is greater than 3%. Moreover, 3% and 10% concentrations of de-icing salt show a consistent trend of damage to plants.
- (4)
- Seven de-icing salts are tested under de-icing at 18% concentration, salt peeling at 3% concentration, and plant dry rate at 3% concentration. A hierarchical analysis model is established on the basis of the priority of the three criteria, and a comparative judgment matrix is constructed. A de-icing salt that meets the requirements is selected. The preferred de-icing salts differ for dissimilar priorities of the three criteria.
Author Contributions
Funding
Conflicts of Interest
References
- Marchand, J.; Sellevold, E.J.; Pigeon, M. The deicer salt scaling deterioration of concrete—An overview. ACI Spec. Publ. 1994, 145, 1–46. [Google Scholar]
- Litvan, G.G. Frost action in cement in the presence of De-Icers. Cem. Concr. Res. 1976, 6, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.; Lee, H.; Sim, J. Experimental comparison of methods to assess the durability of concrete pavement deteriorated from scaling and freeze-thaw effect. KSCE J. Civ. Eng. 2018, 22, 2406–2416. [Google Scholar] [CrossRef]
- Kayama, M.; Quoreshi, A.M.; Kitaoka, S.; Kitahashi, Y.; Sakamoto, Y.; Maruyama, Y.; Kitao, M.; Koike, T. Effects of deicing salt on the vitality and health of two spruce species, Picea abies Karst. and Picea glehnii Masters planted along roadsides in northern Japan. Environ. Pollut. 2003, 124, 127–137. [Google Scholar] [CrossRef]
- Fay, L.; Shi, X. Environmental Impacts of Chemicals for Snow and Ice Control: State of the Knowledge. Water Air Soil Pollut. 2012, 223, 2751–2770. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I.; Kusza, G.; Duzynski, M. Effect of deicing salts on urban soils and health status of roadside trees in the Opole region. Environ. Toxicol. 2004, 19, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cekstere, G.; Osvalde, A. A study of chemical characteristics of soil in relation to street trees status in Riga (Latvia). Urban For. Urban Green. 2013, 12, 69–78. [Google Scholar] [CrossRef]
- Ordóñez-Barona, C.; Sabetski, V.; Millward, A.A.; Steenberg, J. De-icing salt contamination reduces urban tree performance in structural soil cells. Environ. Pollut. 2017, 234, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.F. Investigation and Analysis of the Impact of Snow Melting Agent on Urban Greening. J. Hebei For. Sci. Technol. 2010, 2, 27–29. (In Chinese) [Google Scholar]
- Godwin, K.S.; Hafner, S.D.; Buff, M.F. Long-term trends in sodium and chloride in the Mohawk River, New York: The effect of fifty years of road-salt application. Environ. Pollut. 2003, 124, 273–281. [Google Scholar] [CrossRef]
- Ostendorf, D.W.; Hinlein, E.S.; Rotaru, C.; DeGroot, D.J. Contamination of groundwater by outdoor highway deicing agent storage. J. Hydrol. 2006, 326, 109–121. [Google Scholar] [CrossRef]
- Ostendorf, D.W.; Palmer, R.N.; Hinlein, E.S. Seasonally varying highway de-icing agent contamination in a groundwater plume from an infiltration basin. Hydrol. Res. 2009, 40, 520–532. [Google Scholar] [CrossRef]
- Sanzo, D.; Hecnar, S.J. Effects of road de-icing salt (NaCl) on larval wood frogs (Rana sylvatica). Environ. Pollut. 2006, 140, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Saaty, T.L.; Vargas, L.G. Models, Methods, Concepts & Applications of the Analytic Hierarchy Process; Springer: New York, NY, USA, 2012. [Google Scholar]
- Vaidya, O.S.; Kumar, S. Analytic hierarchy process: An overview of applications. Eur. J. Oper. Res. 2006, 169, 1–29. [Google Scholar] [CrossRef]
- Hassan, Y.; Abd El Halim, A.O.; Razaqpur, A.G.; Bekheet, W.; Farha, M.H. Effects of runway deicer on pavement materials and mixes: Comparison with road salt. J. Transp. Eng. 2002, 128, 385–391. [Google Scholar] [CrossRef]
- Murphy, C.; Wallace, S.; Knight, R.; Cooper, D.; Sellers, T. Treatment performance of an aerated constructed wetland treating glycol from de-icing operations at a UK airport. Ecol. Eng. 2015, 80, 117–124. [Google Scholar] [CrossRef]
- GB/T 23851-2017. Snow Melting Agent (GB/T 23851-2017); China Standard Press, Chaoyang District: Beijing, China, 2017. (In Chinese)





| No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Name | Sodium chloride (SC) | Ethylene glycol (EG) | Urea (U) | Calcium magnesium acetate (CMA) | Sodium formate (SF) | Magnesium chloride (MC) | Calcium chloride (CC) | Potassium acetate (PA) | Ji-suo II (JS) |
| No | De-Icing Salt | De-Icing Accumulation/g | Baseline Percentage (30 min)/% | ||||
|---|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | 150 min | |||
| 1 | Sodium chloride | 8.3 | 9.2 | 11.3 | 11.5 | 11.6 | 100.0 |
| 2 | Ethylene glycol | 8.7 | 10.3 | 12.0 | 12.2 | 12.2 | 104.8 |
| 3 | Urea | 7.6 | 8.8 | 9.6 | 9.7 | 9.9 | 91.6 |
| 4 | Calcium magnesium acetate | 7.9 | 9.0 | 9.8 | 10.4 | 10.5 | 95.2 |
| 5 | Sodium formate | 7.8 | 8.9 | 10.1 | 10.2 | 10.3 | 94.0 |
| 6 | Magnesium chloride | 8.0 | 9.3 | 10.0 | 10.4 | 10.6 | 96.4 |
| 7 | Calcium chloride | 8.5 | 9.7 | 10.1 | 10.3 | 10.4 | 102.4 |
| 8 | Potassium acetate | 5.8 | 6.6 | 7.5 | 7.7 | 6.8 | 69.9 |
| 9 | Ji-suo II | 9.7 | 11.1 | 10.9 | 7.8 | −0.7 | 116.9 |
| No | De-Icing Salt | De-Icing Accumulation/g | ||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | ||
| 1 | Sodium chloride | 15.75 | 18.71 | 17.13 |
| 2 | Ethylene glycol | 10.69 | 13.62 | 8.9 |
| 3 | Urea | 7.91 | 10.75 | 7.05 |
| 4 | Calcium magnesium acetate | 9.31 | 12.55 | 8.73 |
| 5 | Sodium formate | — | — | — |
| 6 | Magnesium chloride | 9.07 | 11.28 | 4.1 |
| 7 | Calcium chloride | 14.28 | 17.45 | 15.51 |
| 8 | Ji-suo II | 10.78 | 8.39 | 4.15 |
| No | De-Icing Salt | Concrete Peeling Amount (kg/m2) | ||
|---|---|---|---|---|
| 3% | 10% | 20% | ||
| 1 | Sodium chloride | 0.46 | 0.40 | 0.04 |
| 2 | Ethylene glycol | 0.52 | 0.44 | 0.12 |
| 3 | Urea | 0.57 | 0.42 | 0.00 |
| 4 | Calcium magnesium acetate | 0.28 | 0.19 | 0.00 |
| 5 | Sodium formate | 0.53 | 0.35 | 0.06 |
| 6 | Magnesium chloride | 0.14 | 0.20 | 0.00 |
| 7 | Calcium chloride | 0.41 | 0.23 | 0.10 |
| 8 | Ji-suo II | 0.49 | 0.38 | 0.05 |
| Significance | Importance of ai Relative to aj | ||||
|---|---|---|---|---|---|
| The Same | Slightly Stronger | Stronger | Evidently Strong | Absolutely Strong | |
| Scale aij | 1 | 3 | 5 | 7 | 9 |
| (a) | ||||
| A | B1 | B2 | B3 | W11 |
| B1 | 1 | 3 | 2 | 0.539 |
| B2 | 1/3 | 1 | 1/2 | 0.164 |
| B3 | 1/2 | 2 | 1 | 0.297 |
| (b) | ||||
| A | B1 | B2 | B3 | W12 |
| B1 | 1 | 3 | 1 | 0.429 |
| B2 | 1/3 | 1 | 1/3 | 0.142 |
| B3 | 1 | 3 | 1 | 0.429 |
| No | De-Icing Salt | B1/Relative Value Calibration | B2/Relative Value Calibration | B3/Relative Value Calibration |
|---|---|---|---|---|
| C1 | Sodium chloride | 100.0/6 | 100.0/3 | 100.0/5 |
| C2 | Ethylene glycol | 104.8/9 | 113.3/2 | 150.0/1 |
| C3 | Urea | 91.6/1 | 124.8/1 | 122.7/3 |
| C4 | Calcium magnesium acetate | 95.2/3 | 60.8/7 | 22.7/9 |
| C5 | Sodium formate | 94.0/2 | 116.1/1 | 163.6/1 |
| C6 | Magnesium chloride | 96.4/4 | 31.5/9 | 50.0/8 |
| C7 | Calcium chloride | 102.4/8 | 89.5/4 | 79.5/6 |
| B1 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | W2 |
|---|---|---|---|---|---|---|---|---|
| C1 | 1 | 1/4 | 6 | 4 | 5 | 3 | 1/3 | 0.150 |
| C2 | 4 | 1 | 9 | 7 | 8 | 6 | 2 | 0.381 |
| C3 | 1/6 | 1/9 | 1 | 1/3 | 1/2 | 1/4 | 1/8 | 0.026 |
| C4 | 1/4 | 1/7 | 3 | 1 | 2 | 1/2 | 1/6 | 0.054 |
| C5 | 1/5 | 1/8 | 2 | 1/2 | 1 | 1/3 | 1/7 | 0.037 |
| C6 | 1/3 | 1/6 | 4 | 2 | 3 | 1 | 1/5 | 0.079 |
| C7 | 3 | 1/2 | 8 | 6 | 7 | 5 | 1 | 0.273 |
| B2 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | W3 |
|---|---|---|---|---|---|---|---|---|
| C1 | 1 | 2 | 3 | 1/5 | 3 | 1/7 | 1/2 | 0.076 |
| C2 | 1/2 | 1 | 2 | 1/6 | 2 | 1/8 | 1/3 | 0.050 |
| C3 | 1/3 | 1/2 | 1 | 1/7 | 1 | 1/9 | 1/4 | 0.032 |
| C4 | 5 | 6 | 7 | 1 | 7 | 1/3 | 4 | 0.258 |
| C5 | 1/3 | 1/2 | 1 | 1/7 | 1 | 1/9 | 1/4 | 0.032 |
| C6 | 7 | 8 | 9 | 3 | 9 | 1 | 6 | 0.440 |
| C7 | 2 | 3 | 4 | 1/4 | 4 | 1/6 | 1 | 0.111 |
| B3 | C1 | C2 | C3 | C4 | C5 | C6 | C7 | W4 |
|---|---|---|---|---|---|---|---|---|
| C1 | 1 | 5 | 3 | 1/5 | 5 | 1/4 | 1/2 | 0.103 |
| C2 | 1/5 | 1 | 1/3 | 1/9 | 1 | 1/8 | 1/6 | 0.027 |
| C3 | 1/3 | 3 | 1 | 1/7 | 3 | 1/6 | 1/4 | 0.055 |
| C4 | 5 | 9 | 7 | 1 | 9 | 2 | 4 | 0.376 |
| C5 | 1/5 | 1 | 1/3 | 1/9 | 1 | 1/8 | 1/6 | 0.027 |
| C6 | 4 | 8 | 6 | 1/2 | 8 | 1 | 3 | 0.268 |
| C7 | 2 | 6 | 4 | 1/4 | 6 | 1/3 | 1 | 0.144 |
| Matrix Order | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| RI | 0 | 0 | 0.58 | 0.9 | 1.12 | 1.24 | 1.32 | 1.41 | 1.45 |
| No. | B1(0.539/0.429) | B2(0.164/0.142) | B3(0.297/0.429) | Wtotal1/Wtotal2 |
|---|---|---|---|---|
| C1 | 0.150 | 0.076 | 0.103 | 0.124/0.119 |
| C2 | 0.381 | 0.050 | 0.027 | 0.222/0.182 |
| C3 | 0.026 | 0.032 | 0.055 | 0.036/0.039 |
| C4 | 0.054 | 0.258 | 0.376 | 0.183/0.221 |
| C5 | 0.037 | 0.032 | 0.027 | 0.033/0.032 |
| C6 | 0.079 | 0.440 | 0.268 | 0.194/0.211 |
| C7 | 0.273 | 0.111 | 0.144 | 0.208/0.195 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, G.; Zhang, J.; Tian, B. Evaluation and Selection of De-Icing Salt Based on Multi-Factor. Materials 2019, 12, 912. https://doi.org/10.3390/ma12060912
Ke G, Zhang J, Tian B. Evaluation and Selection of De-Icing Salt Based on Multi-Factor. Materials. 2019; 12(6):912. https://doi.org/10.3390/ma12060912
Chicago/Turabian StyleKe, Guoju, Jun Zhang, and Bo Tian. 2019. "Evaluation and Selection of De-Icing Salt Based on Multi-Factor" Materials 12, no. 6: 912. https://doi.org/10.3390/ma12060912
APA StyleKe, G., Zhang, J., & Tian, B. (2019). Evaluation and Selection of De-Icing Salt Based on Multi-Factor. Materials, 12(6), 912. https://doi.org/10.3390/ma12060912




