Graphene-Based Inks for Printing of Planar Micro-Supercapacitors: A Review
Abstract
1. Introduction
2. Fundamentals of Micro-Supercapacitors
2.1. Materials
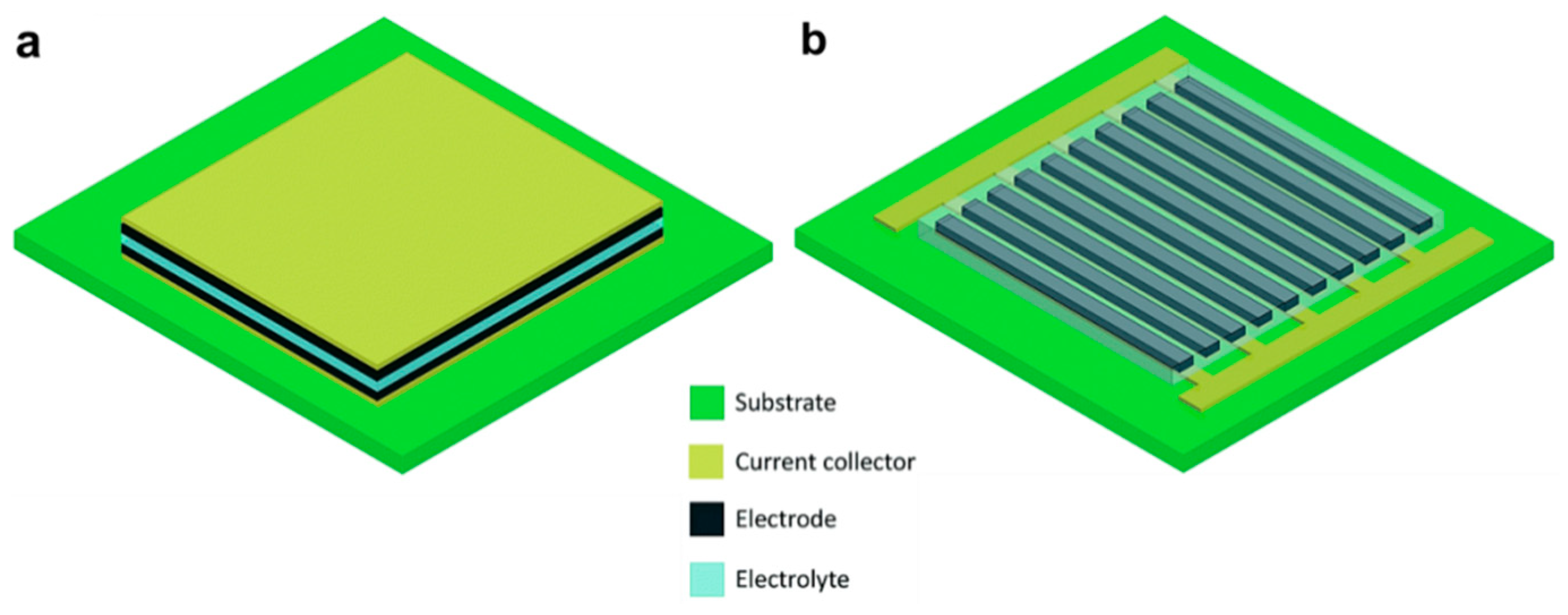
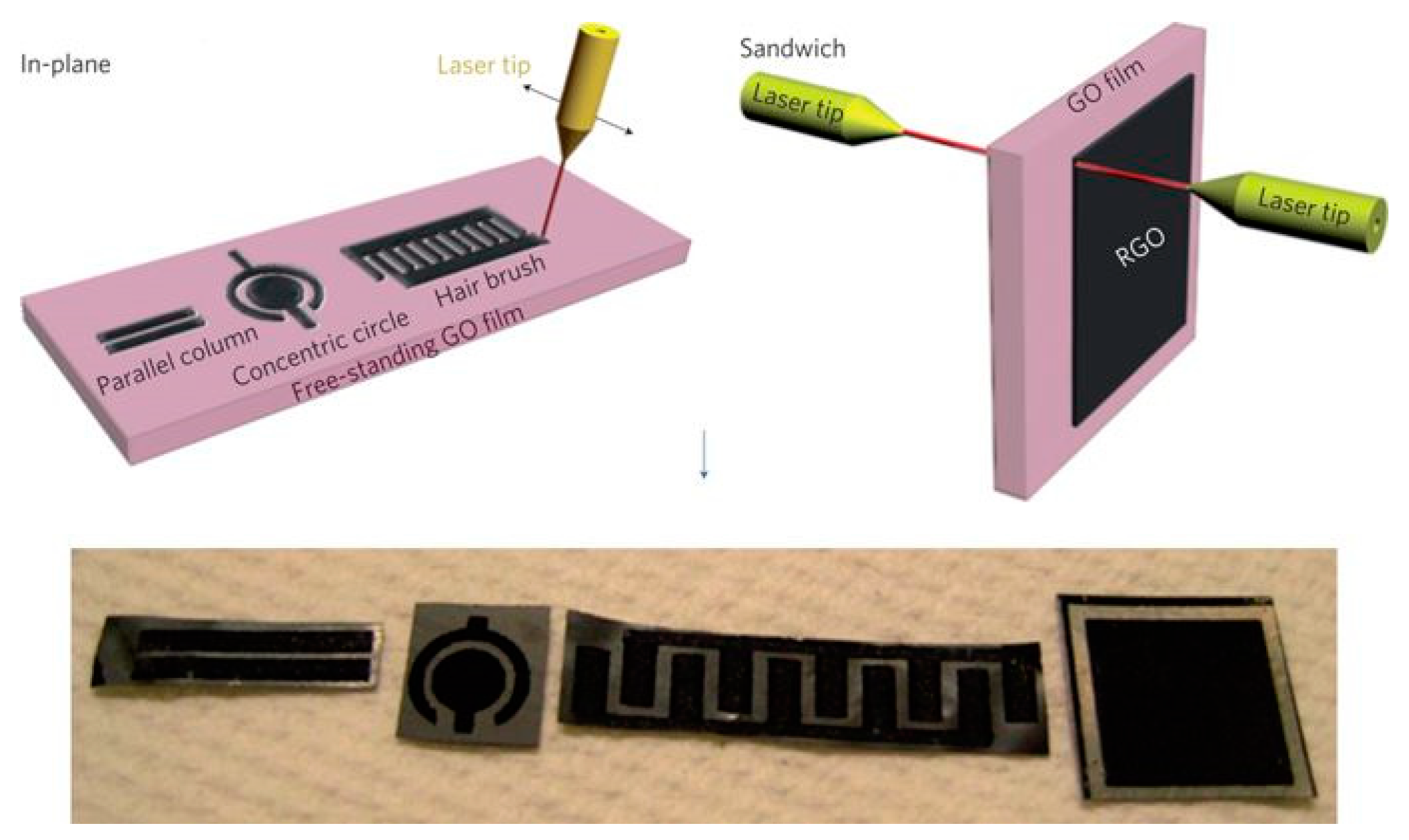
2.2. Designs
2.3. Performance Evaluation
3. Graphene-Based Inks for Electrode Materials
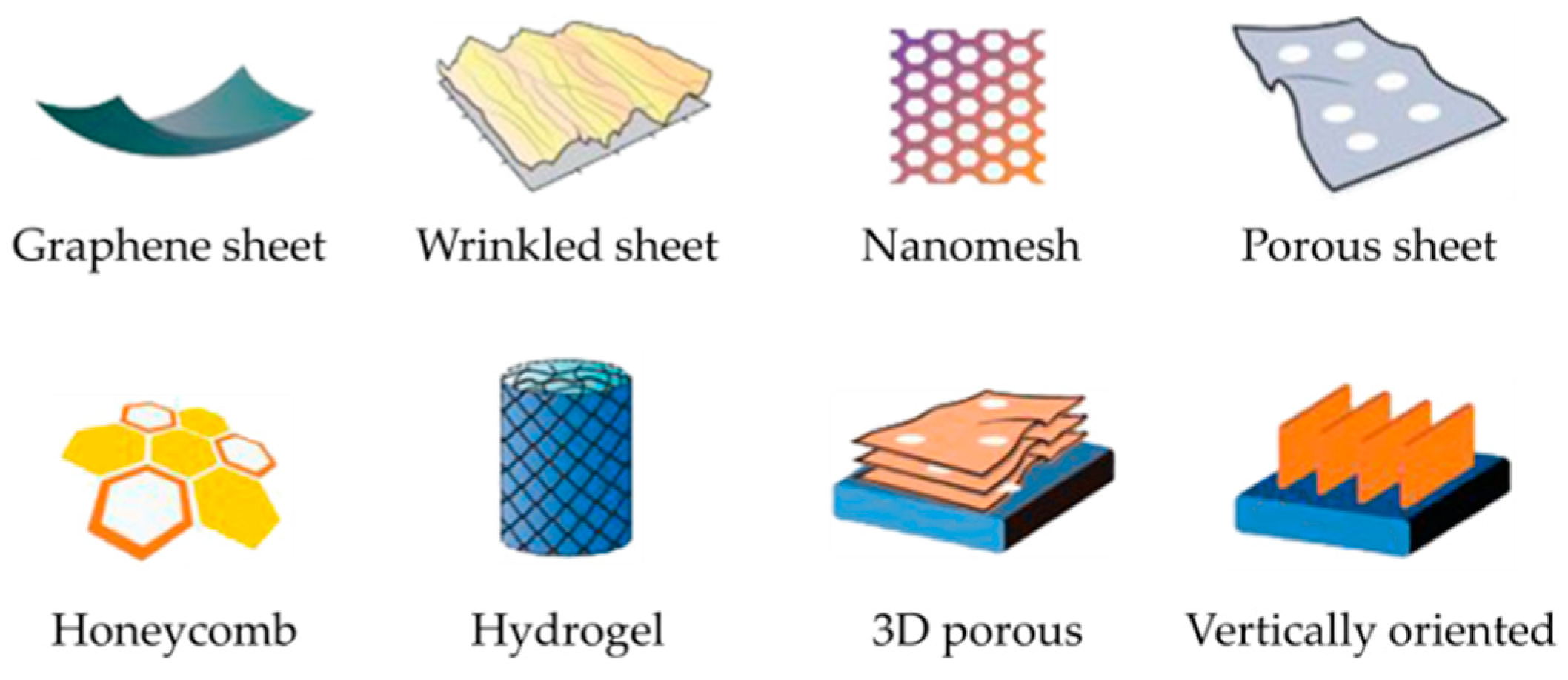
3.1. Pristine Graphene
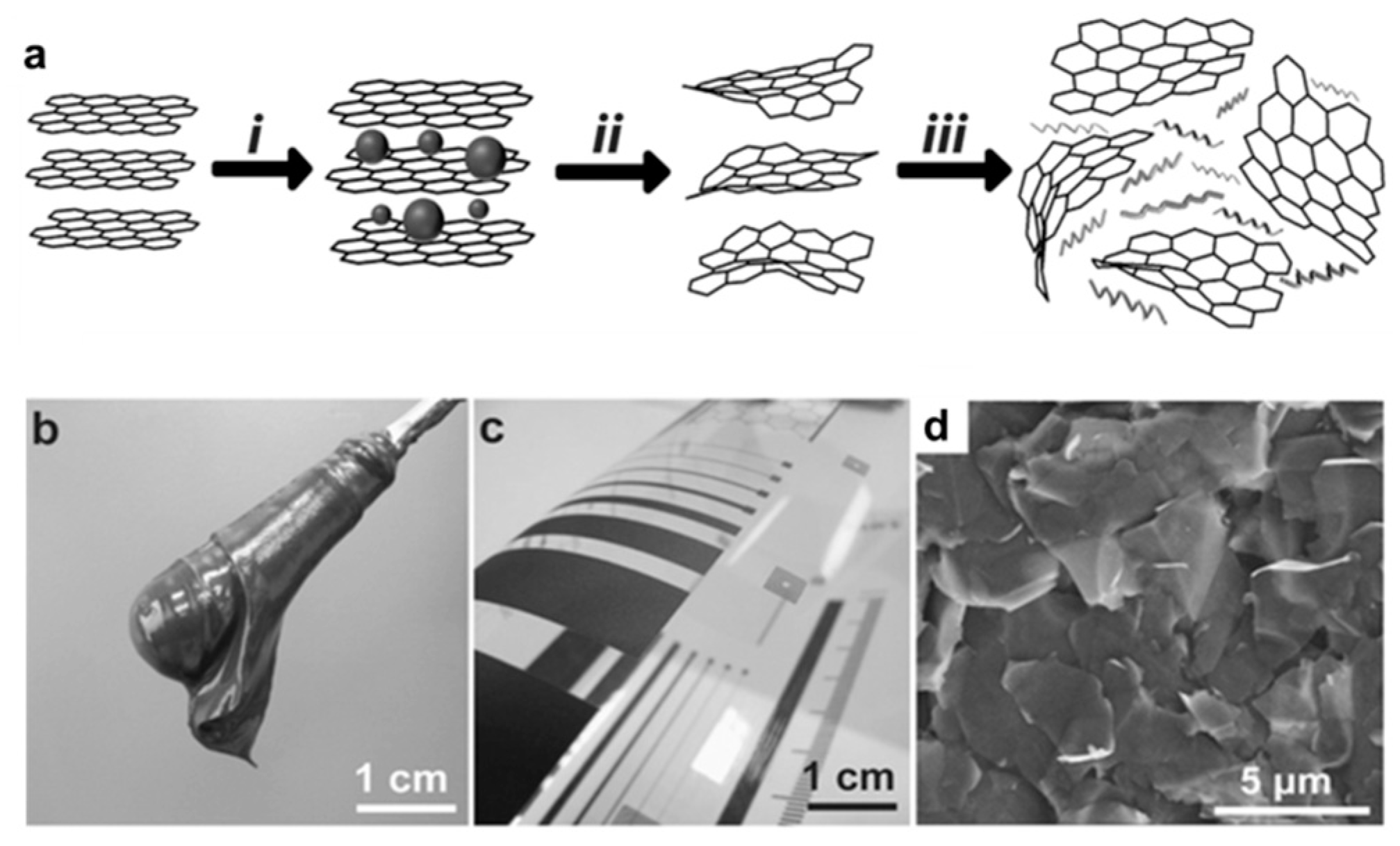
3.2. Graphene Oxide
3.3. Graphene Composites
4. Printing Techniques
4.1. Screen Printing
4.2. Inkjet Printing
4.3. 3D Printing
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gubbi, J.; Buyya, R.; Marusic, S.; Palaniswami, M. Internet of Things (IoT): A vision, architectural elements, and future directions. Future Gener. Comput. Syst. 2013, 29, 1645–1660. [Google Scholar] [CrossRef]
- Zanella, A.; Bui, N.; Castellani, A.; Vangelista, L.; Zorzi, M. Internet of things for smart cities. IEEE Internet Things J. 2014, 1, 22–32. [Google Scholar] [CrossRef]
- Stojkoska, B.L.R.; Trivodaliev, K.V. A review of Internet of Things for smart home: Challenges and solutions. J. Clean. Prod. 2017, 140, 1454–1464. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Harb, J.N.; LaFollette, R.M.; Selfridge, R.H.; Howell, L.L. Microbatteries for self-sustained hybrid micropower supplies. J. Power Sources 2002, 104, 46–51. [Google Scholar] [CrossRef]
- Wakihara, M.; Yamamoto, O. Lithium Ion Batteries: Fundamentals and Performance; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Palacin, M.R. Recent advances in rechargeable battery materials: A chemist’s perspective. Chem. Soc. Rev. 2009, 38, 2565–2575. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Lee, J.A.; Shin, M.K.; Kim, S.H.; Cho, H.U.; Spinks, G.M.; Wallace, G.G.; Lima, M.D.; Lepró, X.; Kozlov, M.E.; Baughman, R.H. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 2013, 4, 1970. [Google Scholar] [CrossRef]
- Beidaghi, M.; Gogotsi, Y. Capacitive energy storage in micro-scale devices: Recent advances in design and fabrication of micro-supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Hu, H.; Pei, Z.; Ye, C. Recent advances in designing and fabrication of planar micro-supercapacitors for on-chip energy storage. Energy Storage Mater. 2015, 1, 82–102. [Google Scholar] [CrossRef]
- Liu, N.; Gao, Y. Recent Progress in Micro-Supercapacitors with In-Plane Interdigital Electrode Architecture. Small 2017, 13. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Morozov, S.; Novoselov, K.; Katsnelson, M.; Schedin, F.; Elias, D.; Jaszczak, J.A.; Geim, A. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Feng, X.; Cheng, H.-M. Recent advances in graphene-based planar micro-supercapacitors for on-chip energy storage. Nat. Sci. Rev. 2014, 1, 277–292. [Google Scholar] [CrossRef]
- Xia, J.; Chen, F.; Li, J.; Tao, N. Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 2009, 4, 505–509. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Chen, T.; Xue, Y.; Roy, A.K.; Dai, L. Transparent and stretchable high-performance supercapacitors based on wrinkled graphene electrodes. ACS Nano 2013, 8, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hayner, C.M.; Kung, M.C.; Kung, H.H. Flexible holey graphene paper electrodes with enhanced rate capability for energy storage applications. ACS Nano 2011, 5, 8739–8749. [Google Scholar] [CrossRef]
- Kim, H.-K.; Bak, S.-M.; Lee, S.W.; Kim, M.-S.; Park, B.; Lee, S.C.; Choi, Y.J.; Jun, S.C.; Han, J.T.; Nam, K.-W. Scalable fabrication of micron-scale graphene nanomeshes for high-performance supercapacitor applications. Energy Environ. Sci. 2016, 9, 1270–1281. [Google Scholar] [CrossRef]
- Chen, C.-M.; Zhang, Q.; Zhao, X.-C.; Zhang, B.; Kong, Q.-Q.; Yang, M.-G.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G.; Schlögl, R. Hierarchically aminated graphene honeycombs for electrochemical capacitive energy storage. J. Mat. Chem. 2012, 22, 14076–14084. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Huang, X.; Liu, Y.; Huang, Y.; Duan, X. Flexible solid-state supercapacitors based on three-dimensional graphene hydrogel films. ACS Nano 2013, 7, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jung, G.; Yoo, S.; Suh, K.S.; Ruoff, R.S. Activated graphene-based carbons as supercapacitor electrodes with macro-and mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Outlaw, R.; Holloway, B. Graphene double-layer capacitor with ac line-filtering performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, W.; Ma, W.; Wen, Z.; Shuai, X.; Chen, J.; Yan, J.; Wang, Z.; Cen, K.; Feng, X. Vertically oriented graphene bridging active-layer/current-collector interface for ultrahigh rate supercapacitors. Adv. Mater. 2013, 25, 5799–5806. [Google Scholar] [CrossRef]
- Aradilla, D.; Delaunay, M.; Sadki, S.; Gérard, J.-M.; Bidan, G. Vertically aligned graphene nanosheets on silicon using an ionic liquid electrolyte: Towards high performance on-chip micro-supercapacitors. J. Mater. Chem. A 2015, 3, 19254–19262. [Google Scholar] [CrossRef]
- Zheng, S.; Li, Z.; Wu, Z.-S.; Dong, Y.; Zhou, F.; Wang, S.; Fu, Q.; Sun, C.; Guo, L.; Bao, X. High packing density unidirectional arrays of vertically aligned graphene with enhanced areal capacitance for high-power micro-supercapacitors. ACS Nano 2017, 11, 4009–4016. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.; Tung, T.T.; Nine, M.J.; Kabiri, S.; Moussa, M.; Tran, D.N.; Losic, D. Scanning atmospheric plasma for ultrafast reduction of graphene oxide and fabrication of highly conductive graphene films and patterns. Carbon 2018, 127, 113–121. [Google Scholar] [CrossRef]
- Tung, T.T.; Feller, J.F.; Kim, T.; Kim, H.; Yang, W.S.; Suh, K.S. Electromagnetic properties of Fe3O4-functionalized graphene and its composites with a conducting polymer. J. Polym. Sci. A 2012, 50, 927–935. [Google Scholar] [CrossRef]
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Graphene inks for printed flexible electronics: Graphene dispersions, ink formulations, printing techniques and applications. Adv. Colloid Interface Sci. 2018, 261, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Fan, Z.; Wei, T.; Zhang, L. High-performance supercapacitor electrodes based on highly corrugated graphene sheets. Carbon 2012, 50, 2179–2188. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Tripathi, K.M.; Tran, T.S.; Kim, Y.J.; Kim, T. Green fluorescent onion-like carbon nanoparticles from flaxseed oil for visible light induced photocatalytic applications and label-free detection of Al (III) ions. ACS Sustain. Chem. Eng. 2017, 5, 3982–3992. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Choi, Y.C.; Lee, S.M.; Chung, D.C.; Bae, D.J.; Lim, S.C.; Lee, Y.H. Supercapacitors using single-walled carbon nanotube electrodes. Adv. Mater. 2001, 13, 497–500. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Cottineau, T.; Toupin, M.; Delahaye, T.; Brousse, T.; Bélanger, D. Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl. Phys. A 2006, 82, 599–606. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.-W. Mesoporous transition metal oxides for supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Tripathi, K.M.; Kim, B.N.; You, I.-K.; Park, B.J.; Han, Y.H.; Kim, T. Three-dimensionally assembled Graphene/α-MnO2 nanowire hybrid hydrogels for high performance supercapacitors. Mater. Res. Bull. 2017, 96, 395–404. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.-G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Van Aken, K.L.; Beidaghi, M.; Gogotsi, Y. Formulation of ionic-liquid electrolyte to expand the voltage window of supercapacitors. Angew. Chem. 2015, 127, 4888–4891. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Design and integration of flexible planar micro-supercapacitors. Nano Res. 2017, 10, 1524–1544. [Google Scholar] [CrossRef]
- Yang, P.; Mai, W. Flexible solid-state electrochemical supercapacitors. Nano Energy 2014, 8, 274–290. [Google Scholar] [CrossRef]
- Le Bideau, J.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- Taghavikish, M.; Subianto, S.; Gu, Y.; Sun, X.; Zhao, X.; Choudhury, N.R. A Poly (ionic liquid) Gel Electrolyte for Efficient all Solid Electrochemical Double-Layer Capacitor. Sci. Rep. 2018, 8, 10918. [Google Scholar] [CrossRef] [PubMed]
- Tooming, T.; Thomberg, T.; Kurig, H.; Jänes, A.; Lust, E. High power density supercapacitors based on the carbon dioxide activated d-glucose derived carbon electrodes and 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid. J. Power Sources 2015, 280, 667–677. [Google Scholar] [CrossRef]
- Mourad, E.; Coustan, L.; Lannelongue, P.; Zigah, D.; Mehdi, A.; Vioux, A.; Freunberger, S.A.; Favier, F.; Fontaine, O. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 2017, 16, 446–453. [Google Scholar] [CrossRef]
- Yoon, Y.; Cho, W.; Lim, J.; Choi, D. Solid-state thin-film supercapacitor with ruthenium oxide and solid electrolyte thin films. J. Power Sources 2001, 101, 126–129. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, D.J.; Kim, H.-K.; Cho, W.I.; Yoon, Y.S. Thin film supercapacitors using a sputtered RuO2 electrode. J. Electrochem. Soc. 2001, 148, A275–A278. [Google Scholar] [CrossRef]
- Sung, J.-H.; Kim, S.-J.; Lee, K.-H. Fabrication of microcapacitors using conducting polymer microelectrodes. J. Power Sources 2003, 124, 343–350. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Wei, S.; He, Y.; Xia, H.; Chen, Q.; Sun, H.-B.; Xiao, F.-S. Direct imprinting of microcircuits on graphene oxides film by femtosecond laser reduction. Nano Today 2010, 5, 15–20. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Dinh, T.M.; Armstrong, K.; Guay, D.; Pech, D. High-resolution on-chip supercapacitors with ultra-high scan rate ability. J. Mater. Chem. A 2014, 2, 7170–7174. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, L.; Liu, L.; Zhu, B.; Dong, H.; Chen, X. All-solid-state flexible ultrathin micro-supercapacitors based on graphene. Adv. Mater. 2013, 25, 4035–4042. [Google Scholar] [CrossRef]
- Cai, J.; Lv, C.; Watanabe, A. Laser direct writing of high-performance flexible all-solid-state carbon micro-supercapacitors for an on-chip self-powered photodetection system. Nano Energy 2016, 30, 790–800. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 2017, 12, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.L.; Tian, Z.; Simon, G.P.; Li, D. Scalable production of graphene via wet chemistry: Progress and challenges. Mater. Today 2015, 18, 73–78. [Google Scholar] [CrossRef]
- Aleeva, Y.; Pignataro, B. Recent advances in upscalable wet methods and ink formulations for printed electronics. J. Mater. Chem. C 2014, 2, 6436–6453. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Tran, T.S.; Park, S.J.; Yoo, S.S.; Lee, T.-R.; Kim, T. High shear-induced exfoliation of graphite into high quality graphene by Taylor–Couette flow. RSC Adv. 2016, 6, 12003–12008. [Google Scholar] [CrossRef]
- Fowkes, F.M. Donor-acceptor interactions at interfaces. J. Adhes. 1972, 4, 155–159. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of defect-free graphene. Acc. Chem. Res. 2012, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, F.; Vaziri, S.; Muhammed, M.; Lemme, M.C.; Östling, M. Efficient inkjet printing of graphene. Adv. Mater. 2013, 25, 3985–3992. [Google Scholar] [CrossRef] [PubMed]
- Majee, S.; Song, M.; Zhang, S.-L.; Zhang, Z.-B. Scalable inkjet printing of shear-exfoliated graphene transparent conductive films. Carbon 2016, 102, 51–57. [Google Scholar] [CrossRef]
- Solomon, H.; Burgess, B.; Kennedy, G.; Staples, R. 1-Methyl-2-pyrrolidone (NMP): Reproductive and developmental toxicity study by inhalation in the rat. Drug Chem. Toxicol. 1995, 18, 271–293. [Google Scholar] [CrossRef]
- Gescher, A. Metabolism of N, N-dimethylformamide: Key to the understanding of its toxicity. Chem. Res. Toxicol. 1993, 6, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.B.; Prabhumirashi, P.L.; Puntambekar, K.; Geier, M.L.; Hersam, M.C. Inkjet printing of high conductivity, flexible graphene patterns. J. Phys. Chem. Lett. 2013, 4, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, W.; Wang, W.; Leng, Y.; Zhao, Y. Inkjet printing patterns of highly conductive pristine graphene on flexible substrates. Ind. Eng. Chem. Res. 2014, 53, 16777–16784. [Google Scholar] [CrossRef]
- Arapov, K.; Rubingh, E.; Abbel, R.; Laven, J.; de With, G.; Friedrich, H. Conductive screen printing inks by gelation of graphene dispersions. Adv. Funct. Mater. 2016, 26, 586–593. [Google Scholar] [CrossRef]
- Hyun, W.J.; Secor, E.B.; Hersam, M.C.; Frisbie, C.D.; Francis, L.F. High-resolution patterning of graphene by screen printing with a silicon stencil for highly flexible printed electronics. Adv. Mater. 2015, 27, 109–115. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Guardia, L.; Fernández-Merino, M.; Paredes, J.; Solis-Fernandez, P.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascón, J. High-throughput production of pristine graphene in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Mohamed, A.; Ardyani, T.; Bakar, S.A.; Brown, P.; Hollamby, M.; Sagisaka, M.; Eastoe, J. Graphene-philic surfactants for nanocomposites in latex technology. Adv. Colloid Interface Sci. 2016, 230, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Lotya, M.; Coleman, J.N. The importance of repulsive potential barriers for the dispersion of graphene using surfactants. New J. Phys. 2010, 12, 125008. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Ayán-Varela, M.; Paredes, J.; Guardia, L.; Villar-Rodil, S.; Munuera, J.; Díaz-González, M.; Fernández-Sánchez, C.; Martínez-Alonso, A.; Tascón, J. Achieving extremely concentrated aqueous dispersions of graphene flakes and catalytically efficient graphene-metal nanoparticle hybrids with flavin mononucleotide as a high-performance stabilizer. ACS Appl. Mater. Interfaces 2015, 7, 10293–10307. [Google Scholar] [CrossRef]
- Brodie, B. Sur le poids atomique du graphite. Ann. Chim. Phys. 1860, 59, 466–472. [Google Scholar]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Berichte der Deutschen Chemischen Gesellschaft 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Le, L.T.; Ervin, M.H.; Qiu, H.; Fuchs, B.E.; Lee, W.Y. Graphene supercapacitor electrodes fabricated by inkjet printing and thermal reduction of graphene oxide. Electrochem. Commun. 2011, 13, 355–358. [Google Scholar] [CrossRef]
- Rocha, V.G.; Garcia-Tunon, E.; Botas, C.; Markoulidis, F.; Feilden, E.; D’Elia, E.; Ni, N.; Shaffer, M.; Saiz, E. Multimaterial 3D Printing of Graphene-Based Electrodes for Electrochemical Energy Storage Using Thermoresponsive Inks. ACS Appl. Mater. Interfaces 2017, 9, 37136–37145. [Google Scholar] [CrossRef]
- Franco, J.; Hunger, P.; Launey, M.E.; Tomsia, A.P.; Saiz, E. Direct write assembly of calcium phosphate scaffolds using a water-based hydrogel. Acta Biomater. 2010, 6, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zangmeister, C.D. Preparation and evaluation of graphite oxide reduced at 220 °C. Chem. Mater. 2010, 22, 5625–5629. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash reduction and patterning of graphite oxide and its polymer composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef]
- Wu, Z.-K.; Lin, Z.; Li, L.; Song, B.; Moon, K.-S.; Bai, S.-L.; Wong, C.-P. Flexible micro-supercapacitor based on in-situ assembled graphene on metal template at room temperature. Nano Energy 2014, 10, 222–228. [Google Scholar] [CrossRef]
- Sun, G.; An, J.; Chua, C.K.; Pang, H.; Zhang, J.; Chen, P. Layer-by-layer printing of laminated graphene-based interdigitated microelectrodes for flexible planar micro-supercapacitors. Electrochem. Commun. 2015, 51, 33–36. [Google Scholar] [CrossRef]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Mirfakhrai, T.; Chen, Z.; Casalongue, H.S.; Dai, H. Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 2011, 4, 729–736. [Google Scholar] [CrossRef]
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding graphene paper supported three-dimensional porous graphene–polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Chang, K.-H.; Tien, H.-W.; Lee, Y.-F.; Li, S.-M.; Wang, Y.-S.; Wang, J.-Y.; Ma, C.-C.M.; Hu, C.-C. Design and tailoring of a hierarchical graphene-carbon nanotube architecture for supercapacitors. J. Mater. Chem. 2011, 21, 2374–2380. [Google Scholar] [CrossRef]
- Xu, Y.; Hennig, I.; Freyberg, D.; Strudwick, A.J.; Schwab, M.G.; Weitz, T.; Cha, K.C.-P. Inkjet-printed energy storage device using graphene/polyaniline inks. J. Power Sources 2014, 248, 483–488. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Z.S.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible in-plane micro-supercapacitors by direct printing of solution-processable electrochemically exfoliated graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Xu, Q.; Hou, Y.; Seyedin, S.; Qin, S.; Wallace, G.G.; Beirne, S.; Razal, J.M.; Chen, J. Development of Graphene Oxide/Polyaniline Inks for High Performance Flexible Microsupercapacitors via Extrusion Printing. Adv. Funct. Mater. 2018, 28, 1706592. [Google Scholar] [CrossRef]
- Perelaer, J.; Smith, P.J.; Mager, D.; Soltman, D.; Volkman, S.K.; Subramanian, V.; Korvink, J.G.; Schubert, U.S. Printed electronics: The challenges involved in printing devices, interconnects, and contacts based on inorganic materials. J. Mater. Chem. 2010, 20, 8446–8453. [Google Scholar] [CrossRef]
- Madou, M.J. Manufacturing Techniques for Microfabrication and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2011; Volume 2. [Google Scholar]
- Sudhakar, Y.; Selvakumar, M.; Bhat, D.K.; Kumar, S.S. Reduced graphene oxide derived from used cell graphite and its green fabrication as an eco-friendly supercapacitor. RSC Adv. 2014, 4, 60039–60051. [Google Scholar] [CrossRef]
- Sekitani, T.; Noguchi, Y.; Zschieschang, U.; Klauk, H.; Someya, T. Organic transistors manufactured using inkjet technology with subfemtoliter accuracy. Proc. Natl. Acad. Sci. USA 2008, 105, 4976–4980. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Kang, J.; Ng, L.W.; Zhu, X.; Howe, R.C.; Jones, C.G.; Hersam, M.C.; Hasan, T. Functional inks and printing of two-dimensional materials. Chem. Soc. Rev. 2018, 47, 3265–3300. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T. Graphene oxide-based electrode inks for 3D-printed lithium-ion batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef]
- Zhang, B.; Seong, B.; Nguyen, V.; Byun, D. 3D printing of high-resolution PLA-based structures by hybrid electrohydrodynamic and fused deposition modeling techniques. J. Micromech. Microeng. 2016, 26, 025015. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Zhao, C.X.; Wong, J.I.; Sun, X.W.; Yang, H.Y. Printed all-solid flexible microsupercapacitors: Towards the general route for high energy storage devices. Nanotechnology 2014, 25, 094010. [Google Scholar] [CrossRef]
- Liu, S.; Xie, J.; Li, H.; Wang, Y.; Yang, H.Y.; Zhu, T.; Zhang, S.; Cao, G.; Zhao, X. Nitrogen-doped reduced graphene oxide for high-performance flexible all-solid-state micro-supercapacitors. J. Mater. Chem. A 2014, 2, 18125–18131. [Google Scholar] [CrossRef]
- Shi, X.; Pei, S.; Zhou, F.; Ren, W.; Cheng, H.-M.; Wu, Z.-S.; Bao, X. Ultrahigh-voltage integrated micro-supercapacitors with designable shapes and superior flexibility. Energy Environ. Sci. 2019. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef]
- Suganuma, K. Introduction to Printed Electronics; Springer Science & Business Media: New York, NY, USA, 2014; Volume 74. [Google Scholar]
- Von Dollen, P.; Barnett, S. A Study of Screen Printed Yttria-Stabilized Zirconia Layers for Solid Oxide Fuel Cells. J. Am. Ceram. Soc. 2005, 88, 3361–3368. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Capasso, A.; Castillo, A.D.R.; Sun, H.; Ansaldo, A.; Pellegrini, V.; Bonaccorso, F. Ink-jet printing of graphene for flexible electronics: An environmentally-friendly approach. Solid State Commun. 2015, 224, 53–63. [Google Scholar] [CrossRef]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing—Process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Li, J.; Sollami Delekta, S.; Zhang, P.; Yang, S.; Lohe, M.R.; Zhuang, X.; Feng, X.; Östling, M. Scalable Fabrication and Integration of Graphene Microsupercapacitors through Full Inkjet Printing. ACS Nano 2017, 11, 8249–8256. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Liu, Z.; Xue, J.; Xin, G.; Yu, Q.; Lian, J.; Chen, M.Y. Annealed graphene sheets decorated with silver nanoparticles for inkjet printing. Chem. Eng. J. 2015, 260, 582–589. [Google Scholar] [CrossRef]
- Li, L.; Gao, M.; Guo, Y.; Sun, J.; Li, Y.; Li, F.; Song, Y.; Li, Y. Transparent Ag@Au–graphene patterns with conductive stability via inkjet printing. J. Mater. Chem. C 2017, 5, 2800–2806. [Google Scholar] [CrossRef]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef]
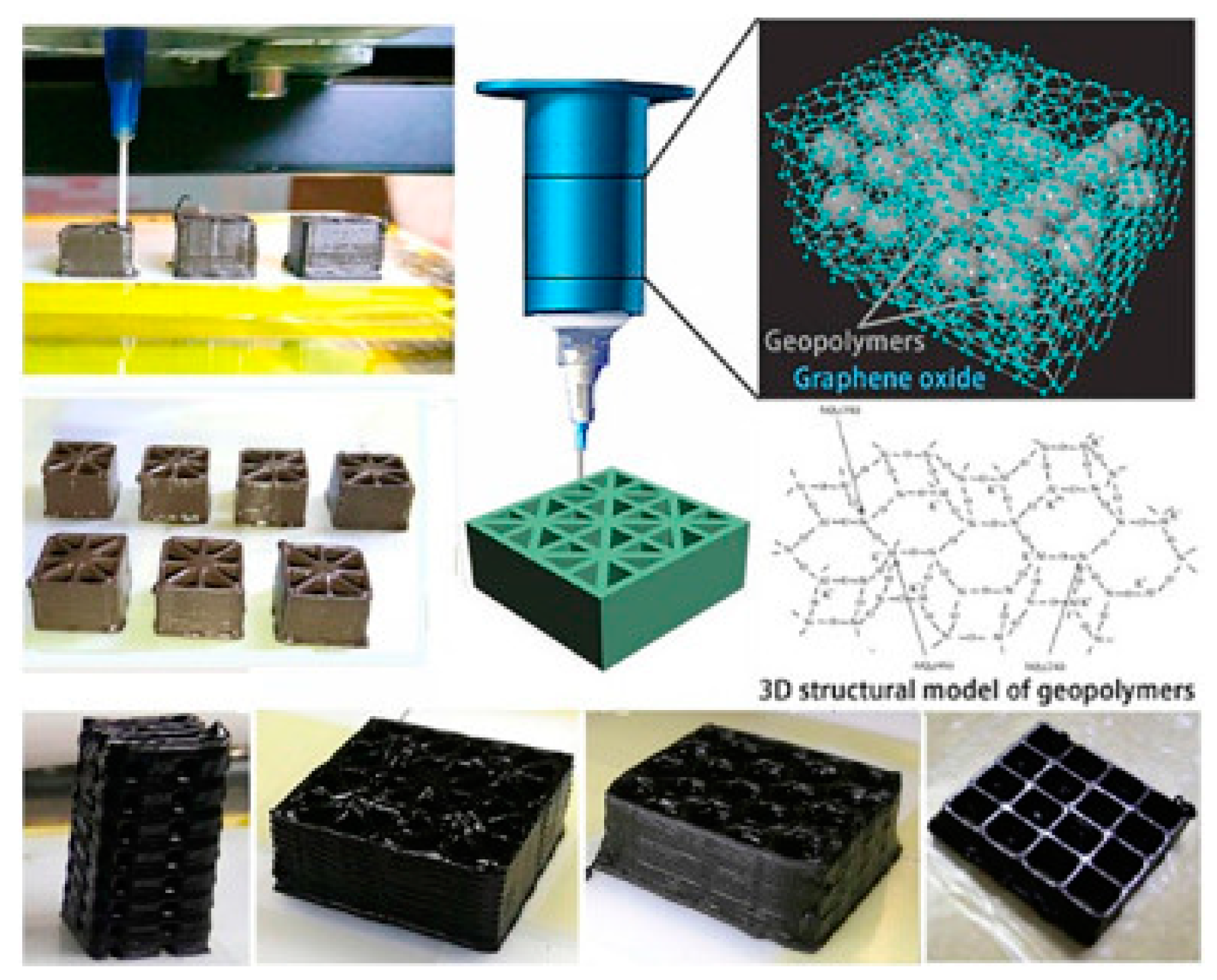
- Zhong, J.; Zhou, G.-X.; He, P.-G.; Yang, Z.-H.; Jia, D.-C. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide. Carbon 2017, 117, 421–426. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Gorkin, R., III; Beirne, S.; Shu, K.; Wallace, G.G. Three dimensional (3D) printed electrodes for interdigitated supercapacitors. Electrochem. Commun. 2014, 41, 20–23. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Su, M.; An, B.; Liu, J.; Su, D.; Li, L.; Li, F.; Song, Y. Printing assembly and structural regulation of graphene towards three-dimensional flexible micro-supercapacitors. J. Mater. Chem. A 2017, 5, 16281–16288. [Google Scholar] [CrossRef]
- Zhang, D.; Chi, B.; Li, B.; Gao, Z.; Du, Y.; Guo, J.; Wei, J. Fabrication of highly conductive graphene flexible circuits by 3D printing. Synth. Met. 2016, 217, 79–86. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Fu, K.; Yao, Y.; Dai, J.; Hu, L. Progress in 3D printing of carbon materials for energy-related applications. Adv. Mater. 2017, 29, 1603486. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Yang, S.; Feng, X.; Müllen, K. Exfoliation of graphene via wet chemical routes. Synth. Met. 2015, 210, 123–132. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D printable graphene composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Rymansaib, Z.; Iravani, P.; Emslie, E.; Medvidović-Kosanović, M.; Sak-Bosnar, M.; Verdejo, R.; Marken, F. All-Polystyrene 3D-Printed Electrochemical Device with Embedded Carbon Nanofiber-Graphite-Polystyrene Composite Conductor. Electroanalysis 2016, 28, 1517–1523. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]

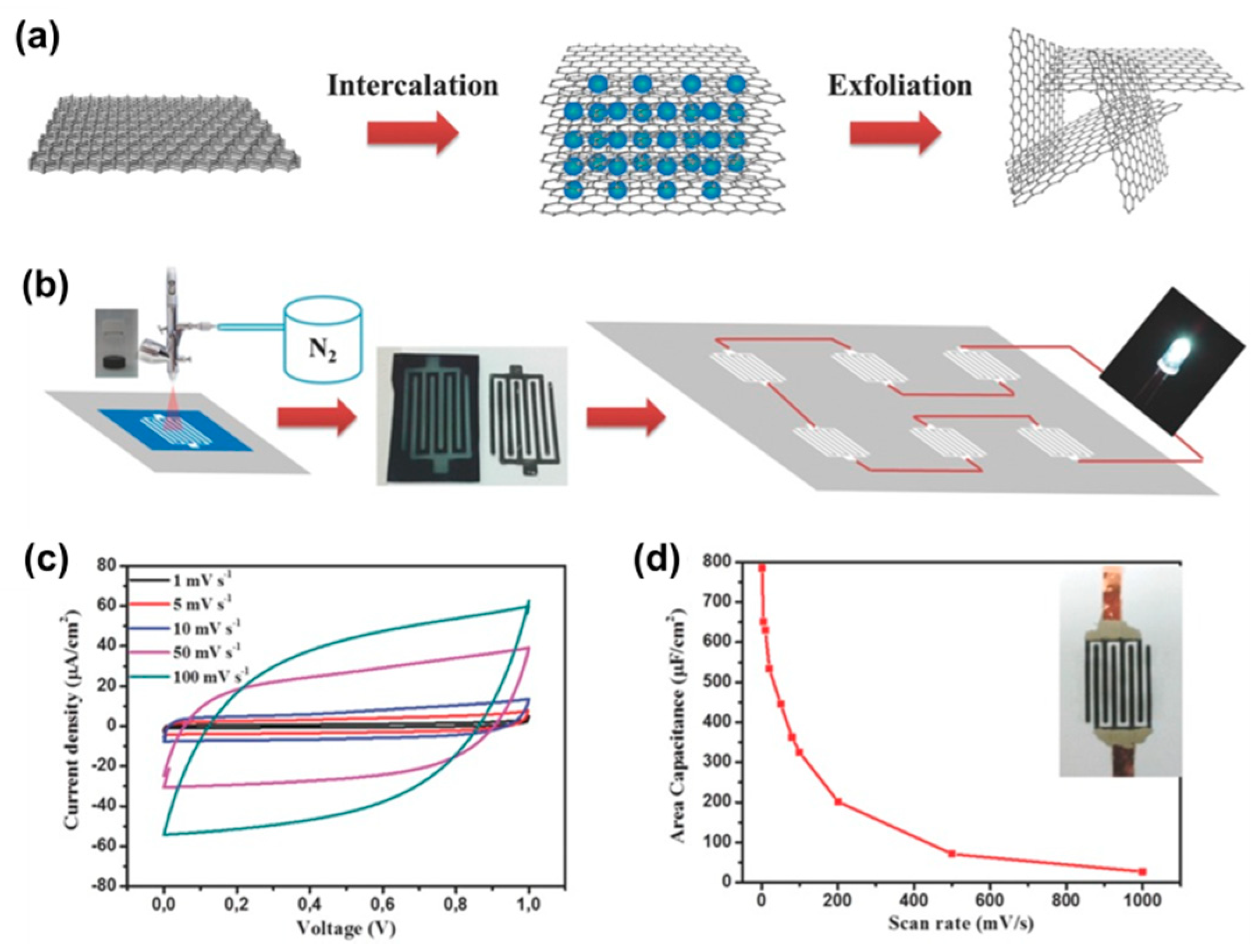
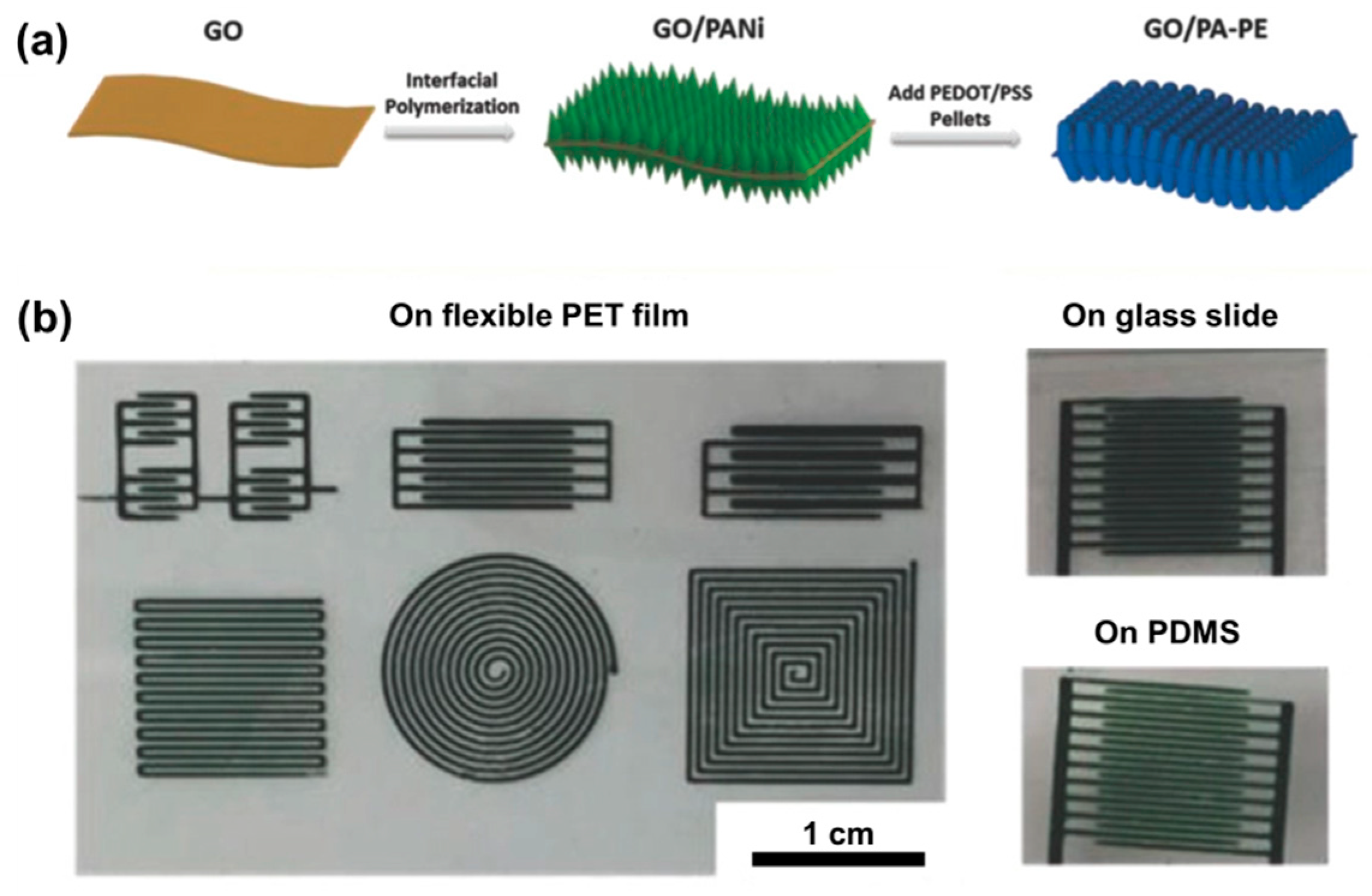
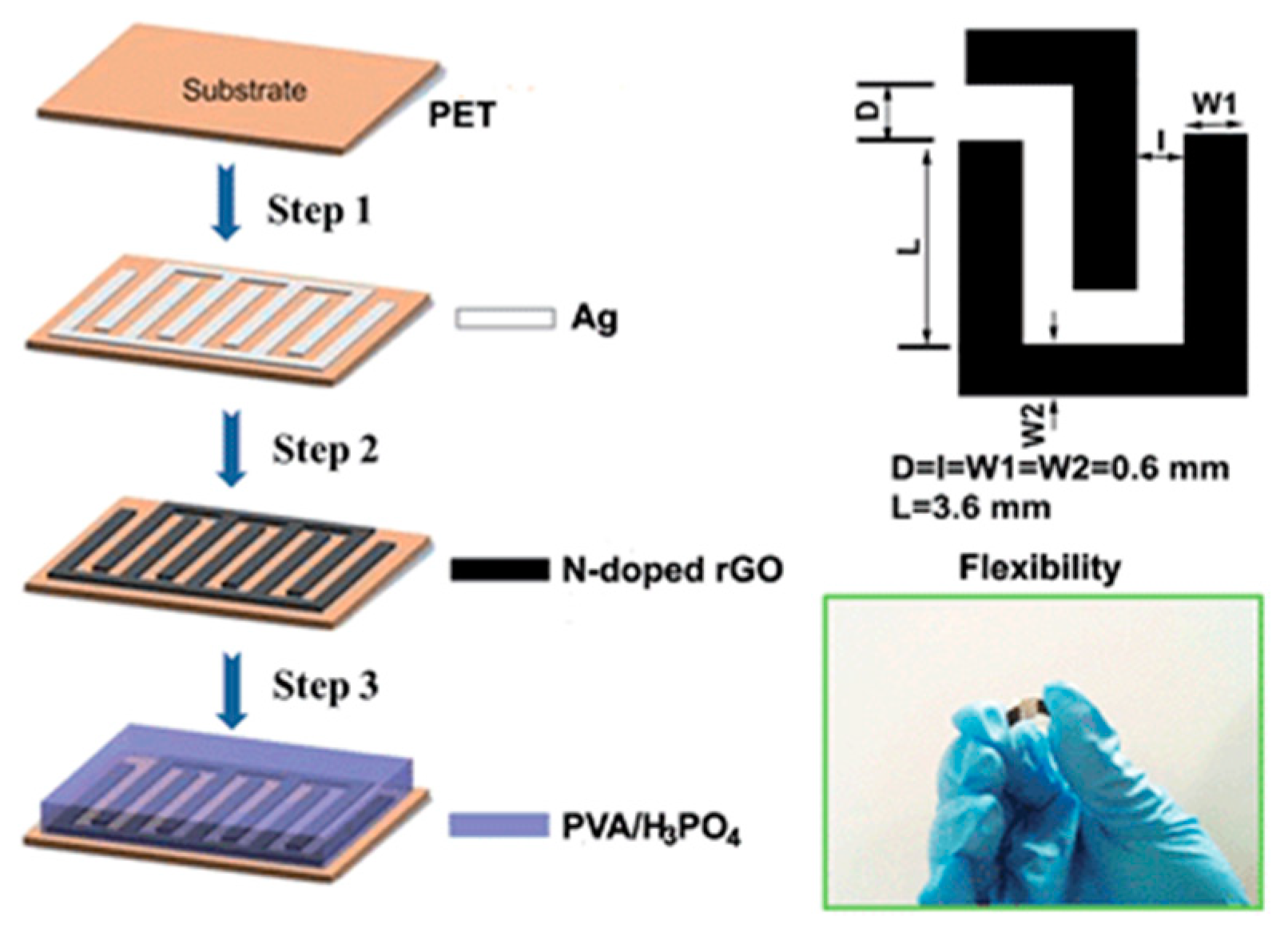
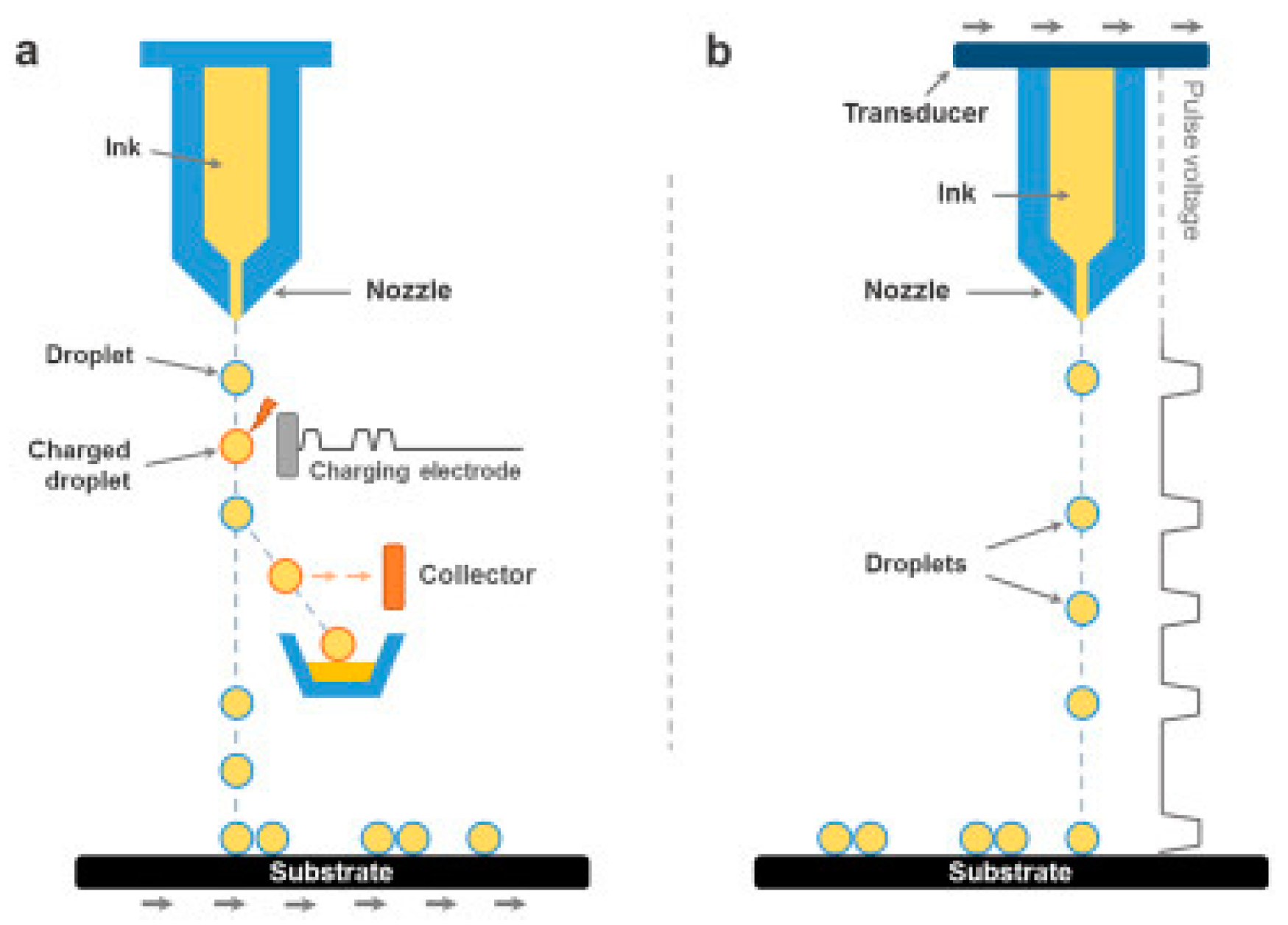
| Reduction Method | Substrate | Electrolyte | Specific Capacitance | Power Density | Reference |
|---|---|---|---|---|---|
| Hydrazine at 60 °C | PET film | PVA/H3PO4 | 462 μF·cm−2 | 324 W·cm−3 | [66] |
| Cu-based redox potential | Polyimide (PI) | PVA/H2SO4 | 0.95 mF·cm−2 | [102] | |
| Hydroiodic acid (HI) | Plastic film | PVA/H2SO4 | 41.8 F·cm−3 | 29.2 mW·cm−2 | [103] |
| CH4 plasma at 700 °C | Silicon wafer | PVA/H3PO4 | 80.7 μF·cm−2 | [104] | |
| Laser writing | Plastic film | PVA/H2SO4 | 3.05 F·cm−3 | 30 W·cm−3 | [105] |
| Laser writing | GO film | H2O | 3.1 F·cm−3 | 1.7 W·cm−3 | [106] |
| Printing Techniques | (a) Screen Printing | (b) Inkjet Printing | (c) 3D Printing |
|---|---|---|---|
| Ink requirements | High viscosity Shear thinning | Low viscosity High surface tension | Shear thinning Quick solidification |
| Resolution | ~10 µm | ~2 µm | ~10 µm |
| Versatility | Mask required | Maskless | Maskless |
| Printing speed | Ultrafast | Fast | Slow |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang Tran, T.; Dutta, N.K.; Roy Choudhury, N. Graphene-Based Inks for Printing of Planar Micro-Supercapacitors: A Review. Materials 2019, 12, 978. https://doi.org/10.3390/ma12060978
Sang Tran T, Dutta NK, Roy Choudhury N. Graphene-Based Inks for Printing of Planar Micro-Supercapacitors: A Review. Materials. 2019; 12(6):978. https://doi.org/10.3390/ma12060978
Chicago/Turabian StyleSang Tran, Tuan, Naba Kumar Dutta, and Namita Roy Choudhury. 2019. "Graphene-Based Inks for Printing of Planar Micro-Supercapacitors: A Review" Materials 12, no. 6: 978. https://doi.org/10.3390/ma12060978
APA StyleSang Tran, T., Dutta, N. K., & Roy Choudhury, N. (2019). Graphene-Based Inks for Printing of Planar Micro-Supercapacitors: A Review. Materials, 12(6), 978. https://doi.org/10.3390/ma12060978






