One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water
Abstract
:1. Introduction
1.1. Ferrite Nanoparticles Applications
1.2. Synthesis Methods
1.3. Applications of Colloidal Dispersions
1.4. Stability of Colloidal Dispersion
- (1)
- (2)
1.5. Present Research
2. Materials and Methods
2.1. Materials
Synthesis of ZnFe2O4 Nanorods in Water Solution
2.2. Methods
2.2.1. X-ray Powder Diffraction (XRD)
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Small Angle X-ray Scattering (SAXS)
2.2.4. Mössbauer Spectroscopy
2.2.5. Near Edge X-ray Absorption Structure (XANES)
2.2.6. Dynamic Light Scattering (DLS) and Zeta Potential Measurement
2.2.7. Vibrating Sample Magnetometry (VSM)
2.2.8. Magnetic Hyperthermia
3. Results and Discussion
3.1. X-ray Powder Diffraction and Transmission Electron Microscopy
3.2. TEM Images and Selected-Area Electron Diffraction (SAED)
3.3. Small Angle X-ray Scattering
3.4. Zeta Potential (ζ) and Dynamic Light Scattering
3.5. Mössbauer Spectroscopy
3.6. X-ray Absorption Spectroscopy
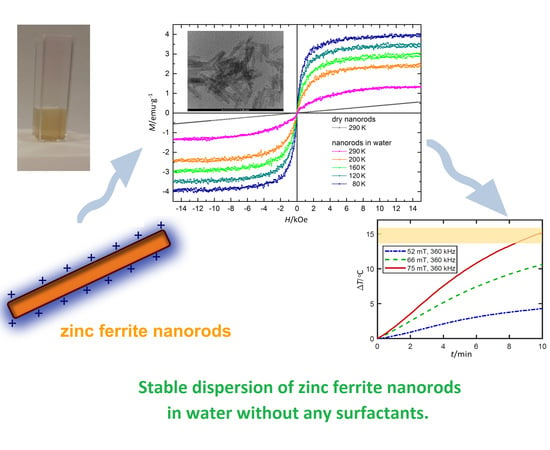
3.7. Magnetic Properties
3.8. Potential Application of ZnFe2O4 Nanorods Dispersed in Water in Magnetic Hyperthermia Therapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-soluble iron oxide nanoparticles with high stability and selective surface functionality. Langmuir 2011, 27, 8990–8997. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Singh, S.; Tandon, P.; Yadav, B.C.; Yadav, R.R. Synthesis, characterization and performance of zinc ferrite nanorods for room temperature sensing applications. J. Alloys Compd. 2015, 618, 475–483. [Google Scholar] [CrossRef]
- Gavilán, H.; Kowalski, A.; Heinke, D.; Sugunan, A.; Sommertune, J.; Varón, M.; Bogart, L.K.; Posth, O.; Zeng, L.; González-Alonso, D.; et al. Colloidal flower-shaped iron oxide nanoparticles: Synthesis strategies and coatings. Part. Part. Syst. Charact. 2017, 34, 1–12. [Google Scholar] [CrossRef]
- Ullrich, S.; Scheeler, S.P.; Pacholski, C.; Spatz, J.P.; Kudera, S. Formation of large 2D arrays of shape-controlled colloidal nanoparticles at variable interparticle distances. Part. Part. Syst. Charact. 2013, 30, 102–108. [Google Scholar] [CrossRef]
- Upadhyay, C.; Verma, H.C.; Sathe, V.; Pimpale, A.V. Effect of size and synthesis route on the magnetic properties of chemically prepared nanosize ZnFe2O4. J. Magn. Magn. Mater. 2007, 312, 271–279. [Google Scholar] [CrossRef]
- Jia, Z.; Ren, D.; Liang, Y.; Zhu, R. A new strategy for the preparation of porous zinc ferrite nanorods with subsequently light-driven photocatalytic activity. Mater. Lett. 2011, 65, 3116–3119. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.-W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Dolcet, P.; Diodati, S.; Zorzi, F.; Voepel, P.; Seitz, C.; Smarsly, B.M.; Mascotto, S.; Nestola, F.; Gross, S. Very fast crystallisation of MFe2O4 spinel ferrites (M = Co, Mn, Ni, Zn) under low temperature hydrothermal conditions: A time-resolved structural investigation. Green Chem. 2018, 20, 2257–2268. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V. Mini-review: Ferrite nanoparticles in the catalysis. Arab. J. Chem. 2014. [Google Scholar] [CrossRef] [Green Version]
- Rezlescu, N.; Rezlescu, E.; Sachelarie, L.; Popa, P.D.; Doroftei, C. Structural and catalytic properties of mesoporous nanocrystalline mixed oxides containing magnesium. Catal. Commun. 2014, 46, 51–56. [Google Scholar] [CrossRef]
- Kmita, A.; Żukrowski, J.; Hodor, K.; Smogór, H.; Sikora, M. Zinc ferrite nanoparticles as perspective functional materials for applications in casting technologies. Metalurgija 2017, 56, 29–32. [Google Scholar]
- Qin, M.; Shuai, Q.; Wu, G.; Zheng, B.; Wang, Z.; Wu, H. Zinc ferrite composite material with controllable morphology and its applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 224, 125–138. [Google Scholar] [CrossRef]
- Kmita, A.; Pribulova, A.; Holtzer, M.; Futas, P.; Roczniak, A. Use of specific properties of zinc ferrite in innovative technologies. Arch. Metall. Mater. 2016, 61. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Yang, Y.; Xiao, W.; Li, Z.; Xue, D.; Li, F.; Ding, J. Synthesis of nonstoichiometric zinc ferrite nanoparticles with extraordinary room temperature magnetism and their diverse applications. J. Mater. Chem. C 2013, 1, 2875–2885. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Zhao, Q.; Hou, Y.; Quan, X.; Chen, G. Structural and photovoltaic properties of highly ordered ZnFe2O4 nanotube arrays fabricated by a facile sol-gel template method. Acta Mater. 2009, 57, 2684–2690. [Google Scholar] [CrossRef]
- Li, Q.; Bo, C.; Wang, W. Preparation and magnetic properties of ZnFe2O4 nanofibers by coprecipitation-air oxidation method. Mater. Chem. Phys. 2010, 124, 891–893. [Google Scholar] [CrossRef]
- Zhao, J.; Mi, L.; Hou, H.; Shi, X.; Fan, Y. The preparation of zinc ferrite nanorods by using single ferrocenyl complex as precursor. Mater. Lett. 2007, 61, 4196–4198. [Google Scholar] [CrossRef]
- Philip, J.; Laskar, J.M. Optical properties and applications of ferrofluids—A review. J. Nanofluids 2012, 1, 3–20. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chemie Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Sawant, V.J.; Bamane, S.R.; Shejwal, R.V.; Patil, S.B. Comparison of drug delivery potentials of surface functionalized cobalt and zinc ferrite nanohybrids for curcumin in to MCF-7 breast cancer cells. J. Magn. Magn. Mater. 2016, 417, 222–229. [Google Scholar] [CrossRef]
- Ghayour, H.; Abdellahi, M.; Ozada, N.; Jabbrzare, S.; Khandan, A. Hyperthermia application of zinc doped nickel ferrite nanoparticles. J. Phys. Chem. Solids 2017, 111, 464–472. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Carrião, M.S.; Drummond, A.L.; Gomes, J.A.; Vieira, E.D.; Sousa, M.H.; Bakuzis, A.F. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv. 2012, 2, 032120. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.S.; Juang, R.S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Kant Sharma, R.; Ghose, R. Synthesis and characterization of nanocrystalline zinc ferrite spinel powders by homogeneous precipitation method. Ceram. Int. 2015, 41, 14684–14691. [Google Scholar] [CrossRef]
- Raeisi Shahraki, R.; Ebrahimi, M.; Seyyed Ebrahimi, S.A.; Masoudpanah, S.M. Structural characterization and magnetic properties of superparamagnetic zinc ferrite nanoparticles synthesized by the coprecipitation method. J. Magn. Magn. Mater. 2012, 324, 3762–3765. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, Q.; Goya, G.F.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 nanocrystals: Synthesis and magnetic properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef]
- Ghasemi, A.; Mousavinia, M. Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol-gel method. Ceram. Int. 2014, 40, 2825–2834. [Google Scholar] [CrossRef]
- Fan, G.; Gu, Z.; Yang, L.; Li, F. Nanocrystalline zinc ferrite photocatalysts formed using the colloid mill and hydrothermal technique. Chem. Eng. J. 2009, 155, 534–541. [Google Scholar] [CrossRef]
- Yan, W.; Jiang, W.; Zhang, Q.; Li, Y.; Wang, H. Structure and magnetic properties of nickel-zinc ferrite microspheres synthesized by solvothermal method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 171, 144–148. [Google Scholar] [CrossRef]
- Kotsikau, D.; Ivanovskaya, M.; Pankov, V.; Fedotova, Y. Structure and magnetic properties of manganese-zinc-ferrites prepared by spray pyrolysis method. Solid State Sci. 2015, 39, 69–73. [Google Scholar] [CrossRef]
- Lee, H.; Jung, J.C.; Kim, H.; Chung, Y.M.; Kim, T.J.; Lee, S.J.; Oh, S.H.; Kim, Y.S.; Song, I.K. Effect of pH in the preparation of ZnFe2O4 for oxidative dehydrogenation of n-butene to 1,3-butadiene: Correlation between catalytic performance and surface acidity of ZnFe2O4. Catal. Commun. 2008, 9, 1137–1142. [Google Scholar] [CrossRef]
- Vékás, L.; Bica, D.; Avdeev, M.V. Magnetic nanoparticles and concentrated magnetic nanofluids: Synthesis, properties and some applications. China Particuology 2007, 5, 43–49. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Bran, C.; Rodríguez-Abreu, C.; Gallo, J.; Vázquez, M.; Rivas, J. A colloidally stable water dispersion of Ni nanowires as an efficient: T2-MRI contrast agent. J. Mater. Chem. B 2017, 5, 3338–3347. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V.; Vázquez, A.; Peña, Y.; Gómez, I. Solubilization, dispersion and stabilization of magnetic nanoparticles in water and non-Aqueous solvents: Recent trends. RSC Adv. 2014, 4, 45354–45381. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Milanovic, M.; Stijepovic, I.; Pavlovic, V.; Srdic, V. Functionalization of zinc ferrite nanoparticles: Influence of modification procedure on colloidal stability. Process. Appl. Ceram. 2016, 10, 287–293. [Google Scholar] [CrossRef]
- Szpak, A.; Kania, G.; Skórka, T.; Tokarz, W.; Zapotoczny, S.; Nowakowska, M. Stable aqueous dispersion of superparamagnetic iron oxide nanoparticles protected by charged chitosan derivatives. J. Nanoparticle Res. 2013, 15, 1372. [Google Scholar] [CrossRef]
- Gittins, D.I.; Caruso, F. Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew. Chem. Int. Ed. Engl. 2001, 40, 3001–3004. [Google Scholar] [CrossRef]
- Laurent, S.; Roch, A.; Robic, C.; Forge, D.; Vander Elst, L.; Muller, R.N.; Port, M. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2009, 110, 2574. [Google Scholar] [CrossRef]
- Glatzel, P.; Sikora, M.; Smolentsev, G.; Fernández-García, M. Hard X-ray photon-in photon-out spectroscopy. Catal. Today 2009, 145, 294–299. [Google Scholar] [CrossRef]
- Koziej, D. Revealing complexity of nanoparticle synthesis in solution by in situ hard X-ray spectroscopy - today and beyond. Chem. Mater. 2016, 28, 2478–2490. [Google Scholar] [CrossRef]
- Thunemann, A.F.; Kegel, J.; Polte, J.; Emmering, F. Superparamagnetic maghemite nanorods: Analysis by coupling field-flow fractional and small-angle X-ray scattering. Anal. Chem. 2008, 80, 5905–5911. [Google Scholar] [CrossRef]
- Gopinath, S.; Philip, J. Prepeartion of metal oxide nanoparticles of different sizes and morphologies, their characterization using small angle X-ray scattering and study of thermal properties. Mater. Chem. Phys. 2014, 145, 213–221. [Google Scholar] [CrossRef]
- Szczerba, W.; Costo, R.; Veintemillas-Verdaguer, S.; Del Puerto Morales, M.; Thünemann, A.F. SAXS analysis of single-and multi-core iron oxide magnetic nanoparticles. J. Appl. Crystallogr. 2017, 50, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Rebbouh, L.; Hermann, R.P.; Grandjean, F.; Hyeon, T.; An, K.; Amato, A.; Long, G.J. Fe57 Mössbauer spectral and muon spin relaxation study of the magnetodynamics of monodispersed γ-Fe2O3 nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 1–12. [Google Scholar] [CrossRef]
- Szczerba, W.; Zukrowski, J.; Przybylski, M.; Sikora, M.; Safonova, O.; Shmeliov, A.; Nicolosi, V.; Schneider, M.; Granath, T.; Oppmann, M.; et al. Pushing up the magnetisation values for iron oxide nanoparticles via zinc doping: X-ray studies on the particle’s sub-nano structure of different synthesis routes. Phys. Chem. Chem. Phys. 2016, 18, 25221–25229. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Guti Errez, V.; Jim Enez-Villacorta, F.; Bonville, P.; Torralvo-Fern, M.J.; Aez-Puche, R. X-ray absorption spectroscopy and Mossbauer spectroscopy studies of superparamagnetic ZnFe2O4 nanoparticles. J. Phys. Chem. C 2011, 115, 1627–1634. [Google Scholar] [CrossRef]
- Bullita, S.; Casu, A.; Casula, M.F.; Concas, G.; Congiu, F.; Corrias, A.; Falqui, A.; Loche, D.; Marras, C. ZnFe2O4 nanoparticles dispersed in a highly porous silica aerogel matrix: A magnetic study. Phys. Chem. Chem. Phys. 2014, 16, 4843. [Google Scholar] [CrossRef] [PubMed]
- Řezníček, R.; Chlan, V.; Štěpánková, H.; Novák, P.; Zukrowski, J.; Kozłowski, A.; Kakol, Z.; Tarnawski, Z.; Honig, J.M. Understanding the Mössbauer spectrum of magnetite below the Verwey transition: Ab initio calculations, simulation, and experiment. Phys. Rev. B 2017, 96. [Google Scholar] [CrossRef]
- Berry, F.J.; Skinner, S.; Thomas, M.F. Mössbauer spectroscopic examination of a single crystal of Fe3O4. J. Phys. Condens. Matter 1998, 10, 215–220. [Google Scholar] [CrossRef]
- Bruce, D.W. Local Structural Characterisation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wilke, M.; Farges, F.; Petit, P.E.; Brown, G.E.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Signorini, L.; Pasquini, L.; Savini, L.; Carboni, R.; Boscherini, F.; Bonetti, E.; Giglia, A.; Pedio, M.; Mahne, N.; Mahne, N.; et al. Size-dependent oxidation in iron/iron oxide core-shell nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 1–8. [Google Scholar] [CrossRef]
- Joly, Y.; Lorenzo, J.E.; Nazarenko, E.; Hodeau, J.-L.; Mannix, D.; Marin, C. Low-temperature structure of magnetite studied using resonant x-ray scattering. Phys. Rev. B 2008, 78, 134110. [Google Scholar] [CrossRef]
- Rijssel, J.V.; Kuipers, B.W.M.; Erné, B.H. Non-regularized inversion method from light scattering applied to ferro fluid magnetization curves for magnetic size distribution analysis. J. Magn. Magn. Mater. 2014, 353, 110–115. [Google Scholar] [CrossRef]
- Spirou, S.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic hyperthermia and radiation therapy: Radiobiological principles and current practice. Nanomaterials 2018, 8, 40. [Google Scholar] [CrossRef]
- Hanini, A.; Lartigue, L.; Gavard, J.; Kacem, K.; Wilhelm, C.; Gazeau, F.; Chau, F.; Ammar, S. Zinc substituted ferrite nanoparticles with Zn0.9Fe2.1O4 formula used as heating agents for in vitro hyperthermia assay on glioma cells. J. Magn. Magn. Mater. 2016, 416, 315–320. [Google Scholar] [CrossRef]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.; Patel, V.; O’Grady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 312001. [Google Scholar] [CrossRef]
- Ota, S.; Kitaguchi, R.; Takeda, R.; Yamada, T.; Takemura, Y. Rotation of magnetization derived from Brownian relaxation in magnetic fluids of different viscosity evaluated by dynamic hysteresis measurements over a wide frequency range. Nanomaterials 2016, 6, 170. [Google Scholar] [CrossRef]
- Brown, W.F., Jr. Thermal fluctuations of a single-domain particle. Phys. Rev. 1963, 130, 1677. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Hankiewicz, J.H.; Celinski, Z.; Stupic, K.F.; Anderson, N.R.; Camley, R.E. Ferromagnetic particles as magnetic resonance imaging temperature sensors. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]










| Sample | dmax/nm | PdI | ζ/mV |
|---|---|---|---|
| Nanorods ZnFe2O4 | 22.5 ± 7.9 | 0.26 | +47.7 ± 2.4 |
| Component No. | C % | IS/mm·s−1 | <IS>/mm·s−1 | H/kGs | <H>/kGs | QS/mm·s−1 | Γ/2/mm·s−1 |
|---|---|---|---|---|---|---|---|
| 1 | 10.3 | −0.1170 | - | - | - | - | 0.312 |
| 2 | 15.4 | 0.5256 | - | - | 0.558 | ||
| 3 | 2.8 | 0.4424 | - | 1.1894 | 0.157 | ||
| 4 | 30.6 | 0.415 | 0.390 | 458.3 | 435.3 | −0.022 | 0.394 |
| 5 | 13.1 | 0.310 | 445.8 | −0.202 | 0.256 | ||
| 6 | 27.8 | 0.399 | 404.9 | −0.152 | 0.771 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Zapotoczny, S.; Sikora, M. One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials 2019, 12, 1048. https://doi.org/10.3390/ma12071048
Kmita A, Lachowicz D, Żukrowski J, Gajewska M, Szczerba W, Kuciakowski J, Zapotoczny S, Sikora M. One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials. 2019; 12(7):1048. https://doi.org/10.3390/ma12071048
Chicago/Turabian StyleKmita, Angelika, Dorota Lachowicz, Jan Żukrowski, Marta Gajewska, Wojciech Szczerba, Juliusz Kuciakowski, Szczepan Zapotoczny, and Marcin Sikora. 2019. "One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water" Materials 12, no. 7: 1048. https://doi.org/10.3390/ma12071048
APA StyleKmita, A., Lachowicz, D., Żukrowski, J., Gajewska, M., Szczerba, W., Kuciakowski, J., Zapotoczny, S., & Sikora, M. (2019). One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials, 12(7), 1048. https://doi.org/10.3390/ma12071048