Interfacial Microstructure in W/2024Al Composite and Inhibition of W-Al Direct Reaction by CeO2 Doping: Formation and Crystallization of Al-Ce-Cu-W Amorphous Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composites
2.2. Characterizations
3. Results and Discussion
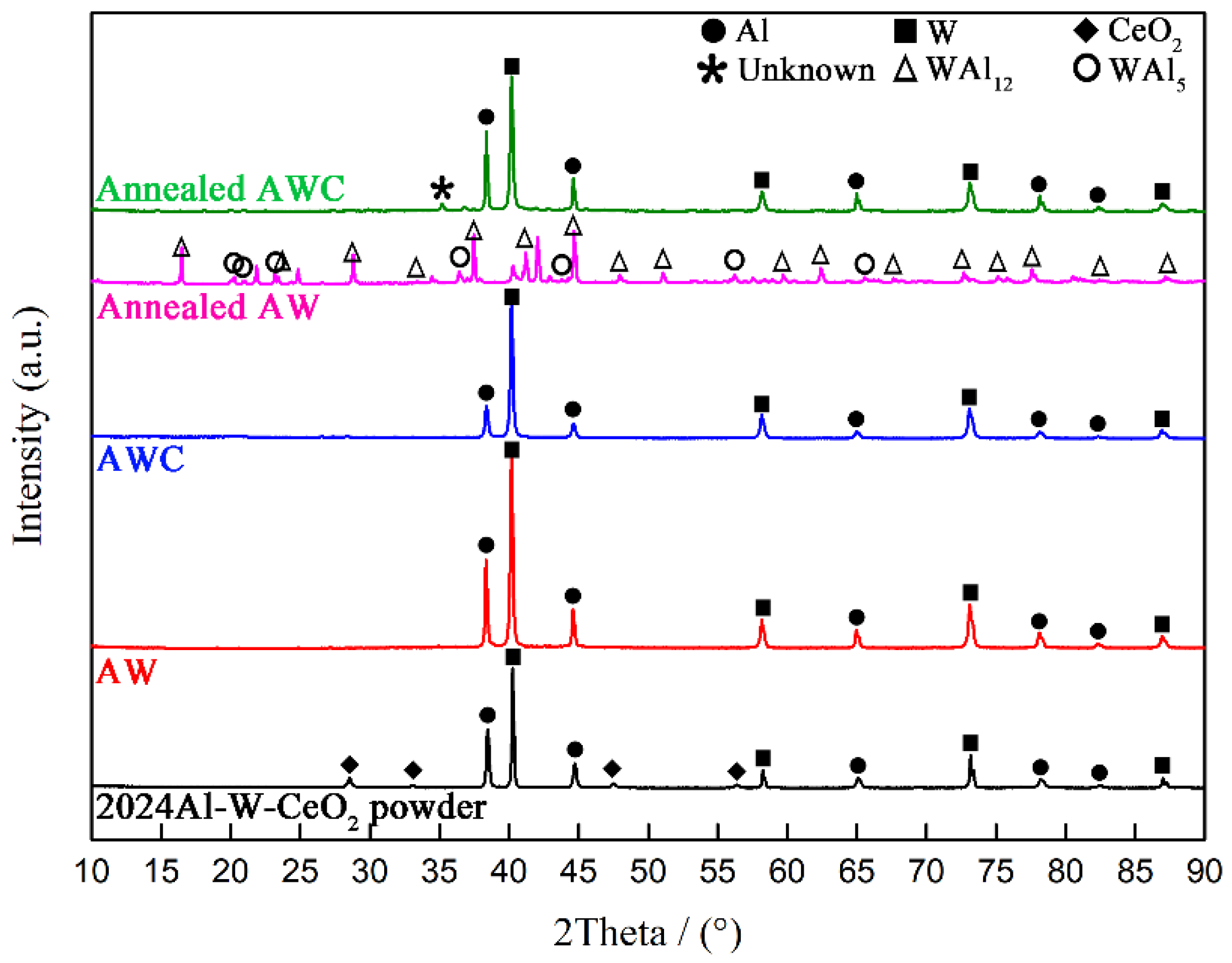
3.1. XRD Patterns and Thermal Measurements
3.2. TEM Investigations at Interfaces
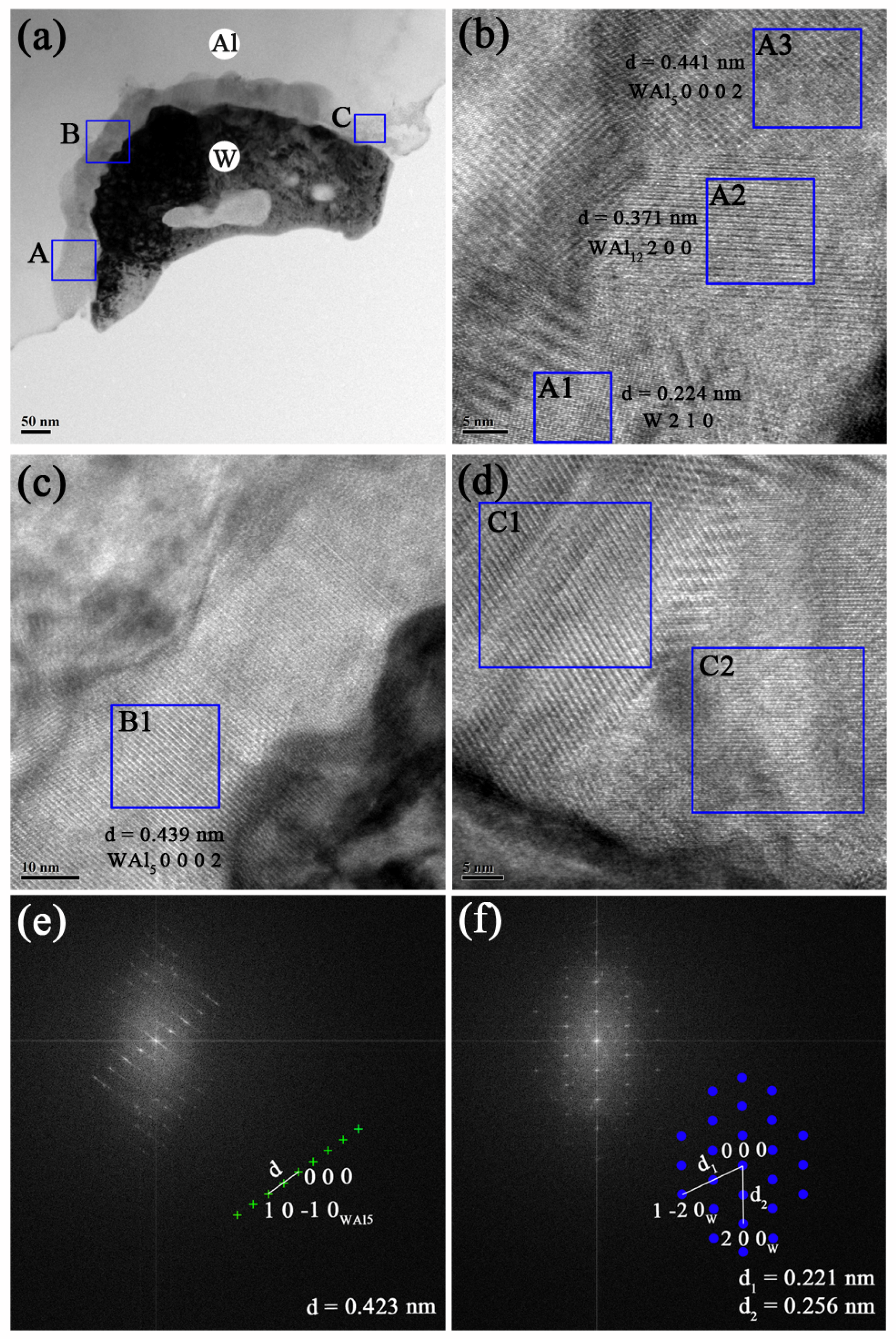
3.2.1. Interfacial Microstructure in Prepared AW
3.2.2. Interfacial Microstructure in the Prepared AWC
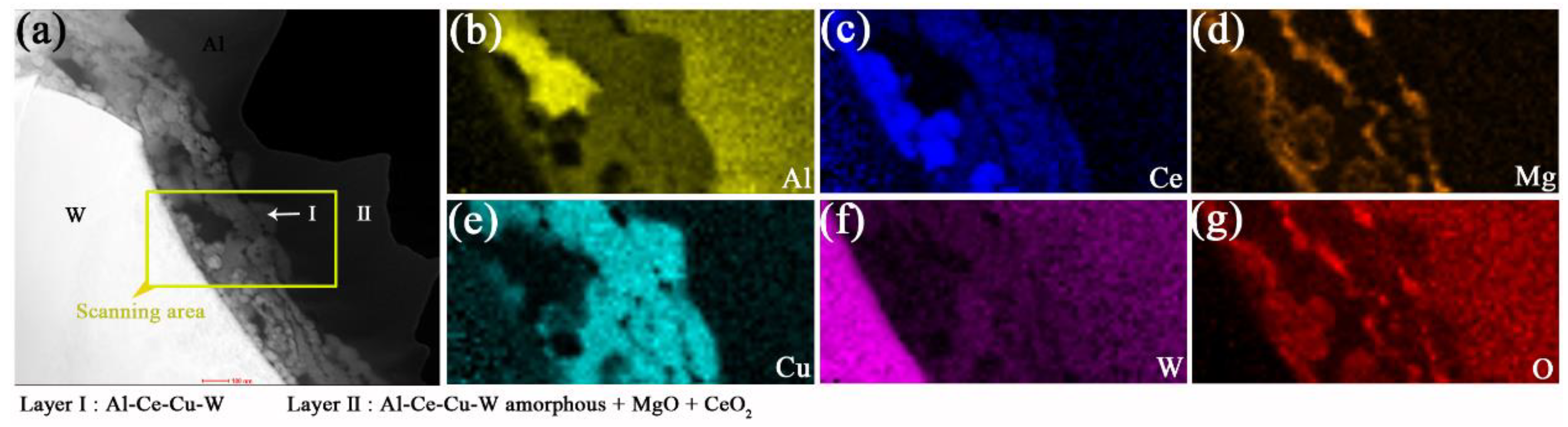
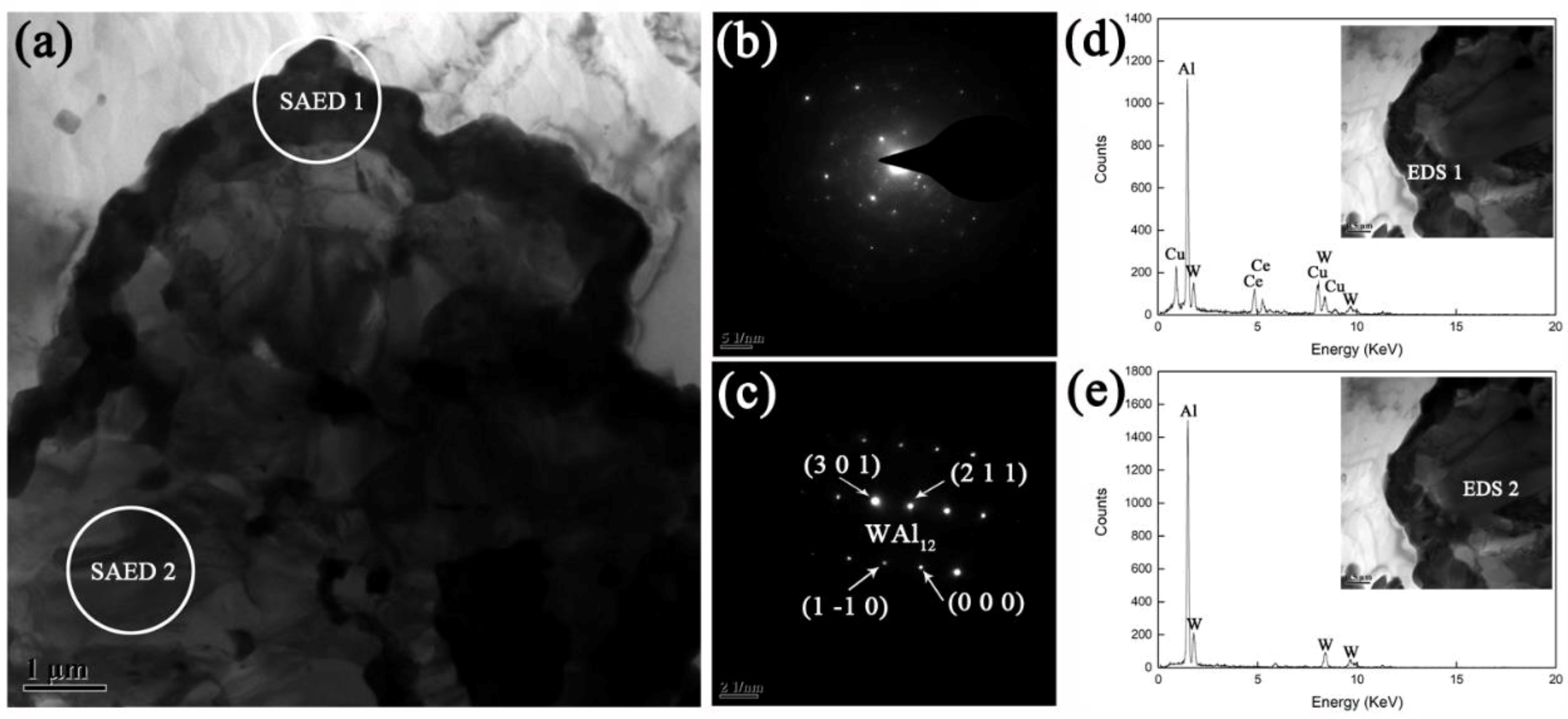
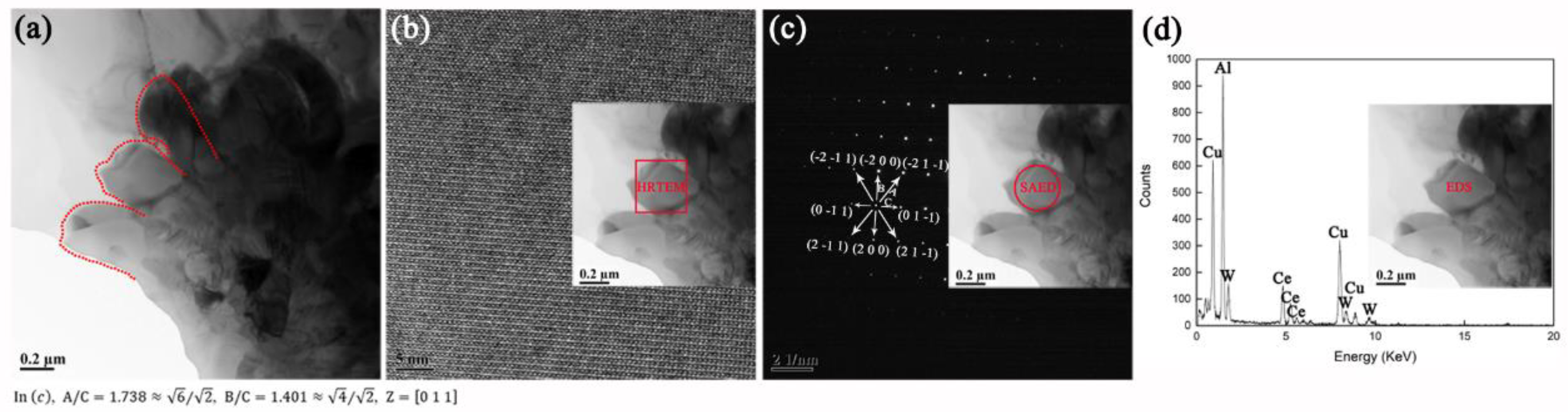
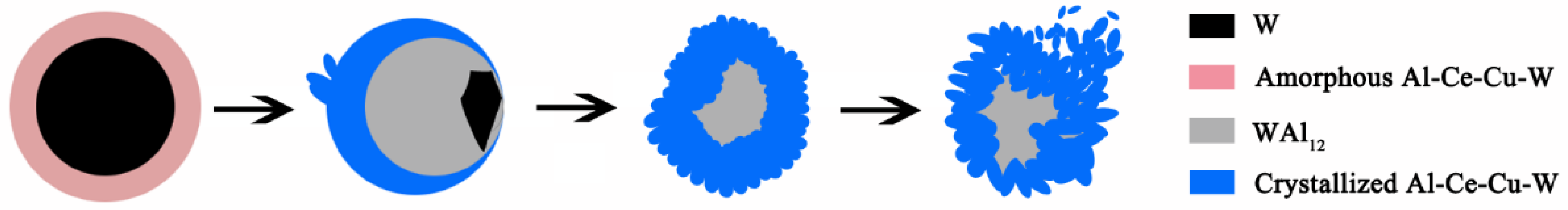
3.2.3. Interfacial Microstructure in Annealed AWC
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ren, S.; He, X.; Qu, X.; Li, Y. Effect of controlled interfacial reaction on the microstructure and properties of the SiCp/Al composites prepared by pressureless infiltration. J. Alloys Compd. 2008, 455, 424–431. [Google Scholar]
- Tan, Z.; Li, Z.; Fan, G.; Guo, Q.; Kai, X.; Ji, G.; Zhang, L.; Zhang, D. Enhanced thermal conductivity in diamond/aluminum composites with a tungsten interface nanolayer. Mater. Des. 2013, 47, 160–166. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.C. Processing and interfacial bonding strength of 2014Al matrix composites reinforced with oxidized SiC particles. Mater. Sci. Eng. A 2006, 420, 8–12. [Google Scholar]
- Dandekar, C.R.; Shin, Y.C. Effect of porosity on the interface behavior of an Al2O3-aluminum composite: A molecular dynamics study. Compos. Sci. Technol. 2011, 71, 350–356. [Google Scholar]
- Contreras, A.; Angeles-Chávez, C.; Flores, O.; Perez, R. Structural, morphological and interfacial characterization of Al-Mg/TiC composites. Mater. Charact. 2007, 58, 685–693. [Google Scholar]
- Jamaludin, S.B.; Hadipramana, J.; Wahid, M.F.M.; Hassin, K.; Rahmat, A. Microstructure and Interface Analysis of Glass Particulate Reinforced Aluminum Matrix Composite. Adv. Mater. Res. 2013, 795, 578–581. [Google Scholar]
- Mao, C.; Sun, X.; Liang, Q.; Yang, J.; Du, J. Interfacial reaction process of the hot-pressed WC/2024Al composite. Rare Met. 2013, 32, 397–401. [Google Scholar]
- Yu, Z.; Wu, G.; Sun, D.; Chen, J.; Jiang, L. Rare-earth oxide coating for sub-micro particulates reinforced aluminum matrix composites. Mater. Sci. Eng. A 2003, 357, 61–66. [Google Scholar] [CrossRef]
- Wang, T.G.; Liang, Q.C.; Qin, Q. Effects of rare earth oxide La2O3 on microstructure and properties of Al2O3/Al composites. Mater. Res. Innov. 2015, 19 (Suppl. 5), S5-891–S5-893. [Google Scholar]
- Yu, Z.; Wu, G.; Jiang, L.; Sun, D. Effect of coating Al2O3 reinforcing particles on the interface and mechanical properties of 6061 alloy aluminium matrix composites. Mater. Lett. 2005, 59, 2281–2284. [Google Scholar]
- Xue, J.; Wang, J.; Han, Y.F.; Chen, C.; Sun, B.D. Behavior of CeO2 additive in in-situ TiB2 particles reinforced 2014 Al alloy composite. Trans. Nonferr. Metals Soc. China 2012, 22, 1012–1017. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, J.; Jing, Q. Effect of mixed rare earth oxides and CaCO3 modification on the microstructure of an in-situ Mg2Si/Al-Si composite. Rare Metals 2009, 28, 169–174. [Google Scholar] [CrossRef]
- Matsuura, M.; Sakurai, M.; Suzuki, K.; Tsai, A.P.; Inoue, A. Local structure change of Ce and Cu in the course of nanocrystalline formation from amorphous Al87Ni8Ce3Cu2. Mater. Sci. Eng. A 1997, 226, 511–514. [Google Scholar] [CrossRef]
- Rios, C.T.; Suriñach, S.; Baró, M.D.; Bolfarini, C.; Botta, W.J.; Kiminami, C.S. Glass forming ability of the Al-Ce-Ni system. J. Non-Cryst. Solids 2008, 354, 4874–4877. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.Q.; Li, Y. Influence of TM and RE elements on glass formation of the ternary Al-TM-RE systems. J. Non-Cryst. Solids 2008, 354, 3473–3479. [Google Scholar] [CrossRef]
- Song, K.; Bian, X.; Lv, X.; Guo, J.; Li, G.H.; Xie, M.T. Compositional dependence of glass-forming ability, medium-range order, thermal stability and liquid fragility of Al-Ni-Ce-based amorphous alloys. Mater. Sci. Eng. A 2009, 506, 87–93. [Google Scholar] [CrossRef]
- Li, G.H.; Wang, W.M.; Bian, X.F.; Zhang, J.T.; Li, R.; Wang, L. Comparing the dynamic and thermodynamic behaviors of Al86Ni9-La5/(La0.5Ce0.5)5 amorphous alloys. J. Alloys Compd. 2009, 478, 745–749. [Google Scholar] [CrossRef]
- Sun, S.P.; Yi, D.Q.; Liu, H.Q.; Zang, B.; Jiang, Y. Calculation of glass forming ranges in Al-Ni-RE (Ce, La, Y) ternary alloys and their sub-binaries based on Miedema’s model. J. Alloys Compd. 2010, 506, 377–387. [Google Scholar] [CrossRef]
- Hu, X.F.; Guo, J.; Fan, G.J.; Feng, T.T. Evaluation of glass-forming ability for Al-based amorphous alloys based on superheated liquid fragility and thermodynamics. J. Alloys Compd. 2013, 574, 18–21. [Google Scholar] [CrossRef]
- Li, C.L.; Murray, J.W.; Voisey, K.T.; Clare, A.T.; McCartney, D.G. Amorphous layer formation in Al86.0Co7.6Ce6.4 glass-forming alloy by large-area electron beam irradiation. Appl. Surf. Sci. 2013, 280, 431–438. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Zhang, D.; Cai, X.; Zhou, G.; Zhang, L. The crystallization behavior of amorphous Al90Fe5Ce5 alloy. J. Mater. Sci. Lett. 2001, 20, 1415–1417. [Google Scholar] [CrossRef]
- Gu, X.J.; Wang, J.Q.; Ye, F.; Lu, K. Influence of pressure on crystallization kinetics in an Al-Ni-Ce-Fe amorphous alloy. J. Non-Cryst. Solids 2001, 296, 74–80. [Google Scholar] [CrossRef]
- Wang, S.H.; Bian, X.F.; Wang, H.R. Crystallization of amorphous Al84.2Ni10La2.1Ce2.8Pr0.3Nd0.6 alloy. Mater. Lett. 2004, 58, 539–542. [Google Scholar] [CrossRef]
- Sahoo, K.L.; Panda, A.K.; Das, S.; Rao, V. Crystallization study of amorphous Al87.5Ni7Mm5Fe0.5 alloy by electrical resistivity measurement. Mater. Lett. 2004, 58, 316–320. [Google Scholar] [CrossRef]
- Wang, S.H.; Bian, X.F. Crystallization of Al-Mg-Ce and Al-Mg-Ni-Ce amorphous alloys. J. Alloys Compd. 2007, 441, 135–138. [Google Scholar] [CrossRef]
- Sun, B.A.; Bian, X.F.; Guo, J.; Zhang, J.Y.; Mao, T. Hump peak formation and the crystallization in amorphous Al87Co10Ce3 alloy. Mater. Lett. 2007, 61, 111–114. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, J.; Chen, H.; Liao, S.; Zhong, X. Crystallization study of amorphous Al82Fe5Ni5Ce8 alloy. J. Alloys Compd. 2008, 454, 359–363. [Google Scholar] [CrossRef]
- Huang, Z.H.; Li, J.F.; Rao, Q.L.; Zhou, Y.H. Primary crystallization of Al-Ni-RE amorphous alloys with different type and content of RE. Mater. Sci. Eng. A 2008, 489, 380–388. [Google Scholar] [CrossRef]
- Wang, S.H.; Bian, X.F. Effect of Si and Co on the crystallization of Al-Ni-RE amorphous alloys. J. Alloys Compd. 2008, 453, 127–130. [Google Scholar] [CrossRef]
- Sha, P.F.; Qi, Z.; Zhang, Z.H. Effect of Ag or Pd additions on the microstructure, crystallization and thermal stability of Al–Ni–Ce amorphous alloys. Intermetallics 2010, 18, 1699–1706. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, W.; Xiong, X.Z.; Kong, L.T.; Li, J.F. Glass forming ability and primary crystallization behavior of Al-Ni-Ce alloys. Intermetallics 2012, 24, 1–6. [Google Scholar] [CrossRef]
- Salehi, M.; Shabestari, S.G.; Boutorabi, S.M.A. Nano-crystal development and thermal stability of amorphous Al-Ni-Y-Ce alloy. J. Non-Cryst. Solids 2013, 375, 7–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, X.Z.; Zhou, W.; Li, J.F. Influence of substitution of La by Ce on the glass forming ability and crystallization behavior of Al-Ni-La alloys. J. Alloys Compd. 2013, 576, 181–186. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, X.Z.; Zhou, W.; Lin, X.; Inoue, A.; Li, J.F. Glass forming ability and crystallization behavior of Al–Ni–RE metallic glasses. Intermetallics 2013, 42, 23–31. [Google Scholar] [CrossRef]
- Mansouri, M.; Simchi, A.; Lee, J.I.; Park, E.S.; Varahram, N. Non-isothermal kinetic studies of crystallization in amorphous Al86Ni10MM4 alloy. J. Non-Cryst. Solids 2014, 387, 36–40. [Google Scholar] [CrossRef]
- Mansouri, M.; Simchi, A.; Varahram, N.; Park, E.S. Development of fcc-Al nanoparticles during crystallization of amorphous Al-Ni alloys containing mischmetal: Microstructure and hardness evaluation. Mater. Sci. Eng. A 2014, 604, 92–97. [Google Scholar] [CrossRef]
- Sidorov, V.; Svec, P.; Janickovic, D.; Mikhailov, V.; Son, L. Magnetic properties and crystallization behavior of Al-Co-Ce(Dy) Amorphous Ribbons. J. Magn. Magn. Mater. 2015, 395, 324–328. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Mandal, R.K.; Srivastava, O.N.; Tiwari, R.S. Crystallization behavior and mechanical properties of (Al90Fe5Ce5)100-xTix amorphous alloys. J. Alloys Compd. 2016, 687, 990–998. [Google Scholar] [CrossRef]
- Mansouri, M.; Bidhendi, H.R.A.; Akhavan, S. Non-isothermal kinetics study of primary crystallization in amorphous Al86Ni10Zr2MM2 alloy. J. Non-Cryst. Solids 2017, 468, 1–4. [Google Scholar] [CrossRef]
- Mansouri, M.; Varahram, N.; Simchi, A. Effect of copper on the thermal stability and non-isothermal crystallization behavior of Al86Ni10-xCuxRE4 (x = 0.5–2.5) amorphous alloys prepared by melt spinning. J. Non-Cryst. Solids 2019, 506, 46–50. [Google Scholar] [CrossRef]




| Element | Cu | Mg | Mn | Fe | Si | Al |
|---|---|---|---|---|---|---|
| Chemical composition | 4.20 | 1.48 | 0.58 | 0.16 | 0.087 | Balance |
| Al-Ce-TM Amorphous System | Crystallization Products | Corresponding Crystallization Conditions |
|---|---|---|
| Al90Fe5Ce5 [21] | Fcc-Al | Pre-existing in amorphous quenched from 1173 °C |
| Al88Ni9Ce2Fe1 [22] | Fcc-Al | Annealing at 138 °C under 1.07 GPa in pressure vessel |
| Fcc-Al + Al3(Ni, Fe) + Al11Ce3 | Annealing at 306 °C under 0.85 GPa in pressure vessel | |
| Al84.2Ni10La2.1Ce2.8Pr0.3Nd0.6 [23] | Fcc-Al + Al3Ni | Continuous heating to 550 K in DSC instrument |
| Fcc-Al + Al3Ni + Al11(La, Ce)3 | Continuous heating to 615/680 K in DSC instrument | |
| Al87.5Ni7Mm5Fe0.5 [24] | Fcc-Al | Annealing at 433 K |
| Fcc-Al + Al11(La, Ce)3 | Annealing at 593 K | |
| Fcc-Al + Al11(La, Ce)3 + Al3Ni + Al4Ce | Annealing at 613 K | |
| Al87Co10Ce3 [26] | Fcc-Al + Co2Al9 | Continuous heating to 262 °C in DSC instrument |
| Fcc-Al + Co2Al9 + Al4Ce | Continuous heating to 274/303/342 °C in DSC instrument | |
| Al82Fe5Ni5Ce8 [27] (powders) | Al11Ce3 + Al3(Fe, Ni) | Annealing at 673 K for 10 h |
| Al87Ni6Ce7 [28] | Fcc-Al + unknown metastable phase 2 | Isochronal heating to 563 K in DSC instrument |
| Al85Ni6Ce9 [28] | Unknown metastable phase 3 | Isochronal heating to 570 K in DSC instrument |
| Al85Ni10Ce5 [30] | Fcc-Al + Al3Ni + Al4Ce | Annealing at ~727 K for 1 h |
| [Al85Ni10Ce5]95Ag5 [30] | Fcc-Al + Al3Ni + Ag3Al17Ce5 | Annealing at ~723 K for 1 h |
| [Al85Ni10Ce5]95Pd5 [30] | Fcc-Al + Al3Ni + unidentified phases | Annealing at ~861 K for 1 h |
| Al86Ni6Y6Ce2 [32] | Fcc-Al | Isochronal annealing up to 548 K in DSC instrument |
| Fcc-Al + Al3Ni | Isochronal annealing up to 618 K in DSC instrument | |
| Fcc-Al + Al3Ni + Al11Re3 | Isochronal annealing up to 688 K in DSC instrument | |
| Al86Ni9(La1−xCex)5 [33] (x = 0−1) | Fcc-Al | Annealing to the end of first DSC exothermic peak |
| Al85.5Ni9.5(La1-xCex)5 [33] (x = 0.2−1) | Fcc-Al + Al4NiCe + Al11Ce3 | Annealing to the end of first DSC exothermic peak |
| Al86Ni10MM4 [35,36,40] | Fcc-Al | Annealing up to 553 K in DSC instrument |
| Fcc-Al + Al11MM3 + Al3Ni | Annealing up to 623/714 K in DSC instrument | |
| Al88Ni10MM2 [36,40] | Fcc-Al | Annealing up to 553 K in DSC instrument |
| Fcc-Al + Al11MM3 + Al3Ni | Annealing up to 714 K in DSC instrument | |
| Al90Fe5Ce5 [38] | Fcc-Al + icosahedral phases | Annealing at 613 K for 2 h |
| (Al90Fe5Ce5)100−xTix [38] (x = 4, 8) | Fcc-Al + icosahedral phases | Annealing at 613 K for 2 h |
| (Al90Fe5Ce5)88Ti12 [38] | Fcc-Al + icosahedral phases + Al20CeTi2 | Pre-existing in amorphous |
| Al86Ni10Zr2MM2 [39] | Fcc-Al | Annealing up to ~570 K in DSC instrument |
| Fcc-Al + Al11MM3 + Al3Ni + Al3Zr | Annealing up to ~690 K in DSC instrument | |
| Al86Ni9.5Cu0.5RE4 [40] | Fcc-Al + Al11MM3 + Al3Ni | Full-crystallization annealing in DSC instrument |
| Al86Ni9Cu1RE4 [40] | Fcc-Al + Al11MM3 + Al3Ni | Full-crystallization annealing in DSC instrument |
| Al86Ni7.5Cu2.5RE4 [40] | Fcc-Al + Al11MM3 + Al3Ni + Al3MMCu | Full-crystallization annealing in DSC instrument |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Mao, C.; Wang, J.; Liang, Q.; Ma, S.; Yang, Z.; Yang, J.; Li, Y. Interfacial Microstructure in W/2024Al Composite and Inhibition of W-Al Direct Reaction by CeO2 Doping: Formation and Crystallization of Al-Ce-Cu-W Amorphous Layers. Materials 2019, 12, 1117. https://doi.org/10.3390/ma12071117
Lv Z, Mao C, Wang J, Liang Q, Ma S, Yang Z, Yang J, Li Y. Interfacial Microstructure in W/2024Al Composite and Inhibition of W-Al Direct Reaction by CeO2 Doping: Formation and Crystallization of Al-Ce-Cu-W Amorphous Layers. Materials. 2019; 12(7):1117. https://doi.org/10.3390/ma12071117
Chicago/Turabian StyleLv, Zheng, Changhui Mao, Jian Wang, Qiushi Liang, Shuwang Ma, Zhimin Yang, Jian Yang, and Yang Li. 2019. "Interfacial Microstructure in W/2024Al Composite and Inhibition of W-Al Direct Reaction by CeO2 Doping: Formation and Crystallization of Al-Ce-Cu-W Amorphous Layers" Materials 12, no. 7: 1117. https://doi.org/10.3390/ma12071117
APA StyleLv, Z., Mao, C., Wang, J., Liang, Q., Ma, S., Yang, Z., Yang, J., & Li, Y. (2019). Interfacial Microstructure in W/2024Al Composite and Inhibition of W-Al Direct Reaction by CeO2 Doping: Formation and Crystallization of Al-Ce-Cu-W Amorphous Layers. Materials, 12(7), 1117. https://doi.org/10.3390/ma12071117





