Enhancing Thermal Oxidation Stability of Silver Nanowire Transparent Electrodes by Using a Cesium Carbonate-Incorporated Overcoating Layer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ag NW Electrode Fabrication and Characterization
2.2. Fabrication of the UV-Curable Passivation Layer
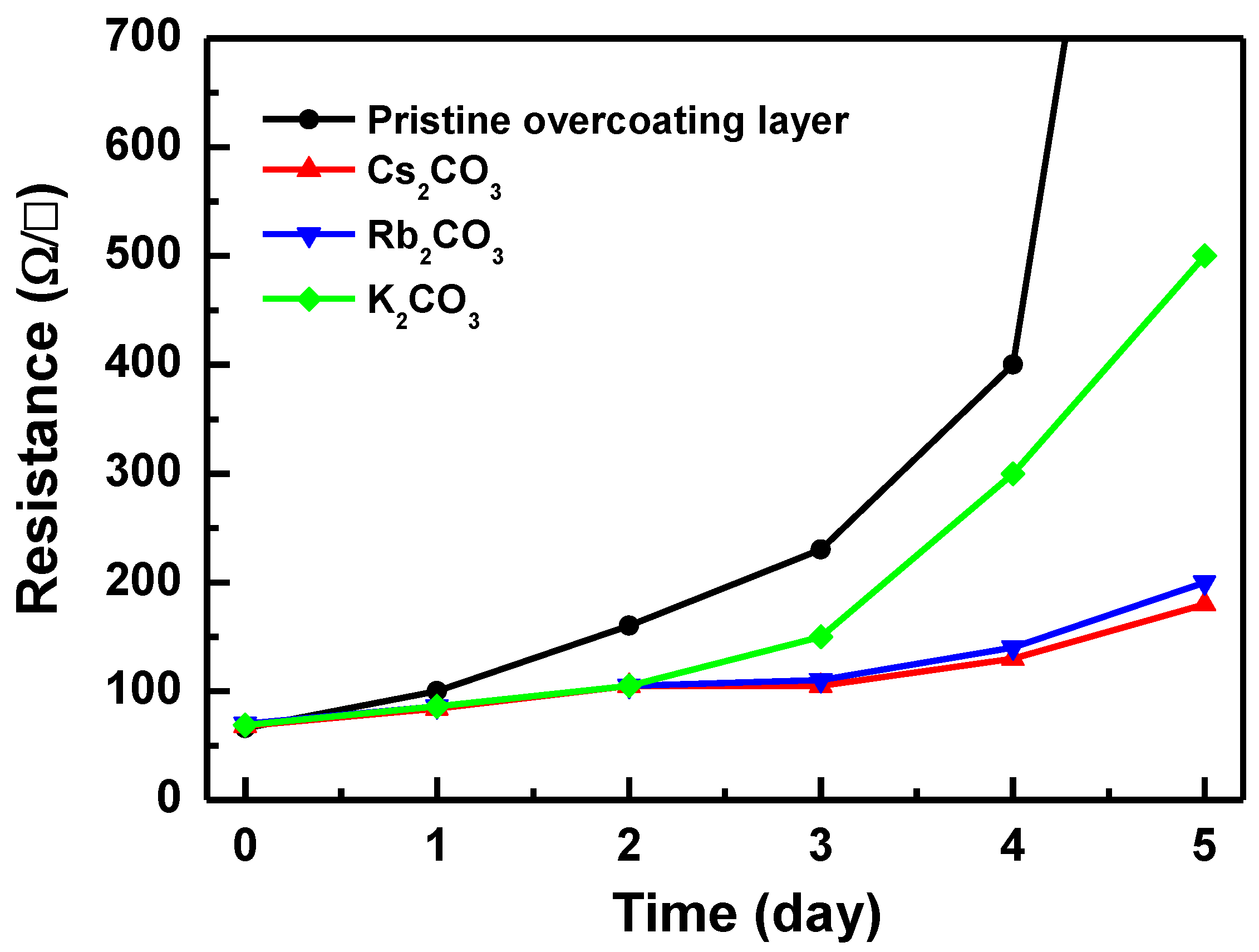
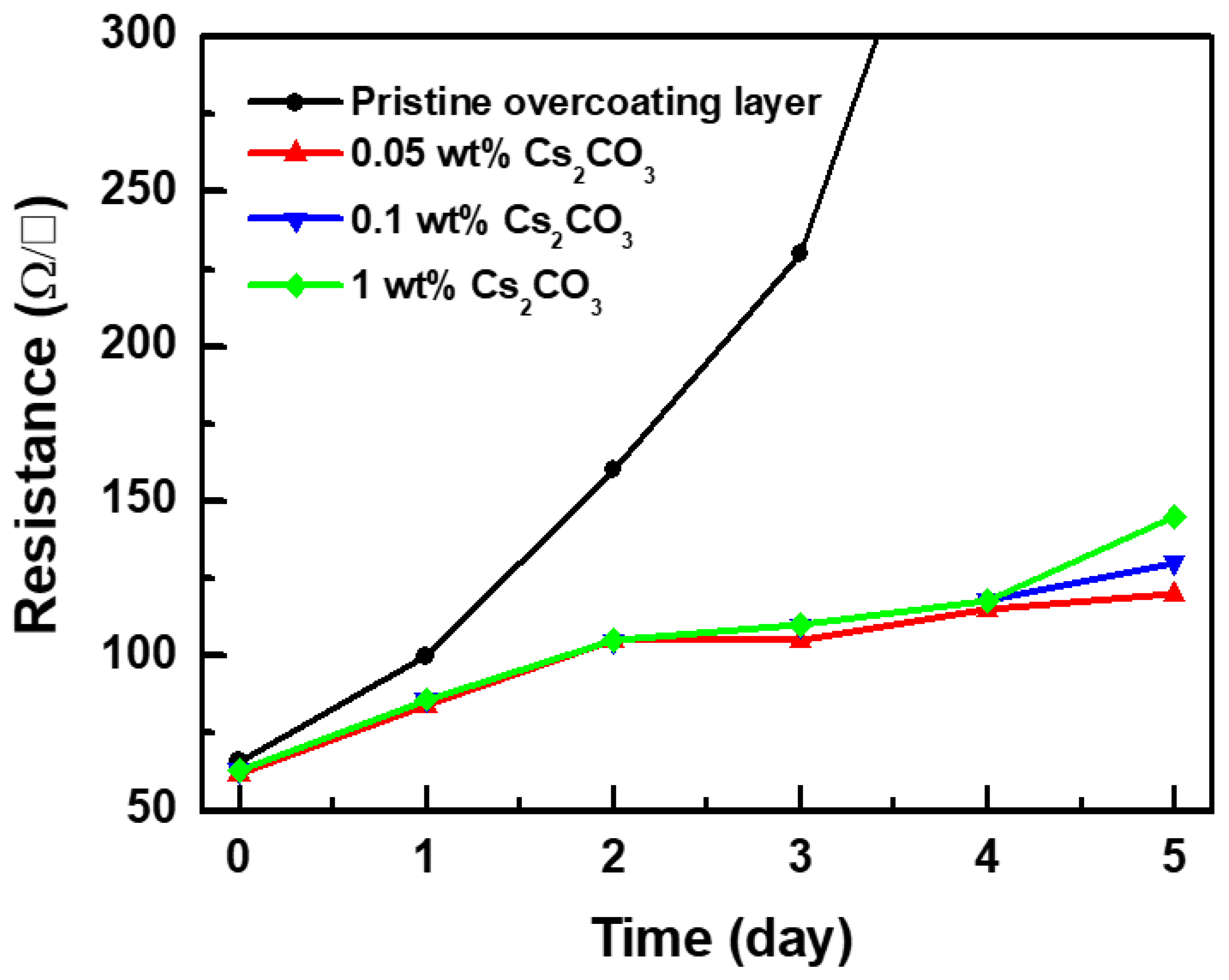
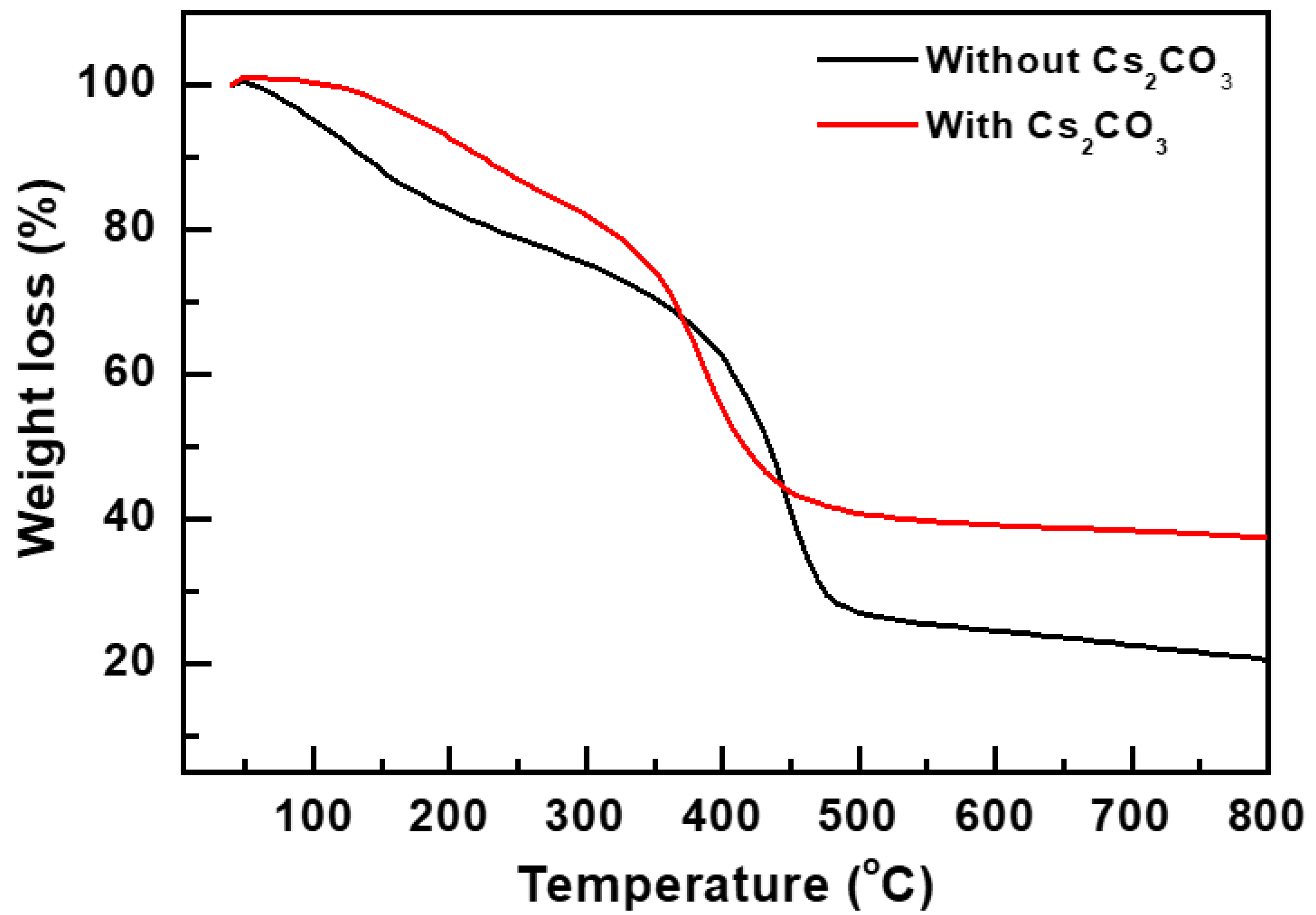
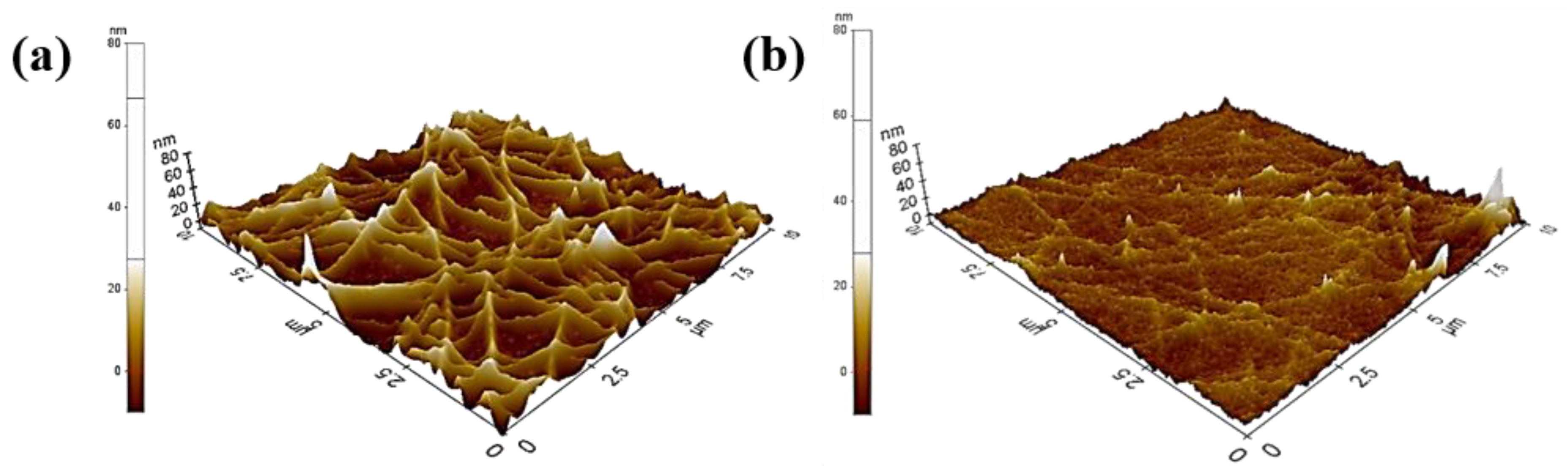
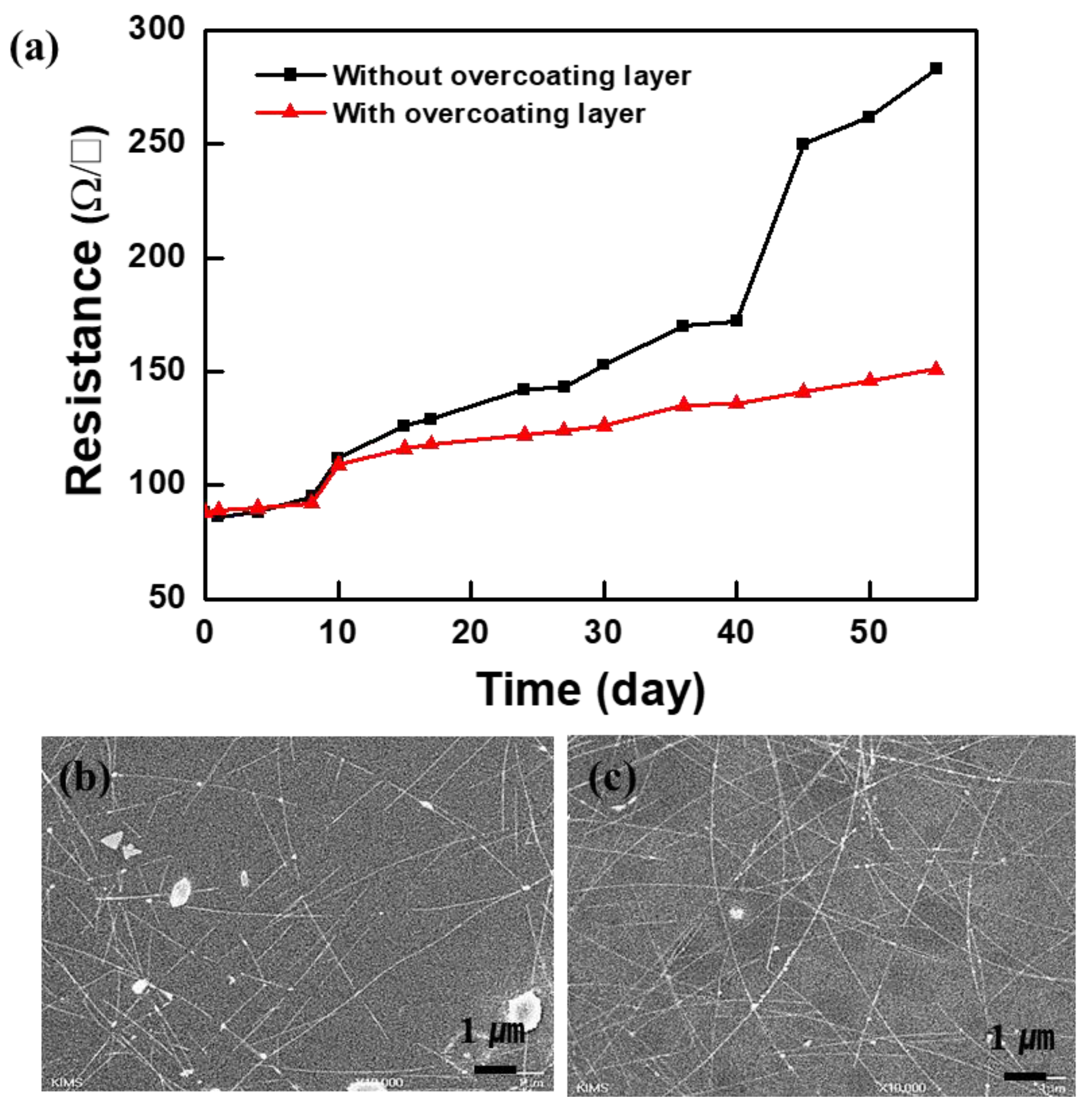
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, R.; Engholm, M. Recent progress on the fabrication and properties of silver nanowire-based transparent electrodes. Nanomaterials 2018, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Jun, L.; Kun, L.; Hu, W.; Ping, C.; Ya-Hui, D.; Zheng, C.; Yun-Fei, L.; Hao-Ran, W.; Yu, D. Inkjet-printed Ag grid combined with Ag nanowires to form a transparent hybrid electrode for organic electronics. Org. Electron. 2017, 41, 179–185. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, C.; Jo, S. Spray deposition of Ag nanowire–graphene oxide hybrid electrodes for flexible polymer–dispersed liquid crystal displays. Materials 2018, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Canlier, A.; Ucak, U.V.; Usta, H.; Cho, C.; Lee, J.; Sen, U.; Citir, M. Development of highly transparent Pd-coated Ag nanowire electrode for display and catalysis applications. Appl. Surf. Sci. 2015, 350, 79–86. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J. Silver nanowire networks embedded in urethane acrylate for flexible capacitive touch sensor. Appl. Surf. Sci. 2016, 363, 1–6. [Google Scholar] [CrossRef]
- Nam, S.; Song, M.; Kim, D.; Cho, B.; Lee, H.M.; Kwon, J.; Park, S.; Nam, K.; Jeong, Y.; Kwon, S. Ultrasmooth, extremely deformable and shape recoverable Ag nanowire embedded transparent electrode. Sci. Rep. 2014, 4, 4788. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.; Jo, S. Hybrid Ag nanowire transparent conductive electrodes with randomly oriented and grid-patterned Ag nanowire networks. Sci. Rep. 2017, 7, 11614. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, H.; Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Highly stable and flexible silver nanowire–graphene hybrid transparent conducting electrodes for emerging optoelectronic devices. Nanoscale 2013, 5, 7750–7755. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; An, Y.; Lee, H.; Lee, E.; Becker, S.; Kim, Y.; Kim, H. Highly Flexible and Transparent Ag Nanowire Electrode Encapsulated with Ultra-Thin Al2O3: Thermal, Ambient, and Mechanical Stabilities. Sci. Rep. 2017, 7, 41336. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, L.; Tao, Z.; Shao, X.; Huang, Y.; Cui, Q.; Guo, X. Neutral-pH PEDOT: PSS as over-coating layer for stable silver nanowire flexible transparent conductive films. Org. Electron. 2014, 15, 3654–3659. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Liu, C.; Saldanha, G.; Zhao, F.; Tong, K.; Liu, J.; Pei, Q. Thermally stable silver nanowire–polyimide transparent electrode based on atomic layer deposition of zinc oxide on silver nanowires. Adv. Funct. Mater. 2015, 25, 7512–7520. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved thermal oxidation stability of solution-processable silver nanowire transparent electrode by reduced graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, K.X.; Fan, S. Analysis of an anti-reflecting nanowire transparent electrode for solar cells. J. Appl. Phys. 2017, 121, 113109. [Google Scholar] [CrossRef]
- Dong, H.; Guo, X.; Li, W.; Wang, L. Cesium carbonate as a surface modification material for organic–inorganic hybrid perovskite solar cells with enhanced performance. RSC Adv. 2014, 4, 60131–60134. [Google Scholar] [CrossRef]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Khaligh, H.H.; Goldthorpe, I.A. Hot-rolling nanowire transparent electrodes for surface roughness minimization. Nanoscale Res. Lett. 2014, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ahn, H.; Yen, S.; Tsai, Y. Thermally induced percolational transition and thermal stability of silver nanowire networks studied by THz spectroscopy. ACS Appl. Mater. Interfaces 2014, 6, 20994–20999. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.-C.; Nam, J.; Kim, J.; Kim, C.S.; Jo, S. Enhancing Thermal Oxidation Stability of Silver Nanowire Transparent Electrodes by Using a Cesium Carbonate-Incorporated Overcoating Layer. Materials 2019, 12, 1140. https://doi.org/10.3390/ma12071140
Jeong Y-C, Nam J, Kim J, Kim CS, Jo S. Enhancing Thermal Oxidation Stability of Silver Nanowire Transparent Electrodes by Using a Cesium Carbonate-Incorporated Overcoating Layer. Materials. 2019; 12(7):1140. https://doi.org/10.3390/ma12071140
Chicago/Turabian StyleJeong, Yong-Chan, Jiyoon Nam, Jongbok Kim, Chang Su Kim, and Sungjin Jo. 2019. "Enhancing Thermal Oxidation Stability of Silver Nanowire Transparent Electrodes by Using a Cesium Carbonate-Incorporated Overcoating Layer" Materials 12, no. 7: 1140. https://doi.org/10.3390/ma12071140
APA StyleJeong, Y.-C., Nam, J., Kim, J., Kim, C. S., & Jo, S. (2019). Enhancing Thermal Oxidation Stability of Silver Nanowire Transparent Electrodes by Using a Cesium Carbonate-Incorporated Overcoating Layer. Materials, 12(7), 1140. https://doi.org/10.3390/ma12071140




