Inorganic and Hybrid (Organic–Inorganic) Lamellar Materials for Heavy Metals and Radionuclides Capture in Energy Wastes Management—A Review
Abstract
1. Introduction
2. Use of Lamellar Materials for the Purification of Aqueous Solutions Containing Heavy Metals
2.1. Natural Clay or Modified Natural Clay and Heavy Metals Adsorption
2.1.1. Classification, Structure and Properties of Clays
- Minerals of type 1:1. They are also called 7 Å minerals because of the thickness of the layer (composed of an arrangement of tetrahedral silicate sheets with octahedral hydroxide sheets). They consist of a siliceous tetrahedral (T) sheet bounded to an aluminous octahedral (O) sheet, or a T.O. stack, another name given to this family of clay minerals. Kaolinite is a notable mineral of this group. In the case of halloysites, the interlayer space can be occupied by water molecules.
- Minerals of type 2:1. This family of minerals (also named T.O.T. type) consists of an arrangement of one octahedral sheet sandwiched between two silicate sheets and presents a layer thickness close to 10 Å. It is the most common type of phyllosilicate encountered in nature. This family includes mica, smectite and illite, which could be distinguished by the contents of their tetrahedral and octahedral sheets. Some members of this family have swelling properties. While smectites and vermiculites can swell by incorporating water molecules, or hydrated cations, into the interlayer space, most micas or illites having interlayer potassium cations have no ability to swell.
- Minerals of type 2:1:1. They have a T.O.T.O. structure with a layer thickness of about 14 Å. For this family of clay minerals, the interlayer space is occupied by an octahedral sheet. Chlorites are part of this family of minerals.
- Interlayered minerals. This last family is characterized by its heterogeneity. Indeed, interlayer minerals have diverse kinds of layers that could be stacked regularly or not. In nature, interlayered minerals illite/smectite or mica/chlorite are frequently found.
- Negative charges relocated at the layer surfaces (basal surfaces) make these surfaces good sites for cation retention by charge compensation (they result from isomorphic substitutions which then lead to deficits of positive charges) (Figure 2).
- Layer edges are composed of silanol and aluminol groups, which, depending on pH, can deprotonate (or even protonate). These changes result in the presence of positive (or negative) charges which can further be neutralized by complexation (Figure 3).
- Exchangeable cations can be supplanted by diverse cationic elements, the interaction between these latter and the surface being electrostatic. Interlayer spaces containing exchangeable cations are negatively charged surfaces that can interact with different cations in a very specific way (electrostatic interactions).
2.1.2. Summary of Uddin’s Review on the Retention of Heavy Metals by Natural Clay Minerals
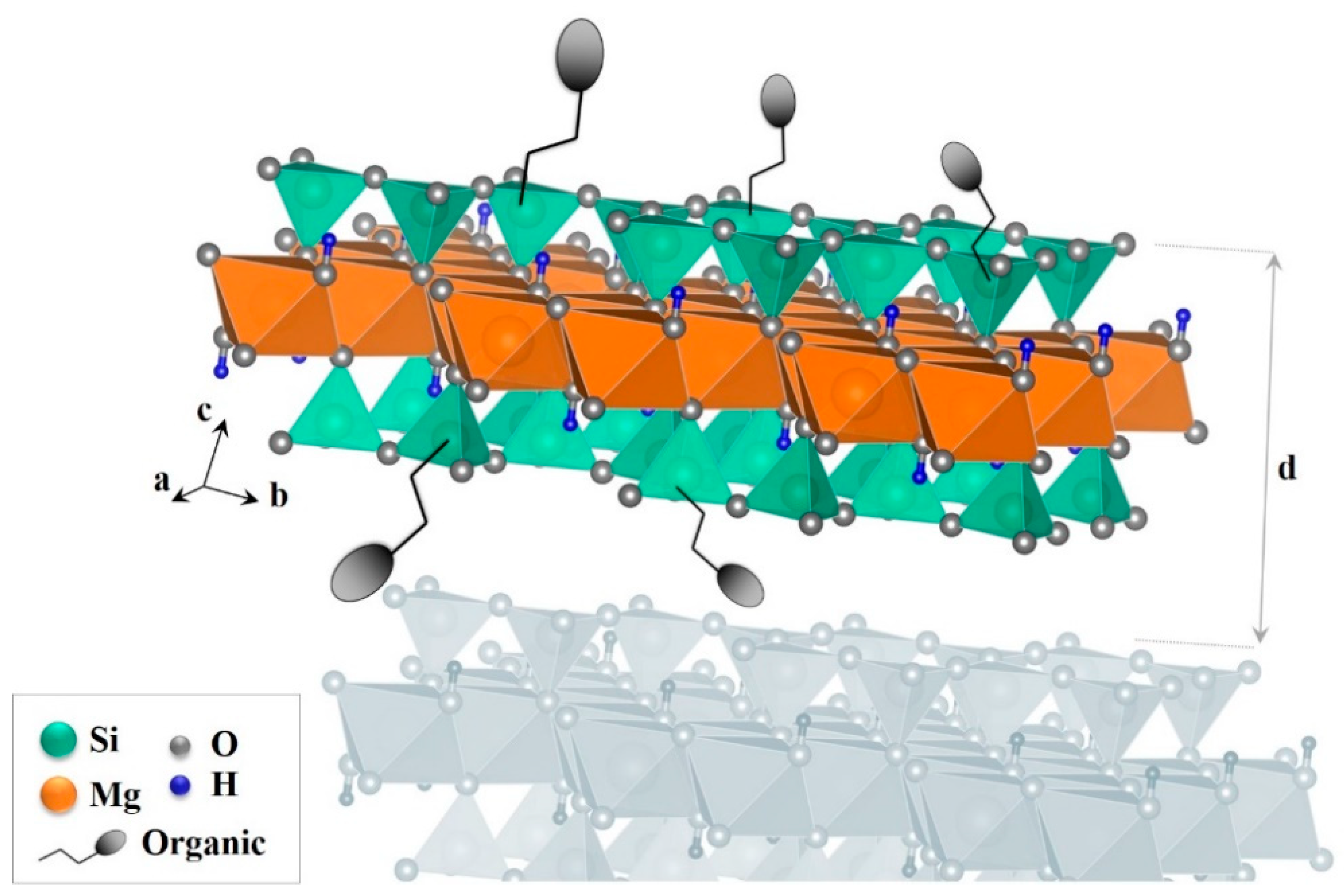
2.2. Heavy Metals Removal by Synthetic Phyllosilicate
2.3. Heavy Metals Retention by Layered Double Hydroxides
3. Use of Lamellar Materials for the Treatment of Effluents Containing Radionuclides
3.1. Clay Minerals Radionuclide Adsorption Investigations
3.2. Removal of Cs and Sr by Layered Double Hydroxides
4. Summary, Discussions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halim, M.; Conte, P.; Piccolo, A. Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances. Chemosphere 2003, 52, 265–275. [Google Scholar] [PubMed]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal-a review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Leung, W.C.; Wong, M.-F.; Chua, H.; Lo, W.; Yu, P.H.F.; Leung, C.K. Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Sci. Technol. 2000, 41, 233–240. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Pedersen, A.J. Characterization and electrodialytic treatment of wood combustion fly ash for removal of cadmium. Biomass Bioenergy 2003, 25, 447–458. [Google Scholar]
- Chowdhury, P.; Athapaththu, S.; Elkamel, A.; Ray, A.K. Visible-solar-light-driven photo-reduction and removal of cadmium ion with Eosin Y-sensitized TiO2 in aqueous solution of triethanolamine. Sep. Purif. Technol. 2017, 174, 109–115. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, X.P.; Jiao, Z.H.; Zhang, Y.S.; Yin, X.B.; Cui, X.M.; Wei, Y.Z. Functionalized Porous Silica-Based Nano/Micro Particles for Environmental Remediation of Hazard Ions. Nanomaterials 2019, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Eccles, H. Treatment of metal-contaminated wastes: Why select a biological process? Trends Biotechnol. 1999, 17, 462–465. [Google Scholar] [CrossRef]
- El-Aryan, Y.F.; El-Said, H.; Abdel-Galil, E.A. Synthesis and characterization of polyaniline-titanium tungstophosphate; Its analytical applications for sorption of Cs+, Co2+, and Eu3+ from waste solutions. Radiochemistry 2014, 56, 614–621. [Google Scholar] [CrossRef]
- Khan Rao, R.A.; Ikram, S.; Uddin, M.K. Removal of Cd(II) from aqueous solution by exploring the biosorption characteristics of gaozaban (Onosma bracteatum). J. Environ. Chem. Eng. 2014, 2, 1155–1164. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Ikram, S.; Uddin, M.K. Removal of Cr(VI) from aqueous solution on seeds of Artimisia absinthium (novel plant material). Desalin. Water Treat. 2015, 54, 3358–3371. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Lin, L.; Khan, Z.H.; Qiu, W.; Song, Z. Synthesis and Characterization of Novel Fe-Mn-Ce Ternary Oxide–Biochar Composites as Highly Efficient Adsorbents for As(III) Removal from Aqueous Solutions. Materials 2018, 11, 2445. [Google Scholar] [CrossRef]
- Kadam, V.; Truong, Y.B.; Easton, C.; Mukherjee, S.; Wang, L.; Padhye, R.; Kyratzis, I.L. Electrospun Polyacrylonitrile/β-Cyclodextrin Composite Membranes for Simultaneous Air Filtration and Adsorption of Volatile Organic Compounds. ACS Appl. Nano Mater. 2018, 1, 4268–4277. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Hao, W.; Li, Y.; Chen, L. Covalent Organic Frameworks Constructed from Flexible Building Blocks with High Adsorption Capacity for Pollutants. ACS Appl. Nano Mater. 2018, 1, 4756–4761. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Mishael, Y.G. Polycyclodextrin–Clay Composites: Regenerable Dual-Site Sorbents for Bisphenol A Removal from Treated Wastewater. ACS Appl. Mater. Interfaces 2018, 10, 27088–27097. [Google Scholar] [CrossRef]
- Luna, F.M.T.; Cecilia, J.A.; Saboya, R.M.A.; Barrera, D.; Sapag, K.; Rodríguez-Castellón, E.; Cavalcante, C.L. Natural and Modified Montmorillonite Clays as Catalysts for Synthesis of Biolubricants. Materials 2018, 11, 1764. [Google Scholar] [CrossRef]
- Srinivasan, R. Advances in Application of Natural Clay and Its Composites in Removal of Biological, Organic, and Inorganic Contaminants from Drinking Water. Adv. Mater. Sci. Eng. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Young, D.A.; Smith, D.E. Simulations of Clay Mineral Swelling and Hydration: Dependence upon Interlayer Ion Size and Charge. J. Phys. Chem. B 2000, 104, 9163–9170. [Google Scholar] [CrossRef]
- Osacky, M.; Geramian, M.; Ivey, D.G.; Liu, Q.; Etsell, T.H. Influence of Nonswelling Clay Minerals (Illite, Kaolinite, and Chlorite) on Nonaqueous Solvent Extraction of Bitumen. Energy Fuels 2015, 29, 4150–4159. [Google Scholar] [CrossRef]
- Tambach, T.J.; Hensen, E.J.M.; Smit, B. Molecular Simulations of Swelling Clay Minerals. J. Phys. Chem. B 2004, 108, 7586–7596. [Google Scholar] [CrossRef]
- Skipper, N.T.; Sposito, L.G.; Chang, F.C. Monte Carlo simulation of interlayer molecular structure in swelling clay minerals. 1. Methodology. Clays. Clays Clay Miner. 1995, 43, 294–303. [Google Scholar] [CrossRef]
- Churchman, G.J.; Gates, W.P.; Theng, B.K.G.; Yuan, G. Chapter 11.1 Clays and Clay Minerals for Pollution Control. Dev. Clay Sci. 2006, 1, 625–675. [Google Scholar]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Clay Materials for Environmental Remediation; Springer International Publishing: Berlin, Germany, 2015; ISBN 978-3-319-16711-4. [Google Scholar]
- Sposito, G.; Skipper, N.T.; Sutton, R.; Park, S.; Soper, A.K.; Greathouse, J.A. Surface geochemistry of the clay minerals. Proc. Natl. Acad. Sci. USA 1999, 96, 3358–3364. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2008; ISBN 978-0-19-531369-7. [Google Scholar]
- Siroux, B. Interactions dans un système césium, strontium/matière organique naturelle/argiles des sols. De la décontamination à la remédiation. Ph.D. Thesis, Université Sorbonne Paris Cité, Université Paris Diderot, Paris, France, 2017. [Google Scholar]
- Mhamdi, M.; Elaloui, E.; Trabelsi-Ayadi, M. Kinetics of cadmium adsorption by smectite of Oued Tfal (Gafsa Basin). Desalin. Water Treat. 2014, 52, 4245–4256. [Google Scholar] [CrossRef]
- Shawabkeh, R.A.; Al-Khashman, O.A.; Al-Omari, H.S.; Shawabkeh, A.F. Cobalt and zinc removal from aqueous solution by chemically treated bentonite. Environmentalist 2007, 27, 357–363. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Hong, X.; Zhao, Y.; Zhang, Q. Removal of hexavalent chromium from aqueous solution using exfoliated polyaniline/montmorillonite composite. Water Sci. Technol. 2014, 70, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Erdem, B.; Özcan, A.; Gök, Ö.; Özcan, A.S. Immobilization of 2,2′-dipyridyl onto bentonite and its adsorption behavior of copper(II) ions. J. Hazard. Mater. 2009, 163, 418–426. [Google Scholar] [CrossRef]
- de Pablo, L.; Chávez, M.L.; Abatal, M. Adsorption of heavy metals in acid to alkaline environments by montmorillonite and Ca-montmorillonite. Chem. Eng. J. 2011, 171, 1276–1286. [Google Scholar] [CrossRef]
- Sathyanarayana, B.; Seshaiah, K. Kinetics and Equilibrium Studies on the Sorption of Manganese(II) and Nickel(II) onto Kaolinite and Bentonite. E-J. Chem. 2010, 8, 373–385. [Google Scholar] [CrossRef]
- Jiang, M.; Jin, X.; Lu, X.-Q.; Chen, Z. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Ozdes, D.; Duran, C.; Senturk, H.B. Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay. J. Environ. Manag. 2011, 92, 3082–3090. [Google Scholar] [CrossRef]
- Arias, F.; Sen, T.K. Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay mineral: A kinetic and equilibrium study. Colloids Surf. A 2009, 348, 100–108. [Google Scholar] [CrossRef]
- Carrado, K.A.; Xu, L.; Csencsits, R.; Muntean, J.V. Use of organo- and alkoxysilanes in the synthesis of grafted and pristine clays. Chem. Mater. 2001, 13, 3766–3773. [Google Scholar] [CrossRef]
- Sales, J.A.A.; Petrucelli, G.C.; Oliveira, F.J.V.E.; Airoldi, C. Some features associated with organosilane groups grafted by the sol–gel process onto synthetic talc-like phyllosilicate. J. Colloid Interface Sci. 2006, 297, 95–103. [Google Scholar] [CrossRef]
- Ukrainczyk, L.; Bellman, R.A.; Anderson, A.B. Template Synthesis and Characterization of Layered Al− and Mg−Silsesquioxanes. J. Phys. Chem. B 1997, 101, 531–539. [Google Scholar] [CrossRef]
- da Fonseca, M.G.; Airoldi, C. New layered inorganic-organic nanocomposites containing n-propylmercapto copper phyllosilicates. J. Mater. Chem. 2000, 10, 1457–1463. [Google Scholar] [CrossRef]
- Silva, C.R.; Fonseca, M.G.; Barone, J.S.; Airoldi, C. Layered Inorganic−Organic Talc-like Nanocomposites. Chem. Mater. 2002, 14, 175–179. [Google Scholar] [CrossRef]
- Brendlé, J. Organic–inorganic hybrids having a talc-like structure as suitable hosts to guest a wide range of species. Dalton Trans. 2018, 47, 2925–2932. [Google Scholar] [CrossRef]
- Kamitani, K.; Uo, M.; Inoue, H.; Makishima, A. Synthesis and spectroscopy of tpps-doped silica gels by the sol-gel process. J. Sol-Gel Sci. Technol. 1993, 1, 85–92. [Google Scholar] [CrossRef]
- Kimura, I.; Taguchi, Y.; Tanaka, M. Preparation of silica particles by sol-gel process in reverse suspension. J. Mater. Sci. 1999, 34, 1471–1475. [Google Scholar] [CrossRef]
- Tamon, T.; Kitamura, T.; Okazaki, M. Preparation of silica aerogel from TEOS. J. Colloid Interface Sci. 1998, 197, 353–359. [Google Scholar] [CrossRef]
- Fukushima, Y.; Tani, M. An organic/inorganic hybrid layered polymer: Methacrylate–magnesium(nickel) phyllosilicate. J. Chem. Soc. Chem. Commun. 1995, 241–242. [Google Scholar] [CrossRef]
- Claverie, M.; Dumas, A.; Careme, C.; Poirier, M.; Le Roux, C.; Micoud, P.; Martin, F.; Aymonier, C. Synthetic talc and talc-like structures: Preparation, features and applications. Chemistry 2018, 24, 519–542. [Google Scholar] [CrossRef]
- Fonseca, M.G.; Airoldi, C. Mercaptopropyl magnesium phyllosilicate Ð thermodynamic data on the interaction with divalent cations in aqueous solution. Thermochim. Acta 2000, 359, 1–9. [Google Scholar] [CrossRef]
- Vieira, E.F.S.; Simoni, J.d.A.; Airoldi, C. Interaction of cations with SH-modified silica gel: Thermochemical study through calorimetric titration and direct extent of reaction determination. J. Mater. Chem. 1997, 7, 2249–2252. [Google Scholar] [CrossRef]
- da Fonseca, M.G.; Silva, C.R.; Barone, J.S.; Airoldi, C. Layered hybrid nickel phyllosilicates and reactivity of the gallery space. J. Mater. Chem. 2000, 10, 789–795. [Google Scholar] [CrossRef]
- Dey, R.K.; Oliveira, A.S.; Patnaik, T.; Singh, V.K.; Tiwary, D.; Airoldi, C. Grafting of organosilane derived from 3-glycidoxypropyltrimethoxysilane and thiourea onto magnesium phyllosilicate by sol–gel process and investigation of metal adsorption properties. J. Solid State Chem. 2009, 182, 2010–2017. [Google Scholar] [CrossRef]
- Lagadic, I.L.; Mitchell, M.K.; Payne, B.D. Highly Effective Adsorption of Heavy Metal Ions by a Thiol-Functionalized Magnesium Phyllosilicate Clay. Environ. Sci. Technol. 2001, 35, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Miehé-Brendlé, J.; Michelin, L.; Delmotte, L. Heavy Metal Retention by Organoclays: Synthesis, Applications, and Retention Mechanism. Chem. Mater. 2005, 17, 5275–5281. [Google Scholar] [CrossRef]
- Badshah, S.; Airoldi, C. Layered organoclay with talc-like structure as agent for thermodynamics of cations sorption at the solid/liquid interface. Chem. Eng. J. 2011, 166, 420–427. [Google Scholar] [CrossRef]
- Fonseca, M.G.D.; Barone, J.S.; Airoldi, C. Self-Organized Inorganic-Organic Hybrids Induced by Silylating Agents with Phyllosilicate-Like Structure and the Influence of the Adsorption of Cations. Clays Clay Miner. 2000, 48, 638–647. [Google Scholar] [CrossRef]
- Moscofian, A.S.O.; Airoldi, C. Synthesized layered inorganic–organic magnesium organosilicate containing a disulfide moiety as a promising sorbent for cations removal. J. Hazard. Mater. 2008, 160, 63–69. [Google Scholar] [CrossRef]
- Melo, M.A., Jr.; Airoldi, C. Energetic features of copper and lead sorption by innovative aminoalcohol-functionalized cobalt phyllosilicates. Dalton Trans. 2010, 39, 10217. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Park, W.-K.; Yang, J.-W. Removal of anionic metals by amino-organoclay for water treatment. J. Hazard. Mater. 2011, 190, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhao, L.; Zhang, S.; Zhang, F.; Dong, M.; Xu, S. Morphologies, Preparations and Applications of Layered Double Hydroxide Micro-/Nanostructures. Materials 2010, 3, 5220–5235. [Google Scholar] [CrossRef] [PubMed]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Valim, J.B. Hidróxidos duplos lamelares: Síntese, estrutura, propriedades e aplicações. Química Nova 1998, 21, 300–311. [Google Scholar] [CrossRef]
- de Roy, A.; Forano, C.; Malki, K.E.; Besse, J.-P. Anionic Clays: Trends in Pillaring Chemistry. In Expanded Clays and Other Microporous Solids; Occelli, M.L., Robson, H.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 108–169. ISBN 978-1-4684-8866-1. [Google Scholar]
- Labajos, F.M.; Sastre, M.D.; Trujillano, R.; Rives, V. New layered double hydroxides with the hydrotalcite structure containing Ni(II) and V(III). J. Mater. Chem. 1999, 9, 1033–1039. [Google Scholar] [CrossRef]
- Miyata, S.; Okada, A. The synthesis of hydrotalcite-like compounds and their physcio-chemical properties—The systems Mg2+ -Al3+ -(SO4)2- and Mg2+ -Al3+ -(CrO4)2-. Clays Clay Miner. 1976, 25, 14–18. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ionics 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Sels, B.F.; Vos, D.E.D.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Vaccari, A. Layered Double Hydroxides: Present and Future; Rives, V., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2001; ISBN 1-59033-060-9. [Google Scholar]
- Hang-Sik, S.; Mi-Joo, K.; Se-Yong, N.; Hi-Chung, M. Phosphorus removal by hydrotalcite-like compounds (HTLcs). Water Sci. Technol. 1996, 34, 161–168. [Google Scholar] [CrossRef]
- Reardon, E.J.; Della Valle, S. Anion Sequestering by the Formation of Anionic Clays: Lime Treatment of Fly Ash Slurries. Environ. Sci. Technol. 1997, 31, 1218–1223. [Google Scholar] [CrossRef]
- Lehmann, M.; Zouboulis, A.I.; Matis, K.A. Removal of metal Ions from dilute aqueous solutions: A comparative study of inorganic sorbent materials. Chemosphere 1999, 39, 881–892. [Google Scholar] [CrossRef]
- Rives, V.; Angeles Ulibarri, M. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates. Coord. Chem. Rev. 1999, 181, 61–120. [Google Scholar] [CrossRef]
- van der Ven, L.; van Gemert, M.L.M.; Batenburg, L.F.; Keern, J.J.; Gielgens, L.H.; Koster, T.P.M.; Fischer, H.R. On the action of hydrotalcite-like clay materials as stabilizers in polyvinylchloride. App. Clay Sci. 2000, 17, 25–34. [Google Scholar] [CrossRef]
- Parker, L.M.; Milestone, N.B.; Newman, R.H. The Use of Hydrotalcite as an Anion Absorbent. Ind. Eng. Chem. Res. 1995, 34, 1196–1202. [Google Scholar] [CrossRef]
- Fujii, S.; Sugie, Y.; Kobune, M.; Touno, A.; Touji, J. Uptakes of Cu2+, Pb2+ and Zn2+ on Synthetic Hydrotalcite in Aqueous Solution. J. Jpn. Chem. Soc. 1992, 1504–1507. [Google Scholar]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appli. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Gu, Z.; Atherton, J.J.; Xu, Z.P. Hierarchical layered double hydroxide nanocomposites: Structure, synthesis and applications. Chem. Commun. 2015, 51, 3024–3036. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf. A 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42– Ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef]
- Ma, S.; Chen, Q.; Li, H.; Wang, P.; Islam, S.M.; Gu, Q.; Yang, X.; Kanatzidis, M.G. Highly selective and efficient heavy metal capture with polysulfide intercalated layered double hydroxides. J. Mater. Chem. A 2014, 2, 10280–10289. [Google Scholar] [CrossRef]
- Li, L.; Qi, G.; Wang, B.; Yue, D.; Wang, Y.; Sato, T. Fulvic acid anchored layered double hydroxides: A multifunctional composite adsorbent for the removal of anionic dye and toxic metal. J. Hazard. Mater. 2018, 343, 19–28. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, B. Self-assembly of graphene oxide aerogels by layered double hydroxides cross-linking and their application in water purification. J. Mater. Chem. A 2014, 2, 8941–8951. [Google Scholar] [CrossRef]
- Kameda, T.; Kondo, E.; Yoshioka, T. Kinetics of Cr(VI) removal by Mg–Al layered double hydroxide doped with Fe2+. J. Water Process Eng. 2014, 4, 134–136. [Google Scholar] [CrossRef]
- Hossein Beyki, M.; Alijani, H.; Fazli, Y. Poly o-phenylenediamine–MgAl@CaFe2O4 nanohybrid for effective removing of lead(II), chromium(III) and anionic azo dye. Process Saf. Environ. Prot. 2016, 102, 687–699. [Google Scholar] [CrossRef]
- Li, Z.; Nagashima, M.; Ikeda, K. Treatment Technology of Hazardous Water Contaminated with Radioisotopes with Paper Sludge Ash-Based Geopolymer—Stabilization of Immobilization of Strontium and Cesium by Mixing Seawater. Materials 2018, 11, 1521. [Google Scholar] [CrossRef]
- Jang, J.G.; Park, S.M.; Lee, H.K. Cesium and Strontium Retentions Governed by Aluminosilicate Gel in Alkali-Activated Cements. Materials 2017, 10, 447. [Google Scholar] [CrossRef]
- Alby, D.; Charnay, C.; Heran, M.; Prelot, B.; Zajac, J. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J. Hazard. Mater. 2018, 344, 511–530. [Google Scholar] [CrossRef]
- Bostick, B.C.; Vairavamurthy, M.A.; Karthikeyan, K.G.; Chorover, J. Cesium adsorption on clay minerals: An EXAFS spectroscopic investigation. Environ. Sci. Technol. 2002, 36, 2670–2676. [Google Scholar] [CrossRef]
- Xia, M.; Zheng, X.; Du, M.; Wang, Y.; Ding, A.; Dou, J. The adsorption of Cs+ from wastewater using lithium-modified montmorillonite caged in calcium alginate beads. Chemosphere 2018, 203, 271–280. [Google Scholar] [CrossRef]
- Cornell, R.M. Adsorption of cesium on minerals: A review. J. Radioanal. Nuclear Chem. Artic. 1993, 171, 483–500. [Google Scholar] [CrossRef]
- Willms, C.; Li, Z.; Allen, L.; Evans, C.V. Desorption of cesium from kaolinite and illite using alkylammonium salts. Appl. Clay Sci. 2004, 25, 125–133. [Google Scholar] [CrossRef]
- Poinssot, C.; Baeyens, B.; Bradbury, M.H. Experimental and modelling studies of caesium sorption on illite. Geochim. Cosmochim. Acta 1999, 63, 3217–3227. [Google Scholar] [CrossRef]
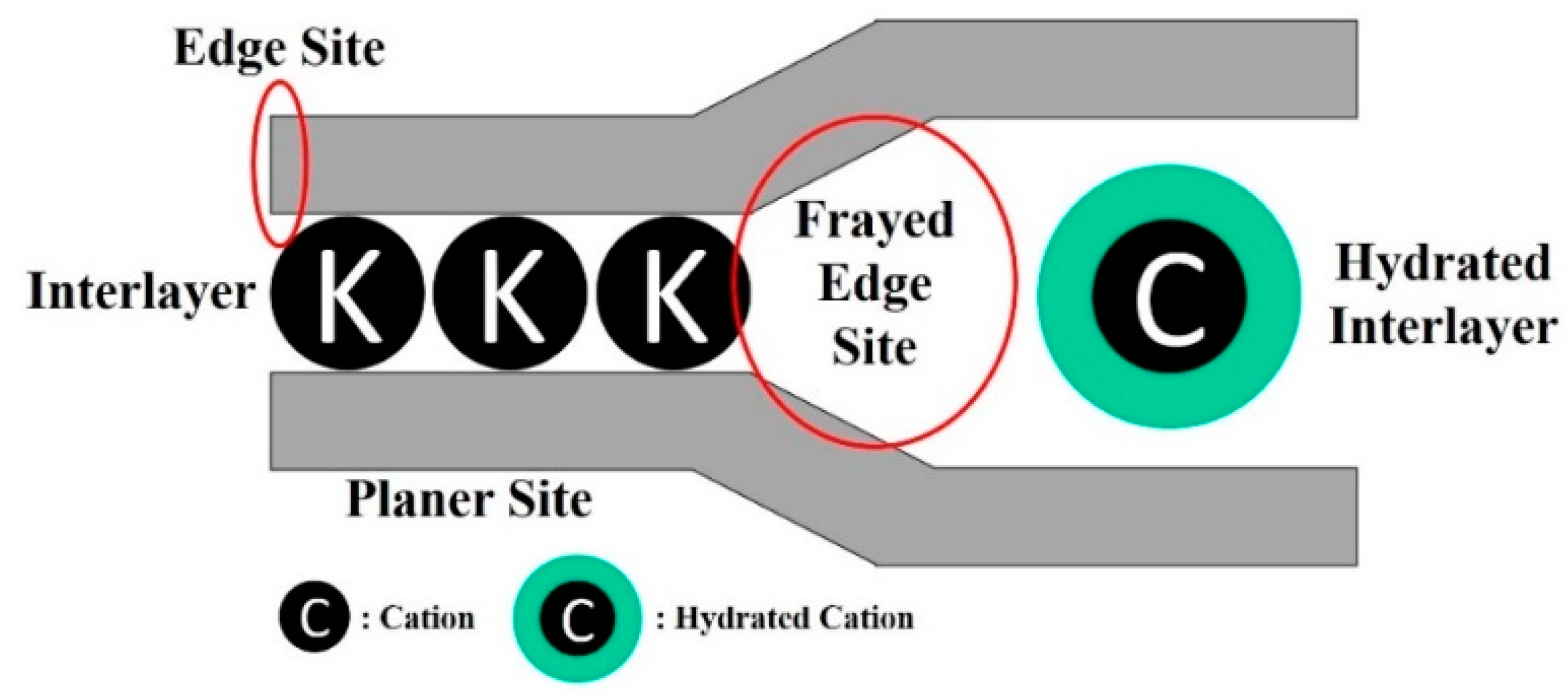
- Okumura, M.; Nakamura, H.; Machida, M. Mechanism of Strong Affinity of Clay Minerals to Radioactive Cesium: First-Principles Calculation Study for Adsorption of Cesium at Frayed Edge Sites in Muscovite. J. Phys. Soc. Jpn. 2013, 82, 033802. [Google Scholar] [CrossRef]
- Park, S.-M.; Alessi, D.S.; Baek, K. Selective adsorption and irreversible fixation behavior of cesium onto 2:1 layered clay mineral: A mini review. J. Hazard. Mater. 2019, 369, 569–576. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.-M.; Jeon, E.-K.; Baek, K. Selective and irreversible adsorption mechanism of cesium on illite. Appl. Geochem. 2017, 85, 188–193. [Google Scholar] [CrossRef]
- Nakao, A.; Thiry, Y.; Funakawa, S.; Kosaki, T. Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand. Soil Sci. Plant Nutr. 2008, 54, 479–489. [Google Scholar] [CrossRef]
- Paulus, W.J.; Komarneni, S.; Roy, R. Bulk synthesis and selective exchange of strontium ions in Na4Mg6Al4Si4O20F4 mica. Nature 1992, 357, 571–573. [Google Scholar] [CrossRef]
- Kodama, T.; Harada, Y.; Ueda, M.; Shimizu, K.; Shuto, K.; Komarneni, S. Selective Exchange and Fixation of Strontium Ions with Ultrafine Na-4-mica. Langmuir 2001, 17, 4881–4886. [Google Scholar] [CrossRef]
- Missana, T.; Garcia-Gutierrez, M.; Alonso, U. Sorption of strontium onto illite/smectite mixed clays. Phys. Chem Earth Parts A/B/C 2008, 33, S156–S162. [Google Scholar] [CrossRef]
- Yu, S.; Mei, H.; Chen, X.; Tan, X.; Ahmad, B.; Alsaedi, A.; Hayat, T.; Wang, X. Impact of environmental conditions on the sorption behavior of radionuclide 90 Sr(II) on Na-montmorillonite. J. Mol. Liq. 2015, 203, 39–46. [Google Scholar] [CrossRef]
- Siroux, B.; Beaucaire, C.; Tabarant, M.; Benedetti, M.F.; Reiller, P.E. Adsorption of strontium and caesium onto an Na-MX80 bentonite: Experiments and building of a coherent thermodynamic modelling. Appl. Geochem. 2017, 87, 167–175. [Google Scholar] [CrossRef]
- Wu, P.; Dai, Y.; Long, H.; Zhu, N.; Li, P.; Wu, J.; Dang, Z. Characterization of organo-montmorillonites and comparison for Sr(II) removal: Equilibrium and kinetic studies. Chem. Eng. J. 2012, 191, 288–296. [Google Scholar] [CrossRef]
- Khan, S.A.; Riaz-ur-Rehman; Khan, M.A. Sorption of strontium on bentonite. Waste Manag. 1995, 15, 641–650. [Google Scholar] [CrossRef]
- Lu, N.; Mason, C.F. Sorption-desorption behavior of strontium-85 onto montmorillonite and silica colloids. Appl. Geochem. 2001, 16, 1653–1662. [Google Scholar] [CrossRef]
- Bellenger, J.-P.; Staunton, S. Adsorption and desorption of 85Sr and 137Cs on reference minerals, with and without inorganic and organic surface coatings. J. Environ. Radioact. 2008, 99, 831–840. [Google Scholar] [CrossRef]
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nuclear Chem. 2012, 293, 613–633. [Google Scholar] [CrossRef]
- Galamboš, M.; Osacký, M.; Rosskopfová, O.; Krajňák, A.; Rajec, P. Comparative study of strontium adsorption on dioctahedral and trioctahedral smectites. J. Radioanal. Nuclear Chem. 2012, 293, 889–897. [Google Scholar] [CrossRef]
- Pshinko, G.N.; Puzyrnaya, L.N.; Kobets, S.A.; Fedorova, V.M.; Kosorukov, A.A.; Demchenko, V.Y. Layered double hydroxide of Zn and Al, intercalated with hexacyanoferrate(II) ions, as a sorbent for removing cesium radionuclides from aqueous solutions. Radiochemistry 2015, 57, 259–265. [Google Scholar] [CrossRef]
- Kameda, T.; Shinmyou, T.; Yoshioka, T. Uptake of Nd3+ and Sr2+ by Li–Al layered double hydroxide intercalated with triethylenetetramine-hexaacetic acid: Kinetic and equilibrium studies. RSC Adv. 2015, 5, 79447–79455. [Google Scholar] [CrossRef]
- Kameda, T.; Shinmyou, T.; Yoshioka, T. Uptake of Nd3+ and Sr2+ by LiAl layered double hydroxides intercalated with ethylenediaminetetraacetate. Mater. Chem. Phys. 2016, 177, 8–11. [Google Scholar] [CrossRef]
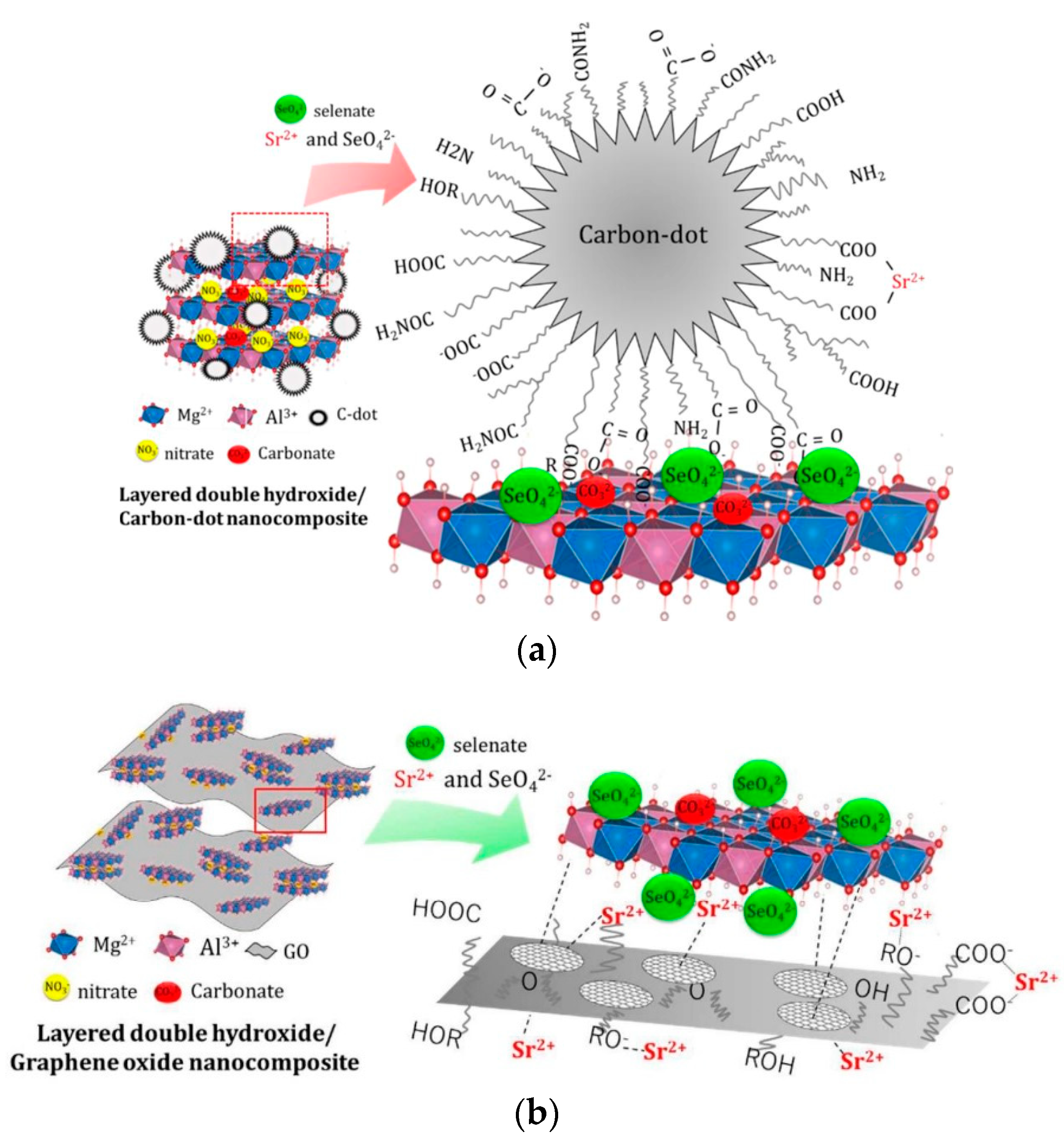
- Koilraj, P.; Kamura, Y.; Sasaki, K. Carbon-Dot-Decorated Layered Double Hydroxide Nanocomposites as a Multifunctional Environmental Material for Co-immobilization of SeO42– and Sr2+ from Aqueous Solutions. ACS Sustain. Chem. Eng. 2017, 5, 9053–9064. [Google Scholar] [CrossRef]
- Koilraj, P.; Kamura, Y.; Sasaki, K. Synergetic co-immobilization of SeO42− and Sr2+ from aqueous solution onto multifunctional graphene oxide and carbon-dot based layered double hydroxide nanocomposites and their mechanistic investigation. J. Mater. Chem. A 2018, 6, 10008–10018. [Google Scholar] [CrossRef]
- Bravo-Suárez, J.J.; Páez-Mozo, E.A.; Oyama, S.T. Review of the synthesis of layered double hydroxides: A thermodynamic approach. Química Nova 2004, 27, 601–614. [Google Scholar] [CrossRef]
- Srankó, D.F. Preparation and Structural Characterization of Alkaline Earth−Iron(III) Layered Double Hydroxides and their Acrylate-Intercalated Derivatives. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2012. [Google Scholar]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Wang, Z.; Sim, A.; Urban, J.J.; Mi, B. Removal and Recovery of Heavy Metal Ions by Two-dimensional MoS2 Nanosheets: Performance and Mechanisms. Environ. Sci. Technol. 2018, 52, 9741–9748. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]






| Heavy Metal | Adsorbent | Maximum Adsorption Capacity (mmol·g−1 ) | Ref. |
|---|---|---|---|
| Cd (II) | Smectite | 8.64 | [34] |
| Co (II) | Chemically treated bentonite | 2.34 | [35] |
| Cr (III/VI) | Polyaniline/Montmorillonite composite | 5.94 | [36] |
| Cu (II) | Bentonite | 0.85 | [37] |
| Hg (II) | Montmorillonite | 1.92 | [38] |
| Mn (II) | Kaolinite | 2.72 | [39] |
| Ni (II) | Kaolinite | 2.40 | [40] |
| Pb (II) | Illite | 1.15 | [41] |
| Zn (II) | Kaolinite | 3.82 | [42] |
| Authors | Date | Phyllosilicate Structure | Organic Compounds | Cations/Anions Adsorbed | Capacity of Cations Sorption (mmol·g−1) | Ref. |
|---|---|---|---|---|---|---|
| Fonseca et al. | 2000 | Talc | Thiol group | Co Ni Cu Zn | Cu2+ = 4.31 Ni2+ = 3.44 Co2+ =3.03 Zn2+ = 2.60 | [54] |
| Fonseca et al. | 2000 | Mg-Talc Cu-Talc | Thiol group | Cu | Cu(Mg-Talc-Thiol) = 5.93 > Cu(Cu-talc-Thiol) | [46] |
| Fonseca et al. | 2000 | Talc | 3-aminopropylamine group = SILMg1 N-propylethylenediamine group = SILMg2 | Co Ni Cu Zn | Cu2+ [8.09, 4.53] > Zn2+ [6.49, 2.41] > Ni2+ [5.55, 3.39] > Co2+ [2.06, 1.24] | [61] |
| Fonseca et al. | 2000 | Ni-Talc Ni located in octahedral sites Or Ni complexed with amino groups and located in the interlayer space | Amino group | Ni | Octahedral Ni [1.78–2.65] Interlamellar Ni [1.58–2.49] | [56] |
| Lagadic et al. | 2000 | Talc | Thiol group | Hg Pb Cd | Hg = 3.01 Cd = 1.87 Pb = 1.76 | [58] |
| Jaber et al. | 2005 | Saponite | Thiol group | Hg Cu Pb | Hg2+:100% Cd2+:90% Pb2+:50% | [59] |
| Sales et al. | 2006 | Talc | (1) Cl-group (2) 5-amino-1.3.4-thiadiazole-2-thiol molecule | Hg | Talc-2 Hg = 0.19 | [44] |
| Moscofian et al. | 2008 | Talc | Nitrogen and sulfur basic centers | Cu Pb Cd | Cu = 3.28 Pb = 1.42 Cd = 0.35 | [62] |
| Dey et al. | 2009 | Talc | Organosilane based on 3-glycidoxypropryltrimethoxysilane and thiourea | Cr Mn Zn | Cr(III) > Mn(II) > Zn(II) | [57] |
| Melo et al. | 2010 | Cobalt-Talc | Nitrogen and oxygen basic centers (Ethanolamines Diethanolamines) | Cu Pb | Ethanolamines: Cu = 2.01 Pb = 2.59 Diethanolamines: Cu = 2.55 Pb = 2.43 | [63] |
| Badshah et al. | 2011 | Talc | Nitrogen and sulfur basic centers | Cu Pb Cd | Cu = 4.01 Pb = 7.08 Cd = 1.86 | [60] |
| Lee et al. | 2011 | Talc | Amino group | CrO42− Fe(CN)63− | Removal efficiency: Chromate: 89.54% Ferricyanide: 97.43% | [64] |
| Heavy Metal | Adsorbent (LDH) | Maximum Adsorption Capacity (mmol·g−1) | Ref |
|---|---|---|---|
| Cd (II) | Graphite oxide aerogels/MgAl | 0.85 | [88] |
| Cr (III/VI) | Fe2+/MgAl | 12.5 | [89] |
| Cu (II) | MoS42−/MgAl | 2.85 | [85] |
| Co (II) | Polysulfide/MgAl | 1.41 | [86] |
| Hg (II) | Polysulfide/MgAl | 4.05 | [86] |
| Ni (II) | Polysulfide/MgAl | 1.81 | [86] |
| Pb (II) | CaFe2O4/polyophenylenediamine/MgAl | 4.83 | [90] |
| Zn (II) | Polysulfide/MgAl | 2.22 | [86] |
| Author | Support | Model Used | Interaction Nature | Capacity |
|---|---|---|---|---|
| Paulus et al. [103] (1992) | Synthetic highly charged Na fluorophlogopite mica (Na4Mg6Al4S4O20F4) | Distribution coefficient Kd (ratio of the amount of strontium sorbed per gram of solid) | Sr2+ trapped into the interlayer space of the mica | 2.31 mmol·g−1 (~44% of the theoretical exchange capacity of Na-4-mica) |
| Khan et al. [109] (1995) | Bentonite (from Shina Bagh. Kala Chita Forest. Attock. Pakistan) | Freundlich and Langmuir isotherms. Dubinin–Radush–Kevich (D-R) equation | Mean free energy for adsorption. E ~ 9 kJ/mol → ion exchange | sorption increases with pH (99% at pH 8.5) |
| Kodama et al. [104] (2001) | Synthetic Na-4-Mica | First-Order Kinetic Model Freundlich modelParabolic Diffusion Model Elovich Model | ion exchange of 2 Na+ by 1 Sr2+ | 0.011 mmol·g−1 |
| Lu et al. [110] (2001) | Ca-montmorillonite (MMT) water collected in 1998 at DP Canyon water collected in 1994 at Fortymile Wash. (Nevada) 85Sr solution | Distribution coefficient Kd | Surface complexation and isotope exchange with Ca2+ | 92–100% of the 85Sr quickly adsorbed |
| Bellenger et al. [111] (2008) | Kaolinite Montmorillonite Illite Al oxide-coating Fe oxide-coating Organic coating | Distribution coefficient Kd | Sr adsorption similar between three phyllosilicates Kd ~ 1000 dm3·kg−1 Sr ads. ↑ by Al-coating Not affected by other coatings Adsorption irreversible | |
| Missana et al. [105] (2008) | Illite/smectite mixtures | Ionic exchange and surface complexation modeling (non-electrostatic model) | Single clay: Ionic exchange | pH [2,3,4,5,6,7,8,9] Kd = 4580 mmol·g−1 pH > 9 Kd = 4000–11500 mmol·g−1 |
| Galambos et al. [112] (2009) | Slovak bentonites | Langmuir isotherm | Cation-exchange mechanism | Sr sorption ↑ when: pH ↑ metal C°↓ |
| Galambos et al. [113] (2012) | Slovak bentonites: Montmorillonite Saponite Hectorite | Distribution coefficient Adsorption percentage Adsorption capacity | Basal surface and edges sites: Cation-exchange mechanism | The authors advised against using Fe-rich smectite to store radioactive waste |
| Wu et al. [108] (2012) | Organo-montmorillonites: Ca-Mt APTES-Mt: grafted with 3-aminopropyl triethoxysilane SDS-Mt: surface modified by sodium dodecyl sulfate HDTMAB-Mt: surface modified by hexadecyl trimethyl ammonium bromide | Pseudo-second-order model SDS-Mt: DKR and Langmuir models Ca-Mt and APTES-Mt: Freundlich model for | Ca-Mt: ion exchange SDS-Mt: surface adsorption APTES-Mt: ligand adsorption | APTES-Mt: 0.75 mmol·g−1 Ca-Mt:0.15 mmol·g−1 SDS-Mt:0.3064 mmol·g−1 HDTMAB-Mt: 0.04 mmol·g−1 |
| Yu et al. [106] (2015) | Na-Montmorillonite | Diffuse-layer model (Sr sorption simulation) Langmuir and Freundlich models (sorption isotherms simulations) | At low pH: outer-sphere surface complexation and ion exchange. At high pH: inner-sphere surface complexation | Sorption capacity of 0.12 mmol·g−1 |
| Siroux et al. [107] (2017) | MX80 bentonite (purified and conditioned under Na-saturated) | Multi-site ion exchange model. Ion exchange theory | 2Na+/Sr2+ exchange | 0.886 mmol·g−1 |
| Noxious Element | Adsorbent | Maximum Adsorption Capacity (mmol·g−1) | Ref |
|---|---|---|---|
| Heavy Metals | |||
| Cd (II) | Smectite | 8.64 | [34] |
| Cr (III/VI) | Fe2+/MgAl (LDH) | 12.5 | [89] |
| Cu (II) | Synthetic Talc with Nitrogen basic centers | 8.09 | [61] |
| Co (II) | Synthetic Talc with Thiol group | 3.03 | [54] |
| Hg (II) | Polysulfide/MgAl (LDH) | 4.05 | [86] |
| Ni (II) | Kaolinite | 2.4 | [40] |
| Pb (II) | Synthetic Talc with Nitrogen and sulfur basic centers | 7.08 | [60] |
| Zn (II) | Synthetic Talc with Nitrogen basic centers | 6.49 | [61] |
| Radionuclides | |||
| Cs | Montmorillonite | 0.78 | [114] |
| Vermiculite | 0.27 | ||
| Illite | 0.15 | ||
| Sr | Synthetic Na-4-Mica | 2.31 | [103] |
| MgAl-NO3 (LDH) | 1.79 | [118] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claverie, M.; Garcia, J.; Prevost, T.; Brendlé, J.; Limousy, L. Inorganic and Hybrid (Organic–Inorganic) Lamellar Materials for Heavy Metals and Radionuclides Capture in Energy Wastes Management—A Review. Materials 2019, 12, 1399. https://doi.org/10.3390/ma12091399
Claverie M, Garcia J, Prevost T, Brendlé J, Limousy L. Inorganic and Hybrid (Organic–Inorganic) Lamellar Materials for Heavy Metals and Radionuclides Capture in Energy Wastes Management—A Review. Materials. 2019; 12(9):1399. https://doi.org/10.3390/ma12091399
Chicago/Turabian StyleClaverie, Marie, Justo Garcia, Thierry Prevost, Jocelyne Brendlé, and Lionel Limousy. 2019. "Inorganic and Hybrid (Organic–Inorganic) Lamellar Materials for Heavy Metals and Radionuclides Capture in Energy Wastes Management—A Review" Materials 12, no. 9: 1399. https://doi.org/10.3390/ma12091399
APA StyleClaverie, M., Garcia, J., Prevost, T., Brendlé, J., & Limousy, L. (2019). Inorganic and Hybrid (Organic–Inorganic) Lamellar Materials for Heavy Metals and Radionuclides Capture in Energy Wastes Management—A Review. Materials, 12(9), 1399. https://doi.org/10.3390/ma12091399