Decomposition Characteristics of SF6 under Flashover Discharge on the Epoxy Resin Surface
Abstract
:1. Introduction
2. Materials and Methods
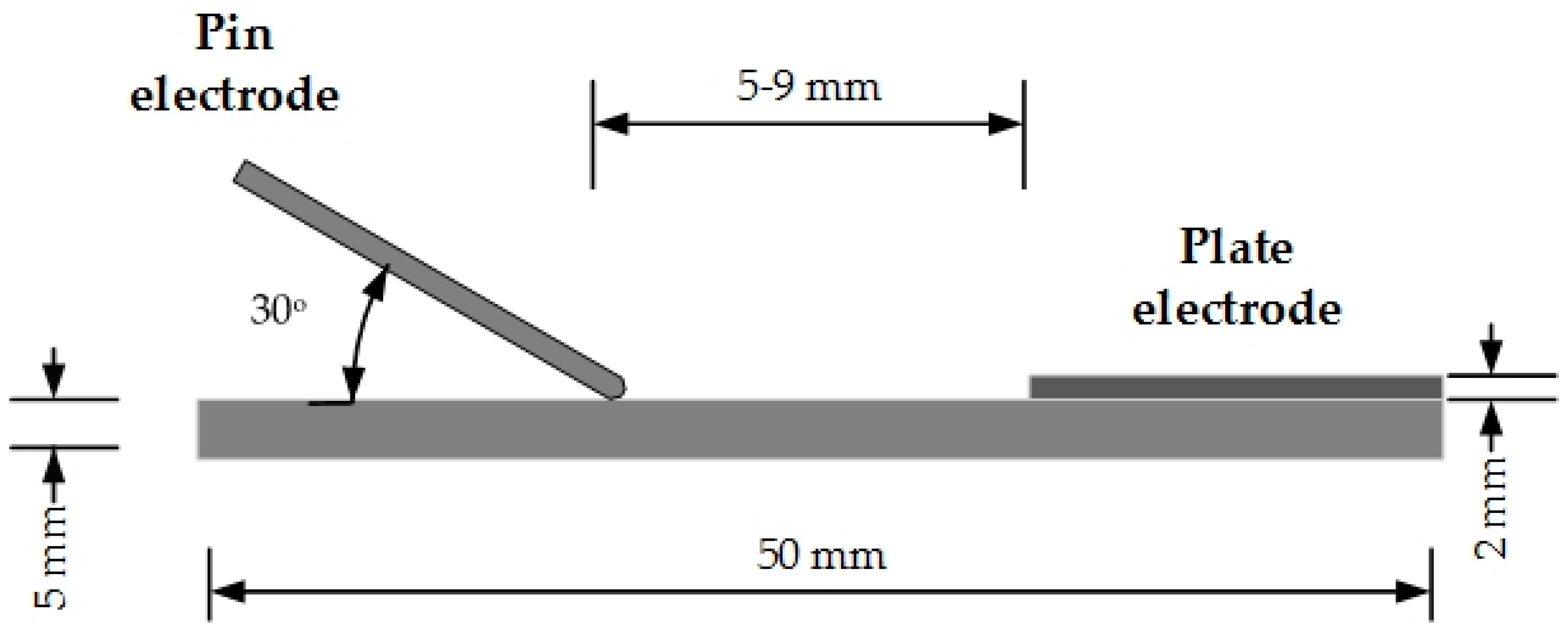
2.1. Materials
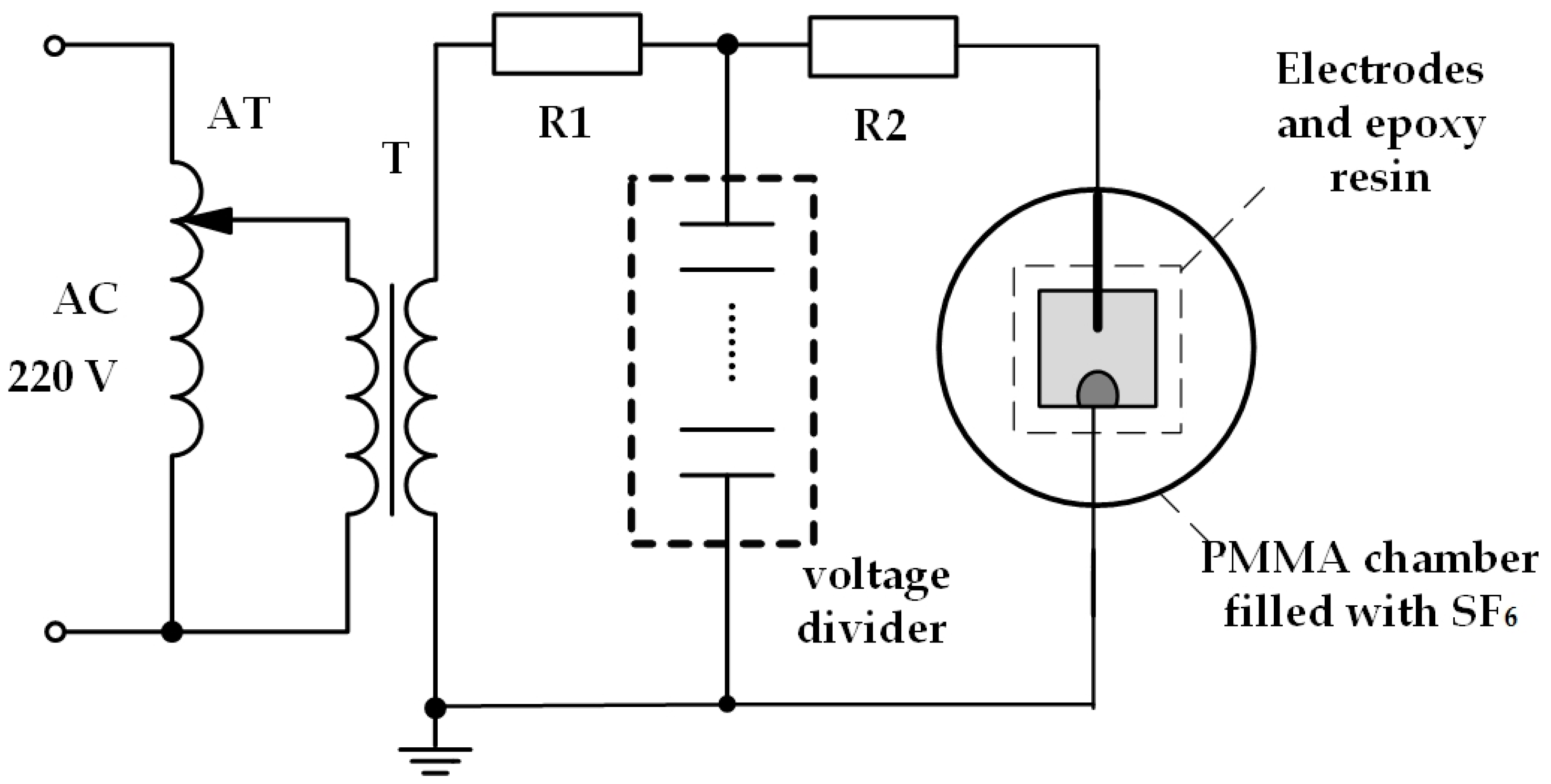
2.2. Experiment Platform
2.3. Experiment Procedures
3. Results and Discussion
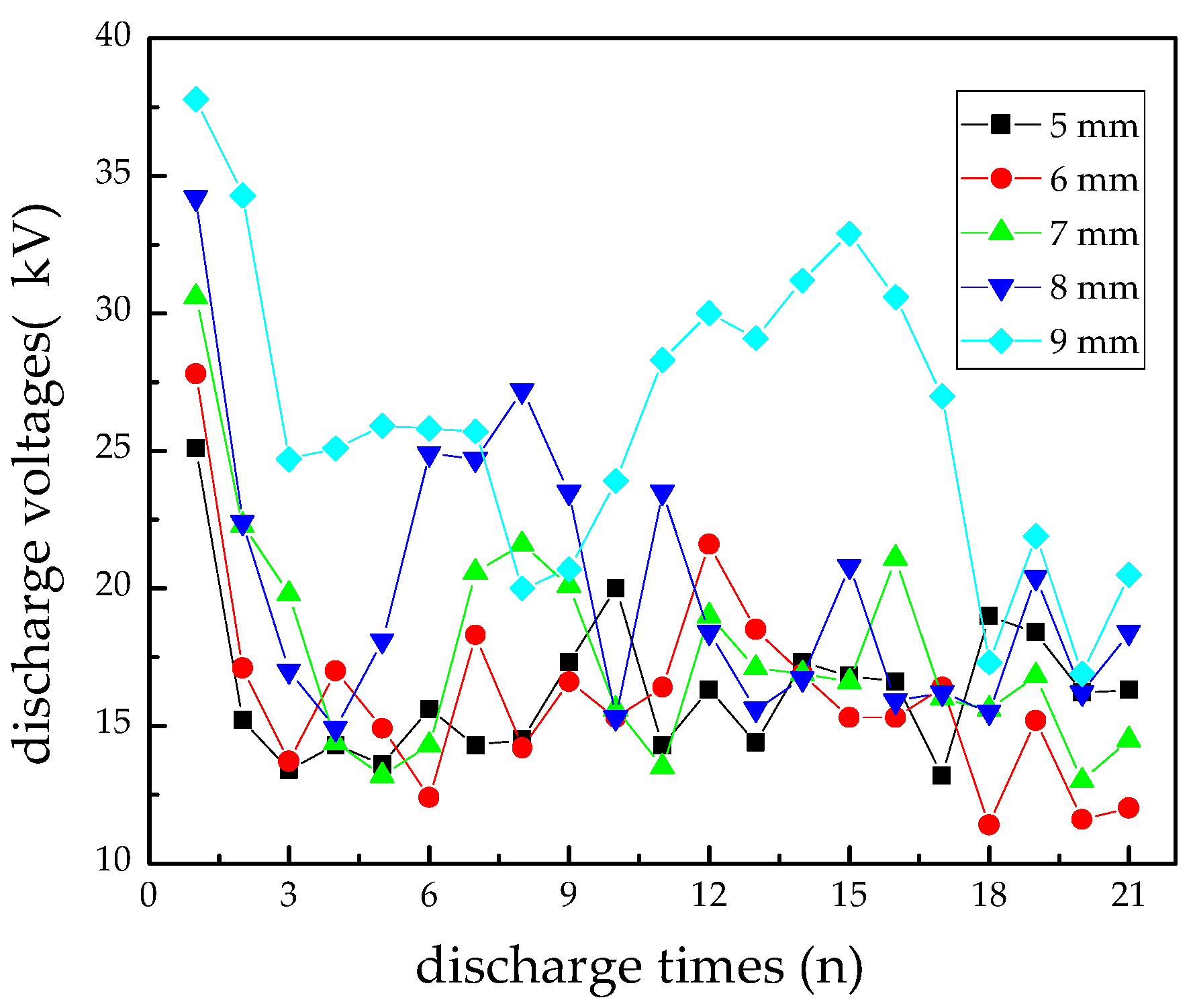
3.1. Discharge Voltages Comparison
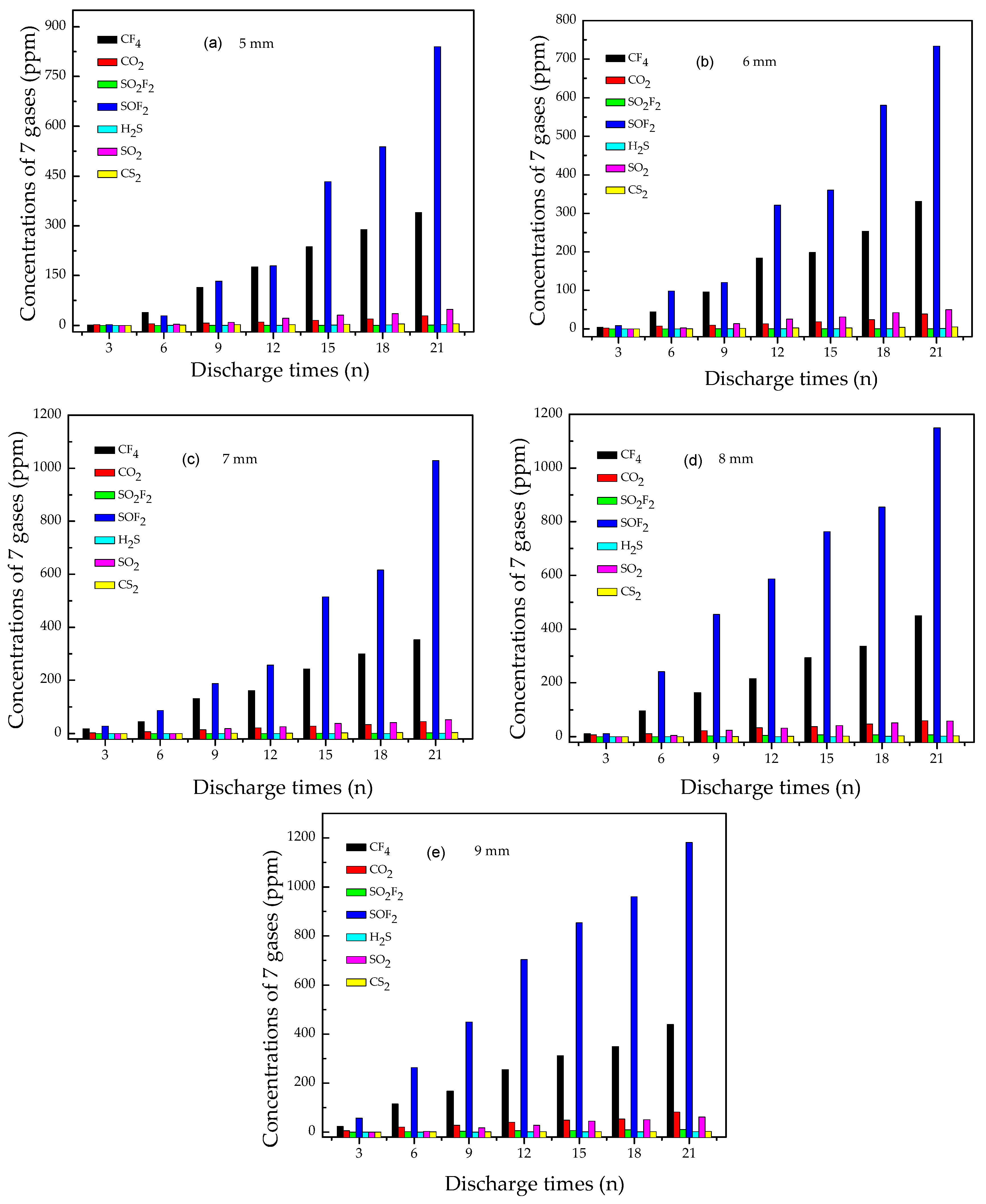
3.2. Concentration Variation of Seven Characteristic Gases
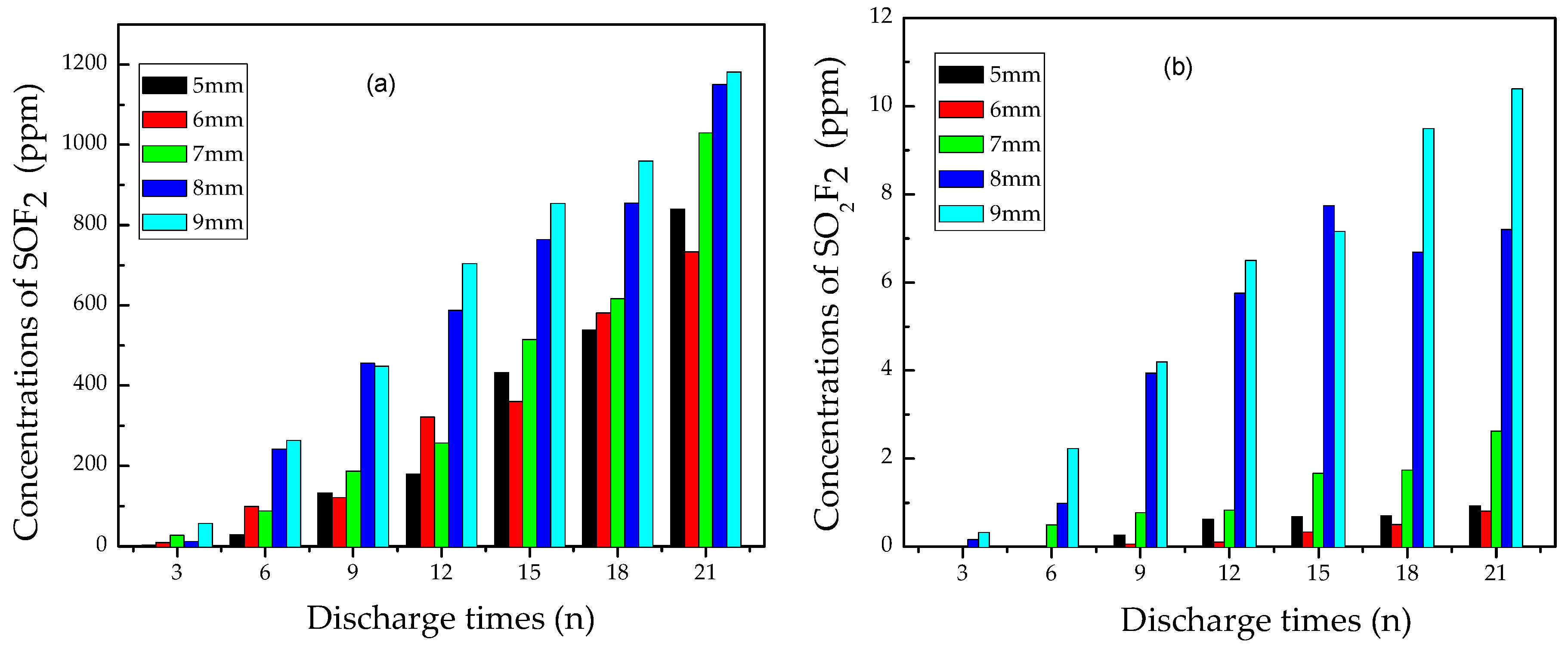
3.2.1. Analysis of SOF2 and SO2F2
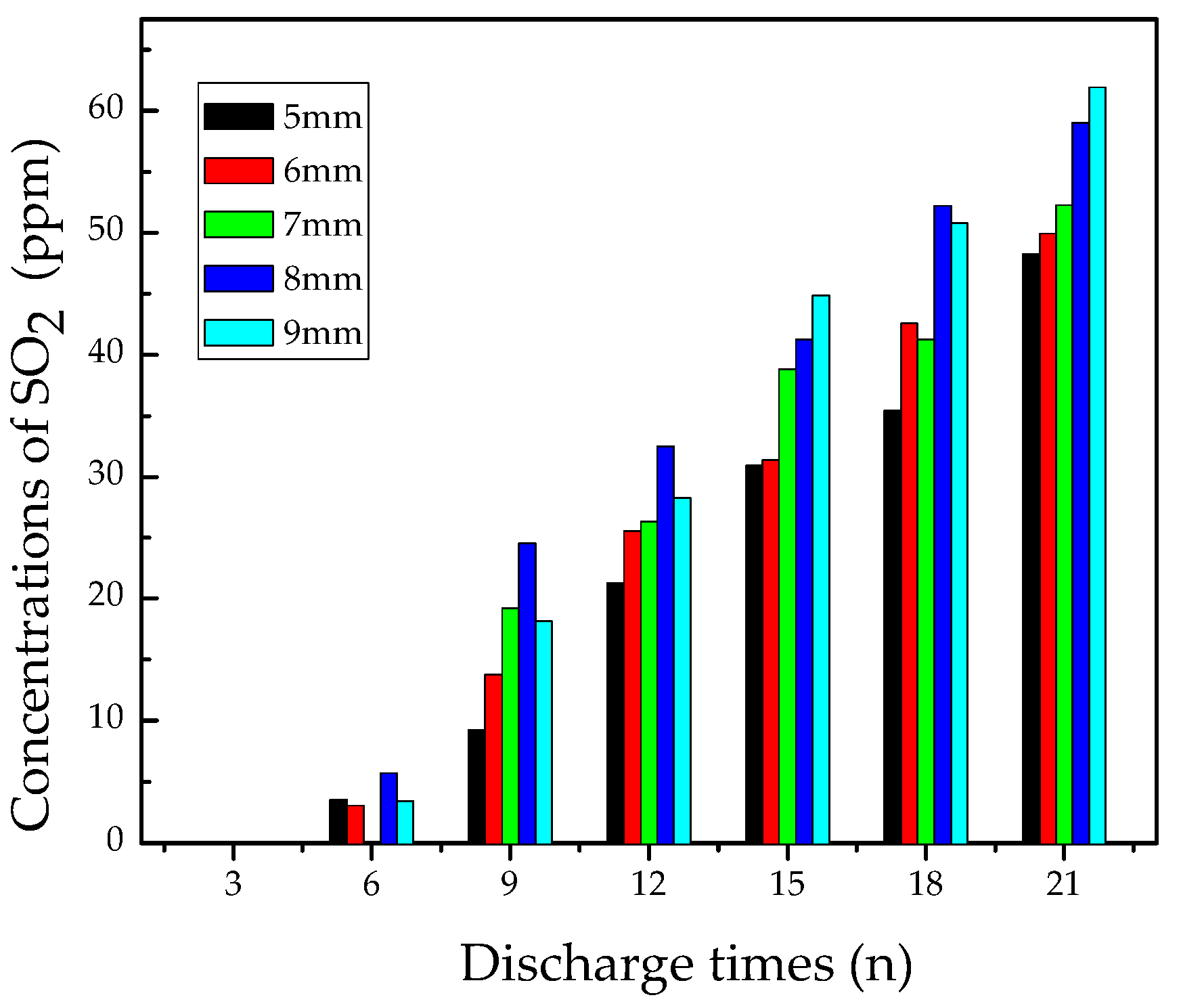
3.2.2. Analysis of SO2
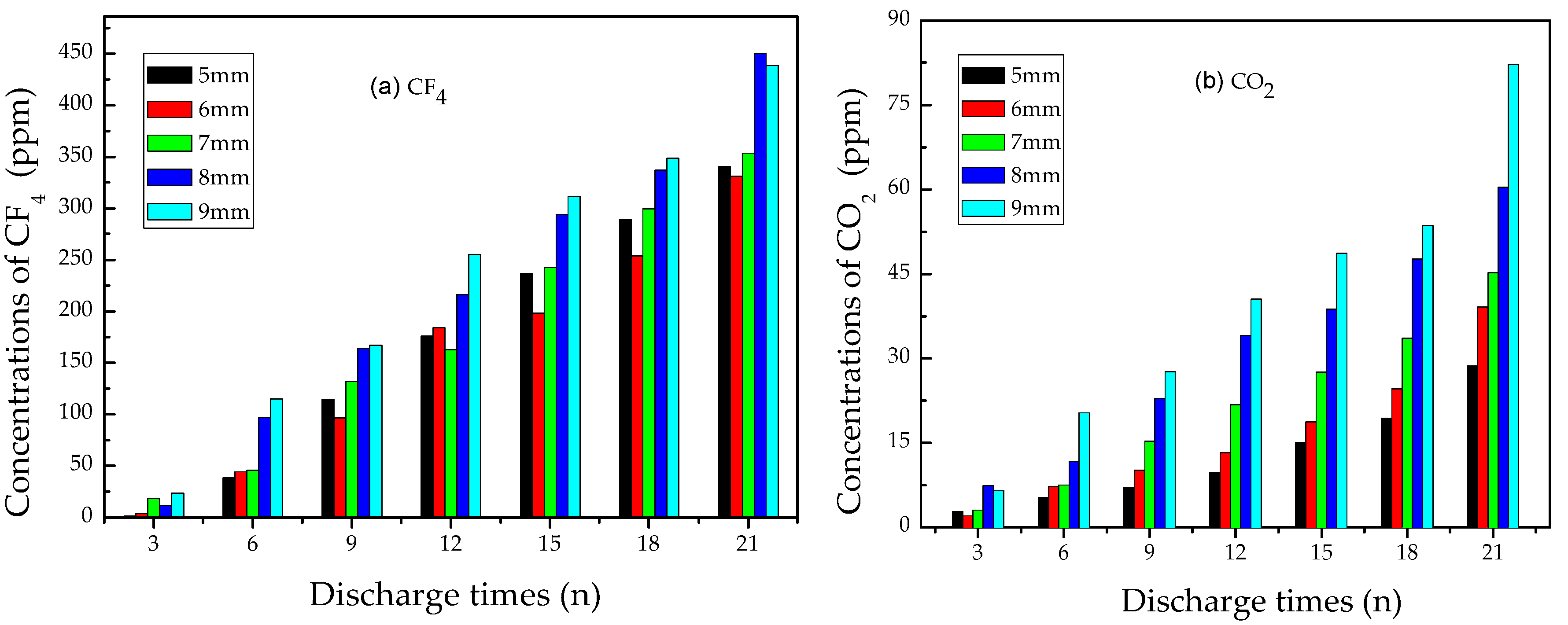
3.2.3. Analysis of CO2 and CF4
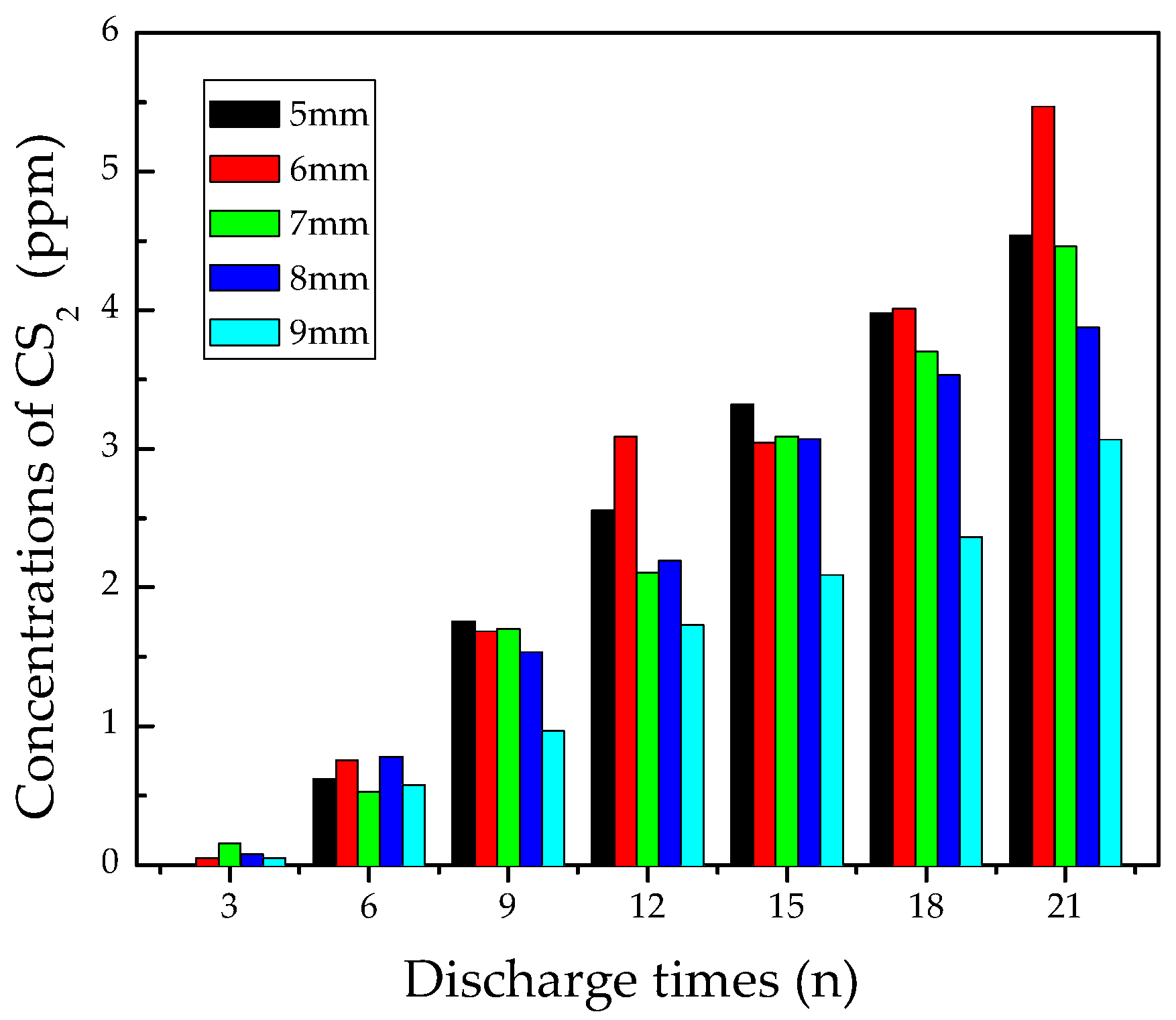
3.2.4. Analysis of CS2
3.2.5. Analysis of H2S
3.3. Basic Rules for Judging the Happening of Flashover Discharge
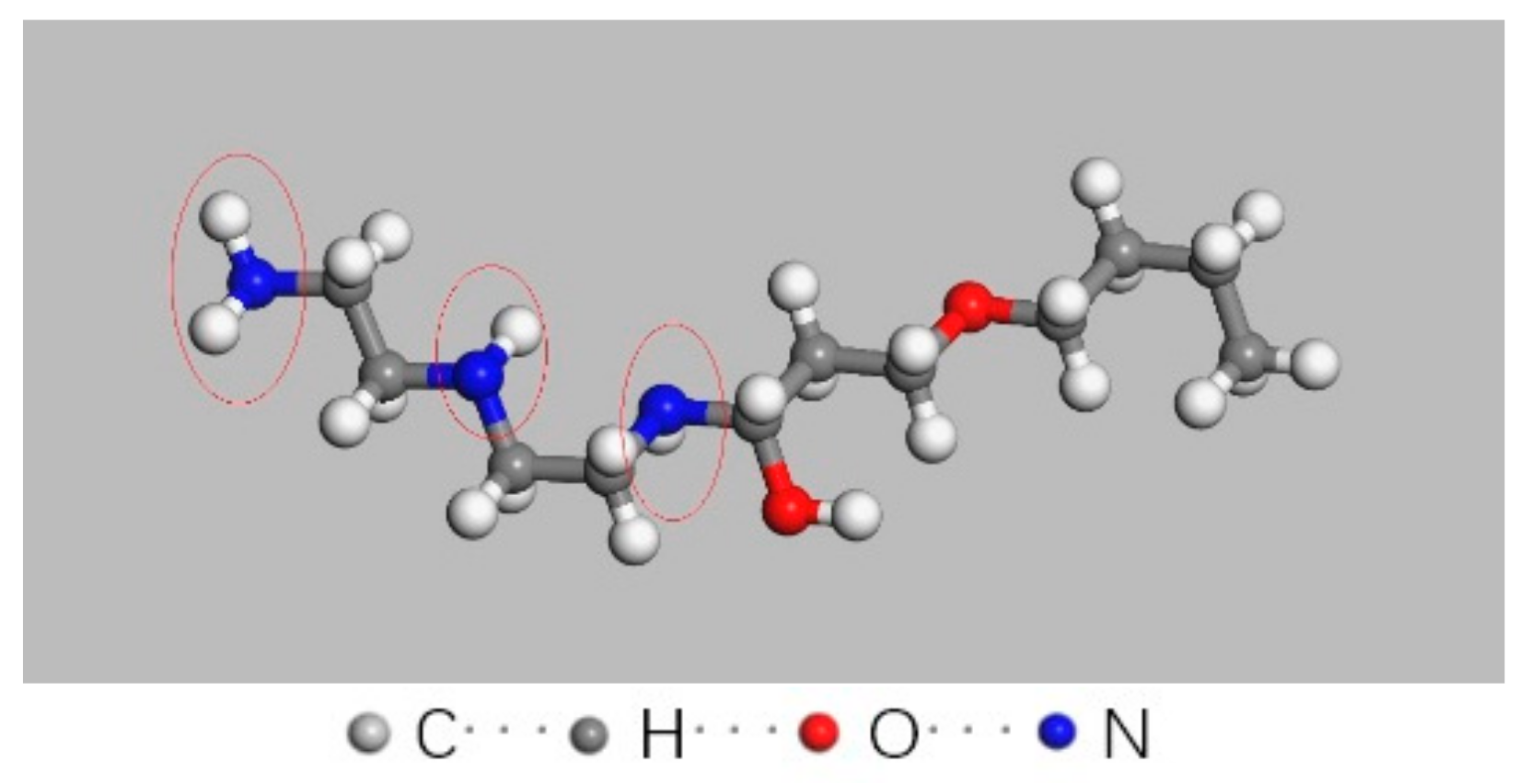
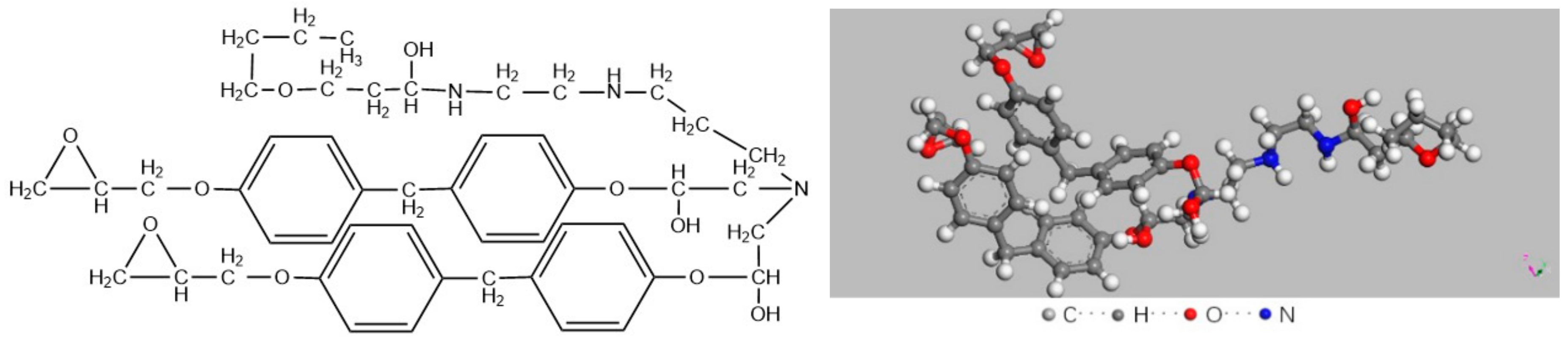
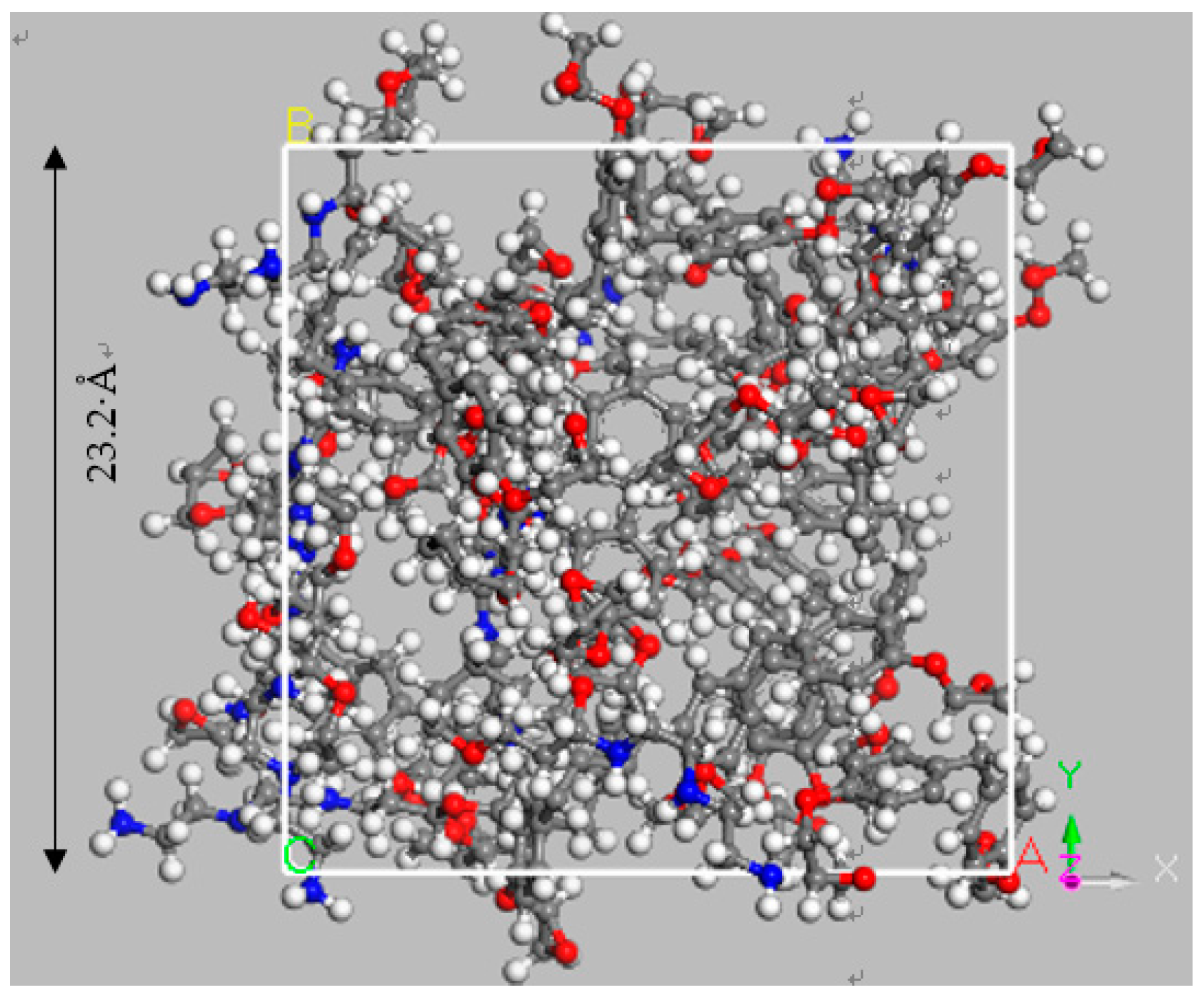
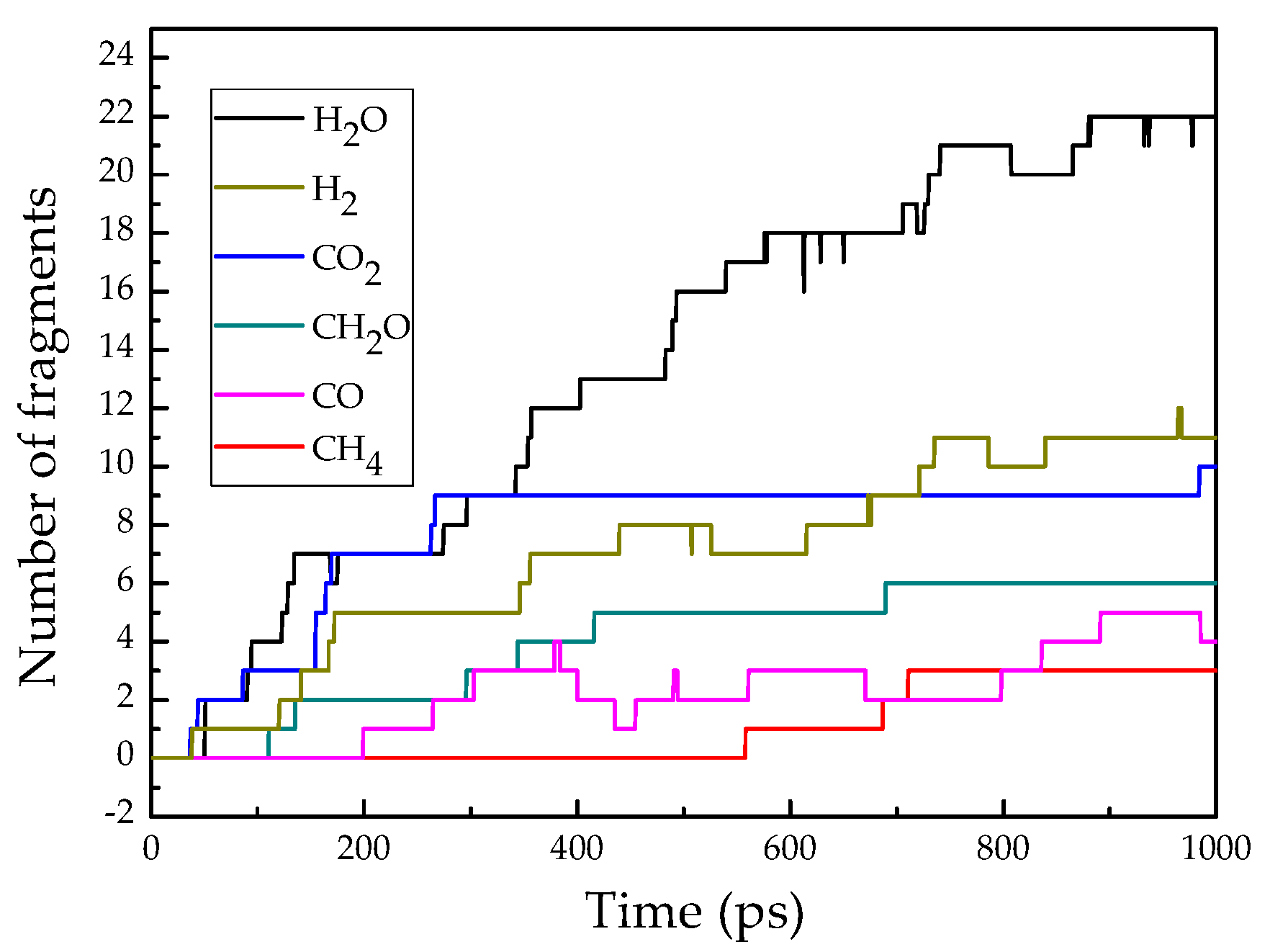
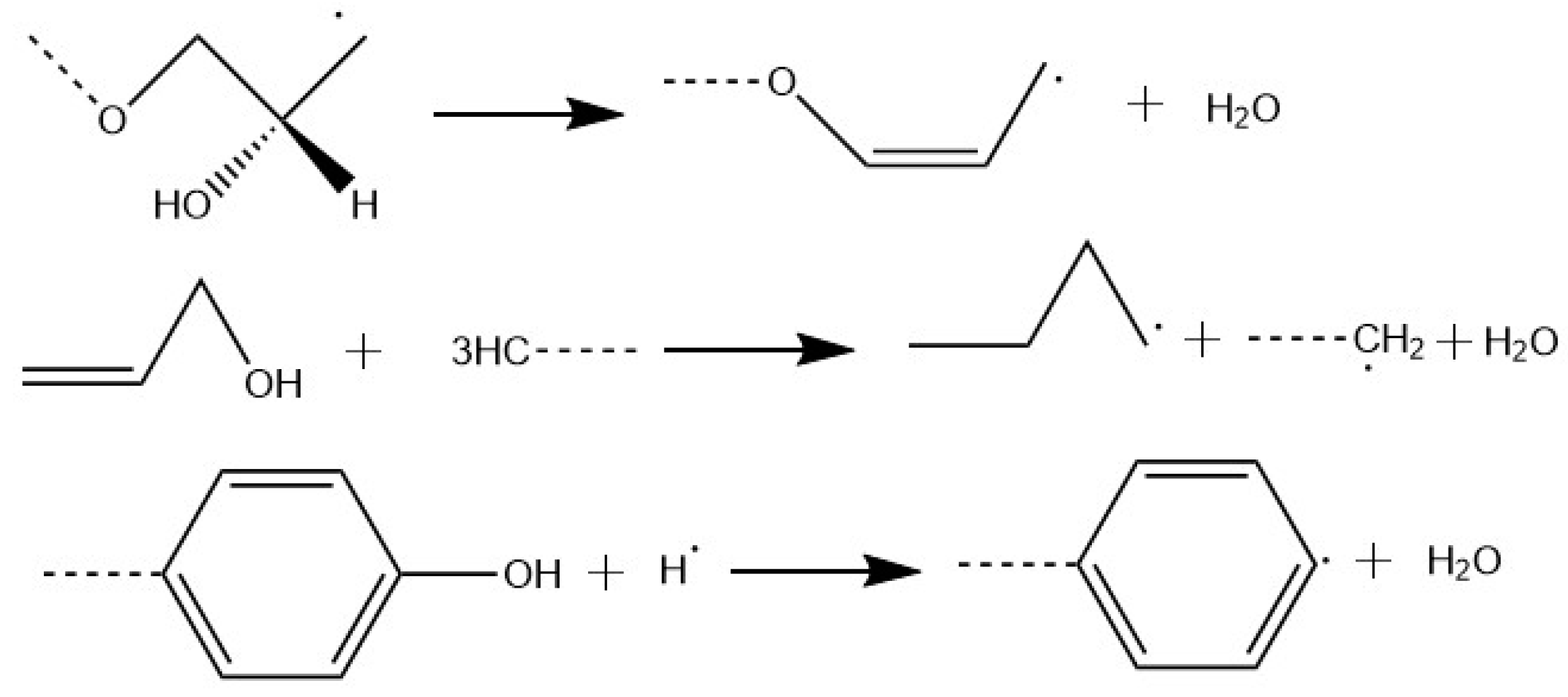
3.4. Simulation for Decomposition of Epoxy Resin under High Energy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qi, B.; Li, C.; Xing, Z.; Wei, Z. Partial Discharge Initiated by Free Moving Metallic Particles on GIS Insulator Surface: Severity Diagnosis and Assessment. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 766–774. [Google Scholar] [CrossRef]
- Albano, M.; Haddad, A.; Griffiths, H.; Coventry, P. Environmentally Friendly Compact Air-Insulated High-Voltage Substations. Energies 2018, 11, 2492. [Google Scholar] [CrossRef]
- Okubo, H. Recent activity and future trend on ageing characteristics of electrical insulation in GIS from manufacturer’s view point. In Proceedings of 1994 4th International Conference on Properties and Applications of Dielectric Materials (ICPADM), Brisbane, Australia, 3–8 July 1994; IEEI: Piscataway, NJ, USA, 1994; Volume 2, pp. 837–840. [Google Scholar]
- Eriksson, A.; Pettersson, K.G.; Krenicky, A.; Baker, R.; Ochoa, J.R.; Leibold, A. Experience with gas insulated substations in the USA. IEEE Trans. Power Deliv. 1994, 10, 210–218. [Google Scholar] [CrossRef]
- Piemontesi, M.; Niemeyer, L. (Eds.) Sorption of SF6 and SF6 decomposition products by activated alumina and molecular sieve 13X. In Proceedings of Conference Record of the 1996 IEEE International Symposium on Electrical Insulation, Montreal, QC, Canada, 16–19 June 1996; IEEI: Piscataway, NJ, USA, 1964; Volume 2, pp. 828–838. [Google Scholar]
- Beyer, C.; Jenett, H.; Klockow, D. Influence of reactive SFx gases on electrode surfaces after electrical discharges under SF6 atmosphere. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Qiu, Y.; Kuffel, E. Comparison of SF6/N2 and SF6/CO2 gas mixtures as alternatives to SF6 gas. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 892–895. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.X.; Ren, X.L. Characteristics of the Concentration Ratio of SO2F2 to SOF2 as the Decomposition Products of SF6 Under Corona Discharge. IEEE. Trans. Plasma Sci. 2012, 40, 56–63. [Google Scholar] [CrossRef]
- Tang, J.; Pan, J.; Yao, Q.; Miao, Y.; Huang, X.; Zeng, F. Feature extraction of SF6 thermal decomposition characteristics to diagnose overheating fault. IET Sci. Meas. Technol. L 2015, 9, 751–757. [Google Scholar] [CrossRef]
- Tang, J.; Rao, X.; Tang, B.; Liu, X.; Gong, X.; Zeng, F.; Yao, Q. Investigation on SF6 spark decomposition characteristics under different pressures. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2066–2075. [Google Scholar] [CrossRef]
- Sauers, I. Evidence for SF4 and SF2 formation in SF6 corona discharges. Presented at the Electrical Insulation and Dielectric Phenomena Conference, Knoxville, TN, USA, 20–24 October 1991. [Google Scholar]
- Derdouri, A.; Casanovas, J.; Grob, R.; Mathieu, J. Spark decomposition of SF6/H2O mixtures. IEEE Trans. Electr. Insul. 1990, 24, 1147–1157. [Google Scholar] [CrossRef]
- Sauers, I.; Christophorou, L.G.; Spyrou, S.M. Negative ion formation in compounds relevant to SF6 decomposition in electrical discharges. Plasma Chem. Plasma Process. 1993, 13, 17–35. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.; Zhou, J. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Belmadani, B.; Casanovas, J.; Casanovas, A.M. SF6 decomposition under power arcs. II. Chemical aspects. IEEE Trans. Electr. Insul. 1991, 26, 1177–1182. [Google Scholar] [CrossRef]
- Chu, F.Y. SF6 Decomposition in Gas-Insulated Equipment. IEEE Trans. Electr. Insul. 1986, 21, 693–725. [Google Scholar] [CrossRef]
- Hergli, R.; Casanovas, J.; Derdouri, A.; Grob, R.; Mathieu, J. Study of the Decomposition of SF6 in the Presence of Water, Subjected to Gamma Irradiation or Corona Discharges. IEEE Trans. Electr. Insul. 2002, 23, 451–465. [Google Scholar] [CrossRef]
- Griffin, G.D.; Sauers, I.; Kurka, K.; Easterly, C.E. Spark decomposition of SF6: Chemical and biological studies. IEEE Trans. Power Deliv.r 1989, 4, 1541–1551. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, W.; Yu, J.; Su, Q.; Zheng, X.; Zhou, Y.; Li, L.; Yao, W.; Wang, B.; Hu, H. Insulation condition monitoring of epoxy spacers in GIS using a decomposed gas CS2. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 2152–2157. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Park, S.-J. Nanodiamond nanocluster-decorated graphene oxide/epoxy nanocomposites with enhanced mechanical behavior and thermal stability. Compos. B Eng. 2017, 114, 111–120. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A: Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Son, Y.-R.; In, I.; An, K.-H.; Kim, B.-J.; Park, S.-J. Recent advanced thermal interfacial materials: A review of conducting mechanisms and parameters of carbon materials. Carbon 2019, 142, 445–460. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.P.; Pan, J.Y.; Zhang, X.X. Correlation Analysis between Formation Process of SF6 Decomposed Components and Partial Discharge Qualities. IEEE. Trans. Dielect. Electr. Insul. 2013, 20, 864–876. [Google Scholar] [CrossRef]
- Fukunaga, K.; Takai, H. Deterioration of epoxy resin as a result of internal partial discharge. Electr. Eng. Jpn. 2010, 111, 11–19. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, X.; Zhang, C.; Wang, R.; Rao, Z.; Shao, T. Aging Characteristics on Epoxy Resin Surface Under Repetitive Microsecond Pulses in Air at Atmospheric Pressure. Plasma Sci. Technol. 2016, 18, 325–330. [Google Scholar] [CrossRef]
- Brunt, R.J.V.; Sauers, I. Gas-phase hydrolysis of SOF2 and SOF4. J. Chem. Phys. 1986, 85, 4377–4380. [Google Scholar] [CrossRef]
- Brunt, R.J.V.; Herron, J.T. Plasma chemical model for decomposition of SF6 in a negative glow corona discharge. Phys. Scr. 1994, T53, 9. [Google Scholar] [CrossRef]
- Fan, L.; Ju, T.; Liu, Y. Mathematical model of influence of oxygen and moisture on feature concentration ratios of SF6 Decomposition Products. In Proceedings of the 2012 IEEE Power and Energy Society General Meeting, San Diego, CA, USA, 22–26 July 2012; pp. 1–5. [Google Scholar]
- Zeng, F.; Tang, J.; Zhang, X.; Sun, H.; Yao, Q.; Miao, Y. Study on the influence mechanism of trace H2O on SF6 thermal decomposition characteristic components. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 367–374. [Google Scholar] [CrossRef]
- Diao, Z.; Zhao, Y.; Chen, B.; Duan, C.; Song, S. ReaxFF reactive force field for molecular dynamics simulations of epoxy resin thermal decomposition with model compound. J. Anal. Appl. pyrolysis 2013, 104, 618–624. [Google Scholar] [CrossRef]


| Gap Distances (mm) | 5 | 6 | 7 | 8 | 9 |
| Roughness/(μm) | 0.7 | 0.761 | 0.859 | 0.807 | 0.887 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Zhang, X.; Xia, R.; Hu, G.; Wu, Y. Decomposition Characteristics of SF6 under Flashover Discharge on the Epoxy Resin Surface. Materials 2019, 12, 1408. https://doi.org/10.3390/ma12091408
Wen H, Zhang X, Xia R, Hu G, Wu Y. Decomposition Characteristics of SF6 under Flashover Discharge on the Epoxy Resin Surface. Materials. 2019; 12(9):1408. https://doi.org/10.3390/ma12091408
Chicago/Turabian StyleWen, Hao, Xiaoxing Zhang, Rong Xia, Guoxiong Hu, and Yunjian Wu. 2019. "Decomposition Characteristics of SF6 under Flashover Discharge on the Epoxy Resin Surface" Materials 12, no. 9: 1408. https://doi.org/10.3390/ma12091408





