Thermal Conductivity of Molten Carbonates with Dispersed Solid Oxide from Differential Scanning Calorimetry
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frangini, S.; Masi, A. Molten carbonates for advanced and sustainable energy applications: Part I. Revisiting molten carbonate properties from a sustainable viewpoint. Int. J. Hydrogen Energy 2016, 41, 18739–18746. [Google Scholar] [CrossRef]
- Fan, L.D.; He, C.X.; Zhu, B. Role of carbonate phase in ceria-carbonate composite for low temperature solid oxide fuel cells: A review. Int. J. Energy Res. 2017, 41, 465–481. [Google Scholar] [CrossRef]
- Sang, L.; Li, F.; Xu, Y. Form-stable ternary carbonates/MgO composite material for high temperature thermal energy storage. Sol. Energy 2019, 180, 1–7. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Solheim, A.; Kjelstrup, S.; Haarberg, G.M. Electrolyte Melt Compositions for Low Temperature Molten Carbonate Thermocells. ACS Appl. Energy Mater. 2018, 1, 5386–5393. [Google Scholar] [CrossRef]
- Serrano-Lopez, R.; Fradera, J.; Cuesta-Lopez, S. Molten salts database for energy applications. Chem. Eng. Process. 2013, 73, 87–102. [Google Scholar] [CrossRef]
- Turchi, C.S.; Vidal, J.; Bauer, M. Molten salt power towers operating at 600–650 °C: Salt selection and cost benefits. Sol. Energy 2018, 164, 38–46. [Google Scholar] [CrossRef]
- Kang, X.; Børset, M.T.; Burheim, O.S.; Haarberg, G.M.; Xu, Q.; Kjelstrup, S. Seebeck coefficients of cells with molten carbonates relevant for the metallurgical industry. Electrochim. Acta 2015, 182, 342–350. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Calandrino, L.; Burheim, O.S.; Solheim, A.; Kjelstrup, S.; Haarberg, G.M. Influence of Electrode Gas Flow Rate and Solid Oxide Ratio in Electrolyte on the Seebeck Coefficient of Molten Carbonate Thermocell. J. Electrochem. Soc. 2017, 164, H5271–H5276. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Wei, X.; Yin, Y.; Qin, B.; Ding, J.; Lu, J. Thermal conductivity improvement of liquid Nitrate and Carbonate salts doped with MgO particles. Energy Procedia 2017, 142, 407–412. [Google Scholar] [CrossRef]
- Ge, Z.W.; Ye, F.; Ding, Y.L. Composite Materials for Thermal Energy Storage: Enhancing Performance through Microstructures. Chemsuschem 2014, 7, 1318–1325. [Google Scholar] [CrossRef]
- Cornwell, K. The possibility of using molten salts for thermoelectric generation. J. Phys. D Appl. Phys. 1968, 1, 173–178. [Google Scholar] [CrossRef]
- Gheribi, A.E.; Torres, J.A.; Chartrand, P. Recommended values for the thermal conductivity of molten salts between the melting and boiling points. Sol. Energy Mater. Sol. Cells 2014, 126, 11–25. [Google Scholar] [CrossRef]
- Khokhlov, V.; Korzun, I.; Dokutovich, V.; Filatov, E. Heat capacity and thermal conductivity of molten ternary lithium, sodium, potassium, and zirconium fluorides mixtures. J. Nucl. Mater. 2011, 410, 32–38. [Google Scholar] [CrossRef]
- Zhang, X.; Fujii, M. Simultaneous measurements of the thermal conductivity and thermal diffusivity of molten salts with a transient short-hot-wire method. Int. J. Thermophys. 2000, 21, 71–84. [Google Scholar] [CrossRef]
- Hoshi, M.; Omotani, T.; Nagashima, A. Transient Method to Measure the Thermal-Conductivity of High-Temperature Melts Using a Liquid-Metal Probe. Rev. Sci. Instrum. 1981, 52, 755–758. [Google Scholar] [CrossRef]
- Tada, Y.; Harada, M.; Tanigaki, M.; Eguchi, W. Laser Flash Method for Measuring Thermal-Conductivity of Liquids - Application to Molten-Salts. Ind. Eng. Chem. Fundam. 1981, 20, 333–336. [Google Scholar] [CrossRef]
- Vyazovkin, S. Thermal Analysis. Anal. Chem. 2006, 78, 3875–3886. [Google Scholar] [CrossRef]
- Haines, P.J.; Reading, M.; Wilburn, F.W. Chapter 5—Differential Thermal Analysis and Differential Scanning Calorimetry. In Handbook of Thermal Analysis and Calorimetry; Brown, M.E., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1998; Volume 1, pp. 279–361. [Google Scholar]
- Hakvoort, G.; Vanreijen, L.L.; Aartsen, A.J. Measurement of the Thermal-Conductivity of Solid Substances by DSC. Thermochim. Acta 1985, 93, 317–320. [Google Scholar] [CrossRef]
- Flynn, J.H.; Levin, D.M. A Method for the Determination of Thermal-Conductivity of Sheet Materials by Differential Scanning Calorimetry (DSC). Thermochim. Acta 1988, 126, 93–100. [Google Scholar] [CrossRef]
- Camirand, C.P. Measurement of thermal conductivity by differential scanning calorimetry. Thermochim. Acta 2004, 417, 1–4. [Google Scholar] [CrossRef]
- Marcus, S.M.; Blaine, R.L. Thermal conductivity of polymers, glasses and ceramics by modulated DSC. Thermochim. Acta 1994, 243, 231–239. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; López-Olmedo, J.P.; Farjas, J.; Roura, P. Determination of thermal conductivity of powders in different atmospheres by differential scanning calorimetry. J. Therm. Anal. Calorim. 2015, 121, 469–473. [Google Scholar] [CrossRef][Green Version]
- Pujula, M.; Sanchez-Rodriguez, D.; Lopez-Olmedo, J.P.; Farjas, J.; Roura, P. Measuring thermal conductivity of powders with differential scanning calorimetry A simplified method. J. Therm. Anal. Calorim. 2016, 125, 571–577. [Google Scholar] [CrossRef]
- Hu, M.; Yu, D.M.; Wei, J.B. Thermal conductivity determination of small polymer samples by differential scanning calorimetry. Polym. Test. 2007, 26, 333–337. [Google Scholar] [CrossRef]
- Sadeghifar, H. In-plane and through-plane electrical conductivities and contact resistances of a Mercedes-Benz catalyst-coated membrane, gas diffusion and micro-porous layers and a Ballard graphite bipolar plate: Impact of humidity, compressive load and polytetrafluoroethylene. Energy Convers. Manag. 2017, 154, 191–202. [Google Scholar]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Thermal conductivity of a graphite bipolar plate (BPP) and its thermal contact resistance with fuel cell gas diffusion layers: Effect of compression, PTFE, micro porous layer (MPL), BPP out-of-flatness and cyclic load. J. Power Sources 2015, 273, 96–104. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. Effect of Polytetrafluoroethylene (PTFE) and micro porous layer (MPL) on thermal conductivity of fuel cell gas diffusion layers: Modeling and experiments. J. Power Sources 2014, 248, 632–641. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Djilali, N.; Bahrami, M. A new model for thermal contact resistance between fuel cell gas diffusion layers and bipolar plates. J. Power Sources 2014, 266, 51–59. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Bahrami, M.; Djilali, N. A statistically-based thermal conductivity model for fuel cell Gas Diffusion Layers. J. Power Sources 2013, 233, 369–379. [Google Scholar] [CrossRef]
- An, X.H.; Cheng, J.H.; Yin, H.Q.; Xie, L.D.; Zhang, P. Thermal conductivity of high temperature fluoride molten salt determined by laser flash technique. Int. J. Heat Mass Transfer 2015, 90, 872–877. [Google Scholar] [CrossRef]
- Trahan, J.; Kuravi, S.; Goswami, D.Y.; Rahman, M.; Stefanakos, E. Thermal Characterization of High Temperature Inorganic Phase Change Materials for Thermal Energy Storage Applications. In Proceedings of the ASME 2012 6th International Conference on Energy Sustainability, Pts A and B 2012, San Diego, CA, USA, 23–26 July 2012; pp. 621–628. [Google Scholar] [CrossRef]
- Glenn, M.J.; Allen, J.A.; Donne, S.W. Thermal Investigation of a Doped Alkali-Metal Carbonate Ternary Eutectic for Direct Carbon Fuel Cell Applications. Energy Fuels 2015, 29, 5423–5433. [Google Scholar] [CrossRef]
- Frangini, S.; Scaccia, S. Influence of lanthanum carbonate additions on thermal stability of eutectic lithium–sodium carbonate near its melting point. Thermochim. Acta 2013, 551, 33–39. [Google Scholar] [CrossRef]
- Ward, A.T.; Janz, G.J. Molten carbonate electrolytes: Electrical conductance, density and surface tension of binary and ternary mixtures. Electrochim. Acta 1965, 10, 849–857. [Google Scholar] [CrossRef]
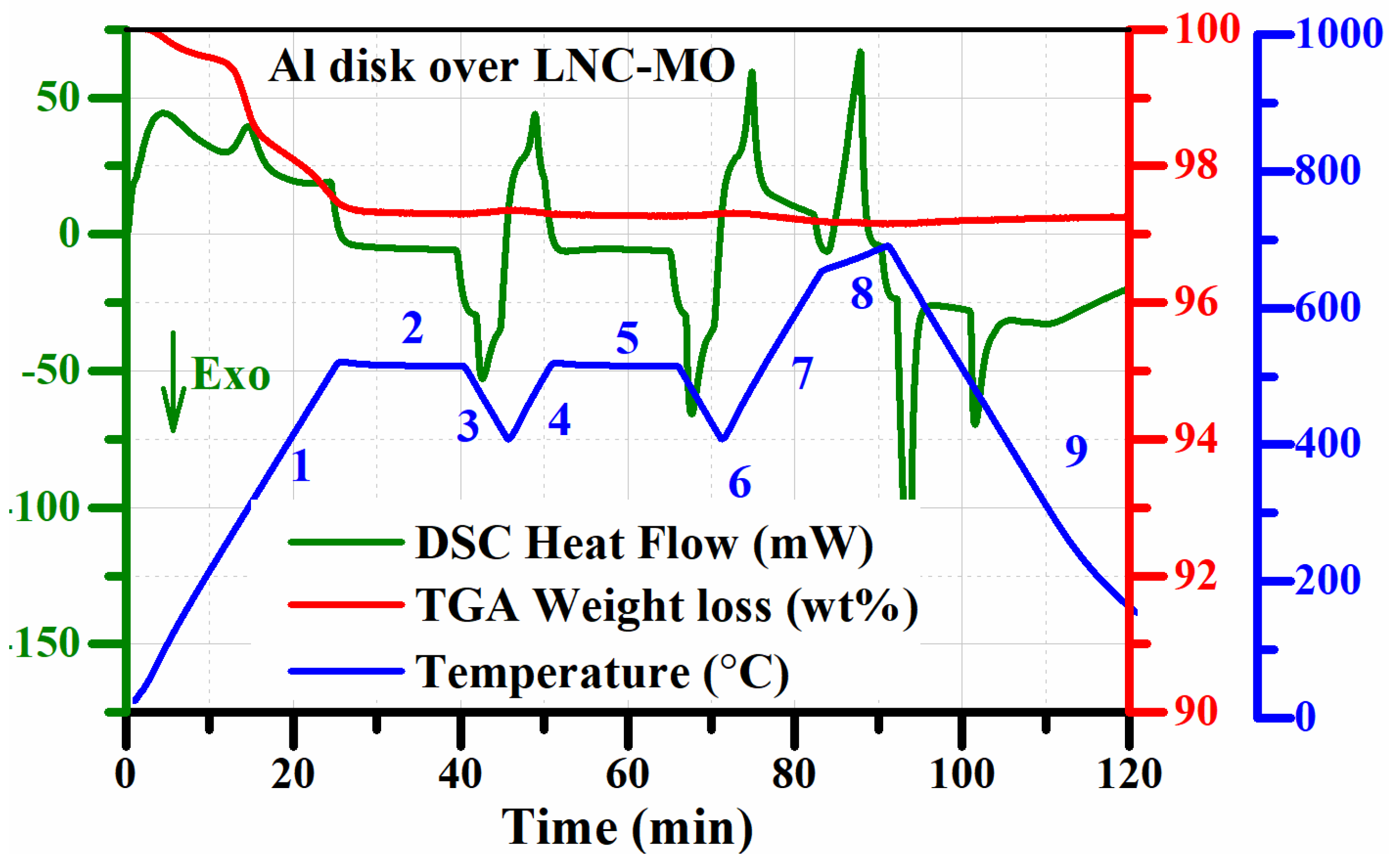
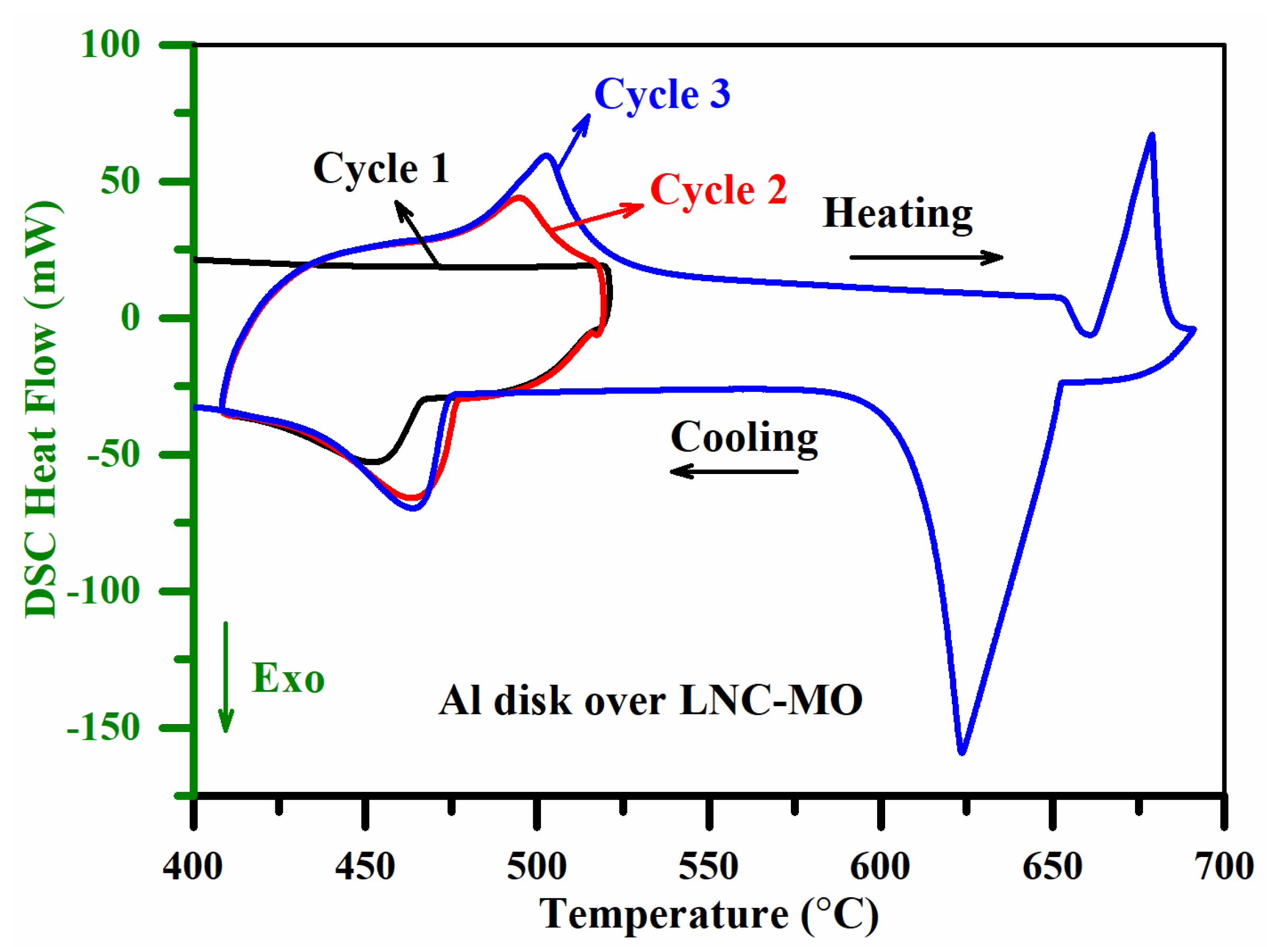

| Sample | Sample Composition | Composition Ratio (vol %) | ||
|---|---|---|---|---|
| Molten Salt | Solid Oxide | Molten Salt | Solid Oxide | |
| LNC-MO | Eutectic molten carbonate (0.53 mol Li2CO3 + 0.47 mol Na2CO3) | MgO | 45 | 55 |
| LNC-AO | Al2O3 | 45 | 55 | |
| LNC-CO | CeO2 | 45 | 55 | |
| LNC-LAO | LiAl2O3 | 45 | 55 | |
| LNC | -- | 100 | -- | |
| Segment | Mode | Temperature (°C) | Rate (°C/min) | Hold Time (min) |
|---|---|---|---|---|
| 1 | Dynamic (Heating) | 30–515 | 20 | --- |
| 2 | Isothermal | 515 | --- | 15 |
| 3 | Dynamic (Cooling) | 515–410 | 20 | --- |
| 4 | Dynamic (Heating) | 410–515 | 20 | --- |
| 5 | Isothermal | 515 | --- | 15 |
| 6 | Dynamic (Cooling) | 515–410 | 20 | --- |
| 7 | Dynamic (Heating) | 410–650 | 20 | --- |
| 8 | Dynamic (Heating) | 650–690 | 5 | --- |
| 9 | Dynamic (Cooling) | 690–30 | 20 | --- |
| Sample | Thermal Conductivity (W m−1K−1) | ||
|---|---|---|---|
| DSC | LFA | Literature [15] | |
| LNC-MO | 0.077 ± 0.004 | 0.078 ± 0.001 | -- |
| LNC-AO | 0.039 ± 0.006 | -- | -- |
| LNC-CO | 0.038 ± 0.004 | -- | -- |
| LNC-LAO | 0.066 ± 0.011 | -- | -- |
| LNC | 0.826 ± 0.001 | -- | 0.887 ± 0.002 |
| Laser Shot Number | Thermal Diffusivity (mm2/s) | Thermal Conductivity (W m−1K−1) |
|---|---|---|
| 1 | 0.301 | 0.078 |
| 2 | 0.300 | 0.078 |
| 3 | 0.297 | 0.077 |
| 4 | 0.298 | 0.077 |
| 5 | 0.304 | 0.079 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandhasamy, S.; Støre, A.; Haarberg, G.M.; Kjelstrup, S.; Solheim, A. Thermal Conductivity of Molten Carbonates with Dispersed Solid Oxide from Differential Scanning Calorimetry. Materials 2019, 12, 1486. https://doi.org/10.3390/ma12091486
Kandhasamy S, Støre A, Haarberg GM, Kjelstrup S, Solheim A. Thermal Conductivity of Molten Carbonates with Dispersed Solid Oxide from Differential Scanning Calorimetry. Materials. 2019; 12(9):1486. https://doi.org/10.3390/ma12091486
Chicago/Turabian StyleKandhasamy, Sathiyaraj, Anne Støre, Geir Martin Haarberg, Signe Kjelstrup, and Asbjørn Solheim. 2019. "Thermal Conductivity of Molten Carbonates with Dispersed Solid Oxide from Differential Scanning Calorimetry" Materials 12, no. 9: 1486. https://doi.org/10.3390/ma12091486
APA StyleKandhasamy, S., Støre, A., Haarberg, G. M., Kjelstrup, S., & Solheim, A. (2019). Thermal Conductivity of Molten Carbonates with Dispersed Solid Oxide from Differential Scanning Calorimetry. Materials, 12(9), 1486. https://doi.org/10.3390/ma12091486





