Coercivity Increase of the Recycled HDDR Nd-Fe-B Powders Doped with DyF3 and Processed via Spark Plasma Sintering & the Effect of Thermal Treatments
Abstract
1. Introduction
2. Experimental
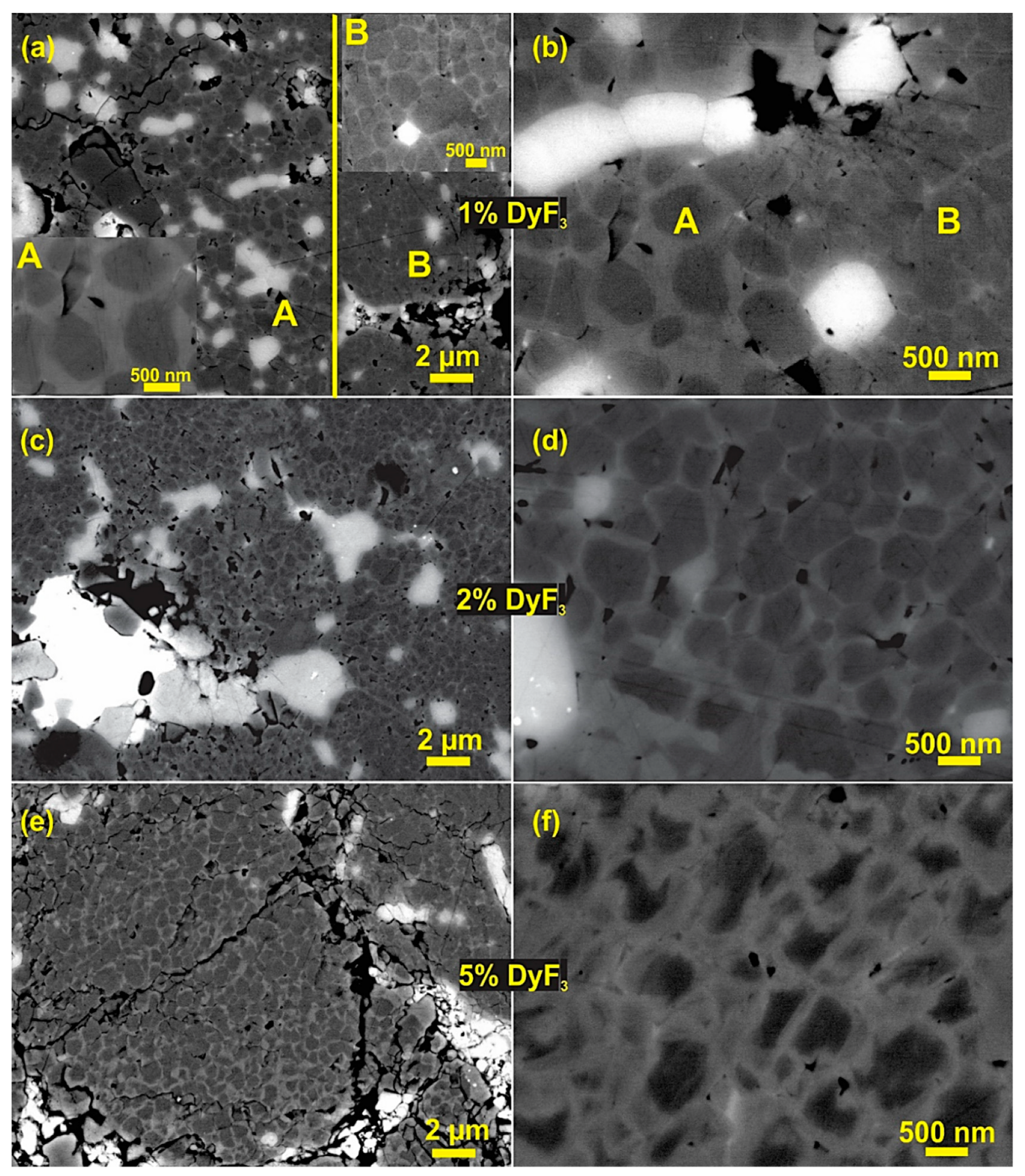
3. Results and Discussion
Characterization of Coarse Recycled HDDR Nd-Fe-B Powder.
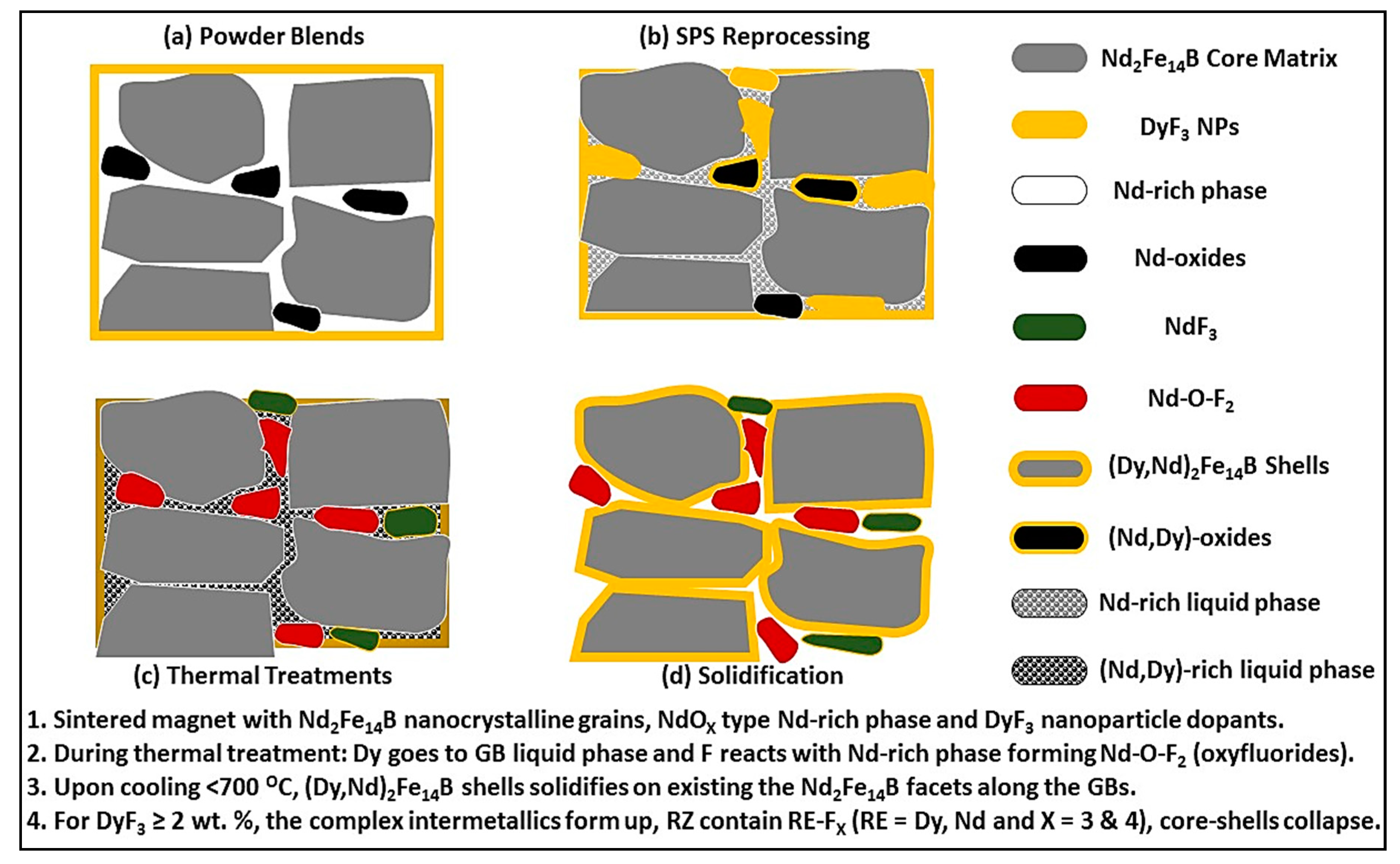
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugimoto, S. Current status and recent topics of rare-earth permanent magnets. J. Phys. D Appl. Phys. 2011, 44, 064001. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Poudyal, N.; Liu, J.P. Advances in nanostructured permanent magnets research. J. Phys. D Appl. Phys. 2013, 46, 043001. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H. The current and future status of rare earth permanent magnets. Scr. Mater. 2018, 154, 273–276. [Google Scholar] [CrossRef]
- Walton, A.; Yi, H.; Rowson, N.A.; Speight, J.D.; Mann, V.S.J.; Sheridan, R.S.; Bradshaw, A.; Harris, I.R.; Williams, A.J. The use of hydrogen to separate and recycle neodymium iron boron-type magnets from electronic waste. J. Clean. Prod. 2015, 104, 236–241. [Google Scholar] [CrossRef]
- Li, X.; Yue, M.; Miha, Z.; Liu, W.; Zhang, D.; Zuo, T. Regeneration of waste sintered Nd-Fe-B magnets to fabricate anisotropic bonded magnets. J. Rare Earths 2015, 33, 736–739. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Muller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.Q.; Yue, M.; Liu, Y.Q.; Zhang, D.T.; Zuo, T.Y. Waste Nd-Fe-B sintered magnet recycling by doping with rare earth rich alloys. IEEE Trans. Magn. 2014, 50, 2105403. [Google Scholar] [CrossRef]
- Sprecher, B.; Xiao, Y.P.; Walton, A.; Speight, J.; Harris, R.; Kleijn, R.; Visser, G.; Kramer, G.J. Life cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ. Sci. Technol. 2014, 48, 3951–3958. [Google Scholar] [CrossRef]
- Périgo, E.A.; da Silva, S.C.; Martin, R.V.; Taklishi, H.; Landgraf FJ, G. Properties of hydrogenation-disproportionation-desorption-recombination NdFeB powders prepared from recycled sintered magnets. J. Appl. Phys. 2012, 111, 07A725. [Google Scholar] [CrossRef]
- Sheridan, R.S.; Sillitoe, R.; Zakotnik, M.; Harris, I.R.; Williams, A.J. Anisotropic powder from sintered NdFeB magnets by the HDDR processing route. J. Magn. Magn. Mater. 2012, 324, 63–67. [Google Scholar] [CrossRef]
- Zakotnik, M.; Harris, I.R.; Williams, A.J. Possible methods of recycling Nd-Fe-B-type sintered magnets using the HD/degassing process. J. Alloys Compd. 2008, 450, 525–531. [Google Scholar] [CrossRef]
- Zakotnik, M.; Harris, I.R.; Williams, A.J. Multiple recycling of NdFeB-type sintered magnets. J. Alloys Compd. 2009, 469, 314–321. [Google Scholar] [CrossRef]
- Itoh, M.; Masuda, M.; Suzuki, S.; Machida, K. Recycling of rare earth sintered magnets as isotropic bonded magnets by melt-spinning. J. Alloys Compd. 2004, 374, 393–396. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Güth, K.; Woodcock, T.G. Recycling used Nd-Fe-B sintered magnets via a hydrogen-based route to produce anisotropic, resin bonded magnets. Adv. Energy Mater. 2013, 3, 151–155. [Google Scholar] [CrossRef]
- Itoh, M.; Masuda, M.; Suzuki, S.; Machida, K. Recycle for sludge scrap of Nd-Fe-B sintered magnet as isotropic bonded magnet. J. Rare Earths 2004, 22, 168–171. [Google Scholar]
- Kim, A.S.; Kim, D.H.; Namkung, S.; Jang, T.S.; Lee, D.H.; Kwon, H.W.; Hwang, D.H. Development of high coercive powder from the Nd-Fe-B sintered magnet scrap. IEEE Trans. Magn. 2004, 40, 2877–2879. [Google Scholar] [CrossRef]
- Ikram, A.; Mehmood, M.F.; Podlogar, M.; Eldosouky, A.; Sheridan, R.S.; Awais, M.; Walton, A.; Krzmanc, M.M.; Tomse, T.; Kobe, S.; et al. The sintering mechanism of fully dense and highly coercive Nd-Fe-B magnets from the recycled HDDR powders reprocessed by spark plasma sintering. J. Alloys Compd. 2019, 774, 1195–1206. [Google Scholar] [CrossRef]
- Sheridan, R.S.; Harris, I.R.; Walton, A. The development of microstructure during hydrogenation–disproportionation–desorption–recombination treatment of sintered neodymium-iron-boron-type magnets. J. Magn. Magn. Mater. 2016, 401, 455–462. [Google Scholar] [CrossRef]
- Bae, K.-H.; Kim, T.-H.; Lee, S.-R.; Kung, S.N.; Jang, T.-S. Magnetic and microstructural characteristics of a DyF3 dip-coated Nd-Fe-B sintered magnet. IEEE Trans. Magn. 2013, 49, 7. [Google Scholar] [CrossRef]
- Park, S.-E.; Kim, T.-H.; Lee, S.-R.; Namkung, S.; Jang, T.-S. Effect of sintering conditions on the magnetic and microstructural properties of Nd–Fe–B sintered magnets doped with DyF3 powders. J. Appl. Phys. 2012, 111, 07A707. [Google Scholar] [CrossRef]
- Pratomo, S.B.; Oktadinata, H.; Pawawoi. Effect of DyF3 and TbF3 additions on the coercivity enhancement in grain boundary diffusion processed Nd-Fe-B permanent magnets. AIP Conf. Proc. 2018, 1964, 020019. [Google Scholar] [CrossRef]
- Sueptitz, R.; Sawatzki, S.; Moore, M.; Uhlemann, M.; Gutfleisch, O.; Gebert, A. Effect of DyF3 on the corrosion behavior of hot-pressed Nd-Fe-B permanent magnets. Mater. Corros. 2015, 66, 152–157. [Google Scholar] [CrossRef]
- Mcguiness, P.; Akdogan, O.; Asali, A.; Bance, S.; Bittner, F.; Coey, J.M.D.; Dempsey, N.M.; Fidler, J.; Givord, D.; Gutfleisch, O.; et al. Replacement and original magnet engineering options (ROMEOs): A European seventh framework project to develop advanced permanent magnets without, or with reduced use of, critical raw materials. JOM 2015, 67, 1306–1317. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Dong, X.; Liu, Q.; Komuro, M. Effect of DyF3 additions on the coercivity and grain boundary structure in sintered Nd-Fe-B magnets. Scr. Mater. 2011, 64, 1137–1140. [Google Scholar] [CrossRef]
- Cao, X.J.; Chen, L.; Guo, S.; Li, X.B.; Yi, P.P.; Yan, A.R.; Yan, G.L. Coercivity enhancement of sintered Nd-Fe-B magnets by efficiently diffusing DyF3 based on electrophoretic deposition. J. Alloys Compd. 2015, 631, 315–320. [Google Scholar] [CrossRef]
- Xu, F.; Wang, J.; Dong, X.; Zhang, L.; Wu, J. Grain boundary microstructure in DyF3-diffusion processed Nd–Fe–B sintered magnets. J. Alloys Compd. 2011, 509, 7909–7914. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Dong, X.; Xu, F.; Komuro, M. Increased coercivity in sintered Nd-Fe-B magnets with NdF3 additions and the related grain boundary phase. Scr. Mater. 2009, 61, 1048–1051. [Google Scholar] [CrossRef]
- Soderžnik, M.; Korent, M.; Soderžnik, K.Ž.; Katter, M.; Üstüner, K.; Kobe, S. High-coercivity Nd-Fe-B magnets obtained with the electrophoretic deposition of submicron TbF3 followed by the grain-boundary diffusion process. Acta Mater. 2016, 115, 278–284. [Google Scholar] [CrossRef]
- Cao, X.J.; Chen, L.; Guo, S.; Di, J.H.; Ding, G.F.; Chen, R.J.; Yan, A.R.; Chen, K.Z. Improved thermal stability of TbF3-coated sintered Nd-Fe-B magnets by electrophoretic deposition. AIP Adv. 2018, 8, 056222. [Google Scholar] [CrossRef]
- Cao, X.; Chen, L.; Guo, S.; Chen, R.; Yan, G.; Yan, A. Impact of TbF3 diffusion on coercivity and microstructure in sintered Nd-Fe-B magnets by electrophoretic deposition. Scr. Mater. 2016, 116, 40–43. [Google Scholar] [CrossRef]
- Žagar, K.; Kocjan, A.; Kobe, S. Magnetic and microstructural investigation of high-coercivity net-shape Nd-Fe-B-type magnets produced from spark-plasma-sintered melt-spun ribbons blended with DyF3. J. Magn. Magn. Mater. 2016, 403, 90–96. [Google Scholar] [CrossRef]
- Sawatzki, S.; Dirba, I.; Wendrock, H.; Schultz, L.; Gutfleisch, O. Diffusion processes in hot-deformed Nd-Fe-B magnets with DyF3 additions. J. Magn. Magn. Mater. 2014, 358–359, 163–169. [Google Scholar] [CrossRef]
- Takagi, K.; Akada, M.; Soda, R.; Ozaki, K. Preparation of Nd-Fe-B sintered magnets from HDDR-processed powder. J. Magn. Magn. Mater. 2015, 393, 461–466. [Google Scholar] [CrossRef]
- Seelam, U.M.R.; Ohkubo, T.; Abe, T.; Hirosawa, S.; Hono, K. Faceted shell structure in grain boundary diffusion-processed sintered Nd-Fe-B magnets. J. Alloys Compd. 2014, 617, 884–892. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauss, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Gerven, T.V.; Jones, P.T.; et al. REE recovery from end-of-life NdFeB permanent magnet scrap: A critical review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Reimer, M.V.; Schenk-Mathes, H.Y.; Hoffmann, M.F.; Elwert, T. Recycling decisions in 2020, 2030, and 2040—When can substantial NdFeB extraction be expected in the EU? Metals 2018, 8, 867. [Google Scholar] [CrossRef]
- Wenga, Z.; Haque, N.; Mudd, G.M.; Jowitt, S.M. Assessing the energy requirements and global warming potential of the production of rare earth elements. J. Clean. Prod. 2016, 139, 1282–1297. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The rare earth elements: Demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Jin, H.Y.; Afiuny, P.; Dove, S.; Furlan, G.; Zakotnik, M.; Yih, Y.; Sutherland, J.W. Life cycle assessment of neodymium-iron-boron magnet-to-magnet recycling for electric vehicle motors. Environ. Sci. Technol. 2018, 52, 3796–3802. [Google Scholar] [CrossRef]
- Lalana, E.H.; Degri, M.J.J.; Bradshaw, A.; Walton, A. Recycling of rare earth magnets by hydrogen processing and re-sintering. In European Congress and Exhibition on Powder Metallurgy. European PM Conference Proceedings; The European Powder Metallurgy Association: Shrewsbury, UK, 2016. [Google Scholar]
- Rabatho, J.P.; Tongamp, W.; Takasaki, Y.; Haga, K.; Shibayama, A. Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J. Mater. Cycles Waste Manag. 2013, 15, 171–178. [Google Scholar] [CrossRef]
- Zakotnik, M.; Tudor, C.O.; Peiró, L.T.; Afiuny, P.; Skomski, R.; Hatch, G.P. Analysis of energy usage in Nd-Fe-B magnet to magnet recycling. Environ. Technol. Innov. 2016, 5, 117–126. [Google Scholar] [CrossRef]
- Diehl, O.; Schönfeldt, M.; Brouwer, E.; Dirks, A.; Rachut, K.; Gassmann, J.; Güth, K. Towards an alloy recycling of Nd-Fe-B permanent magnets in a circular economy. J. Sustain. Metall. 2018, 4, 163–175. [Google Scholar] [CrossRef]
- Lixandru, A.; Poenaru, I.; Guth, K.; Gauss, R.K.; Gutfleisch, O. A systematic study of HDDR processing conditions for the recycling of end-of-life Nd-Fe-B magnets. J. Alloys Compd. 2017, 724, 51–61. [Google Scholar] [CrossRef]
- Li, W.F.; Ohkubo, T.; Hono, K.; Nishiuchi, T.; Hirosawa, S. The role of grain boundaries in the coercivity of hydrogenation disproportionation desorption recombination processed Nd-Fe=B powders. J. Appl. Phys. 2009, 105, 07A706. [Google Scholar] [CrossRef]
- Li, W.F.; Ohkubo, T.; Hono, K.; Nishiuchi, T.; Hirosawa, S. Coercivity mechanism of hydrogenation disproportionation desorption recombination processed Nd-Fe-B based magnets. Appl. Phys. Lett. 2008, 93, 052505. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, S.-R.; Lee, M.-W.; Jang, T.-S.; Kim, J.W.; Kim, Y.D.; Kim, H.-J. Dependence of magnetic, phase-transformation and microstructural characteristics on the Cu content of Nd-Fe-B sintered magnet. Acta Mater. 2014, 66, 12–21. [Google Scholar] [CrossRef]
- Vent-Schmidt, T.; Fang, Z.; Lee, Z.; Dixon, D.; Riedel, S. Extending the row of lanthanide tetrafluorides: A combined matrix-isolation and quantum-chemical study. Chem. Eur. J. 2016, 22, 2406–2416. [Google Scholar] [CrossRef]
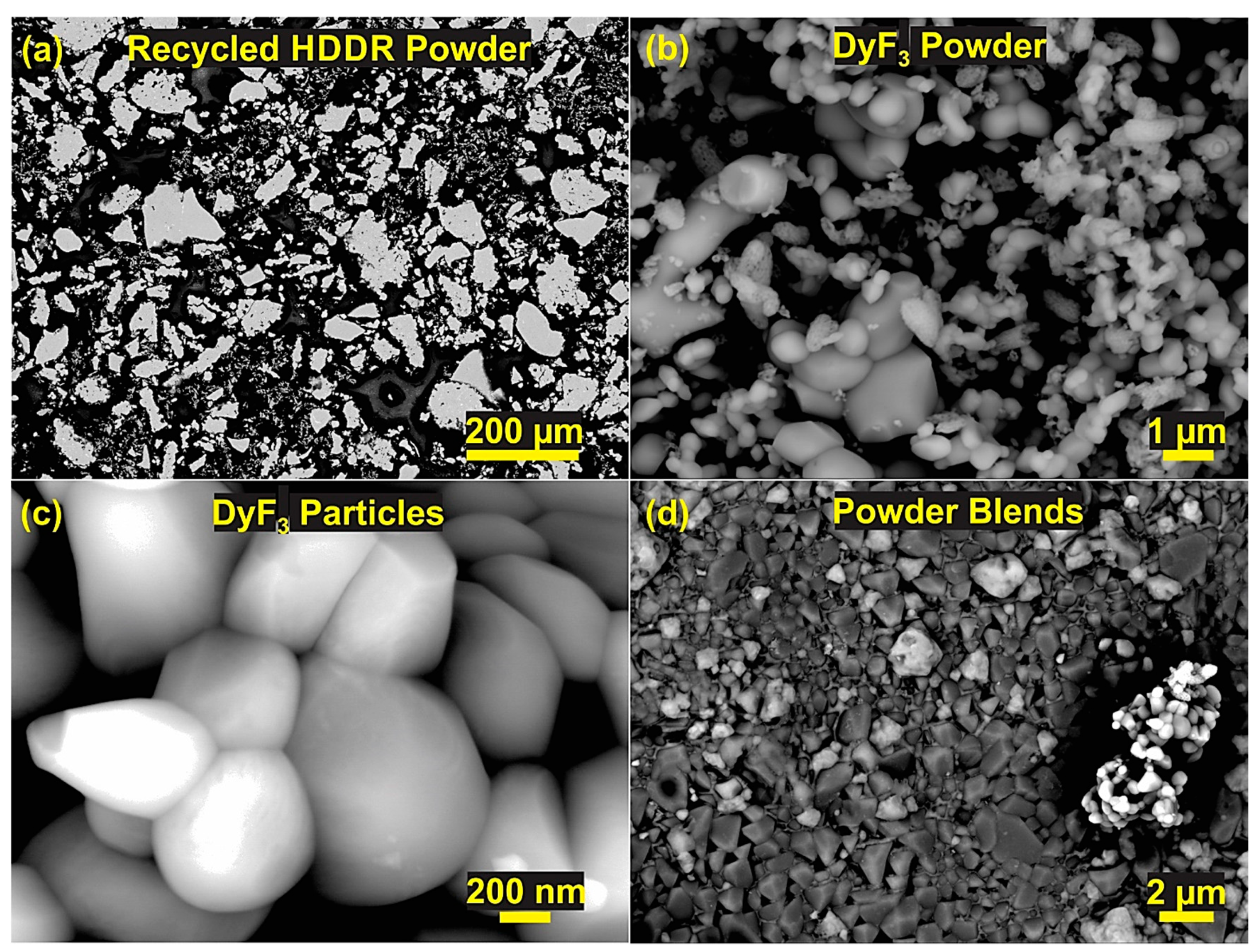
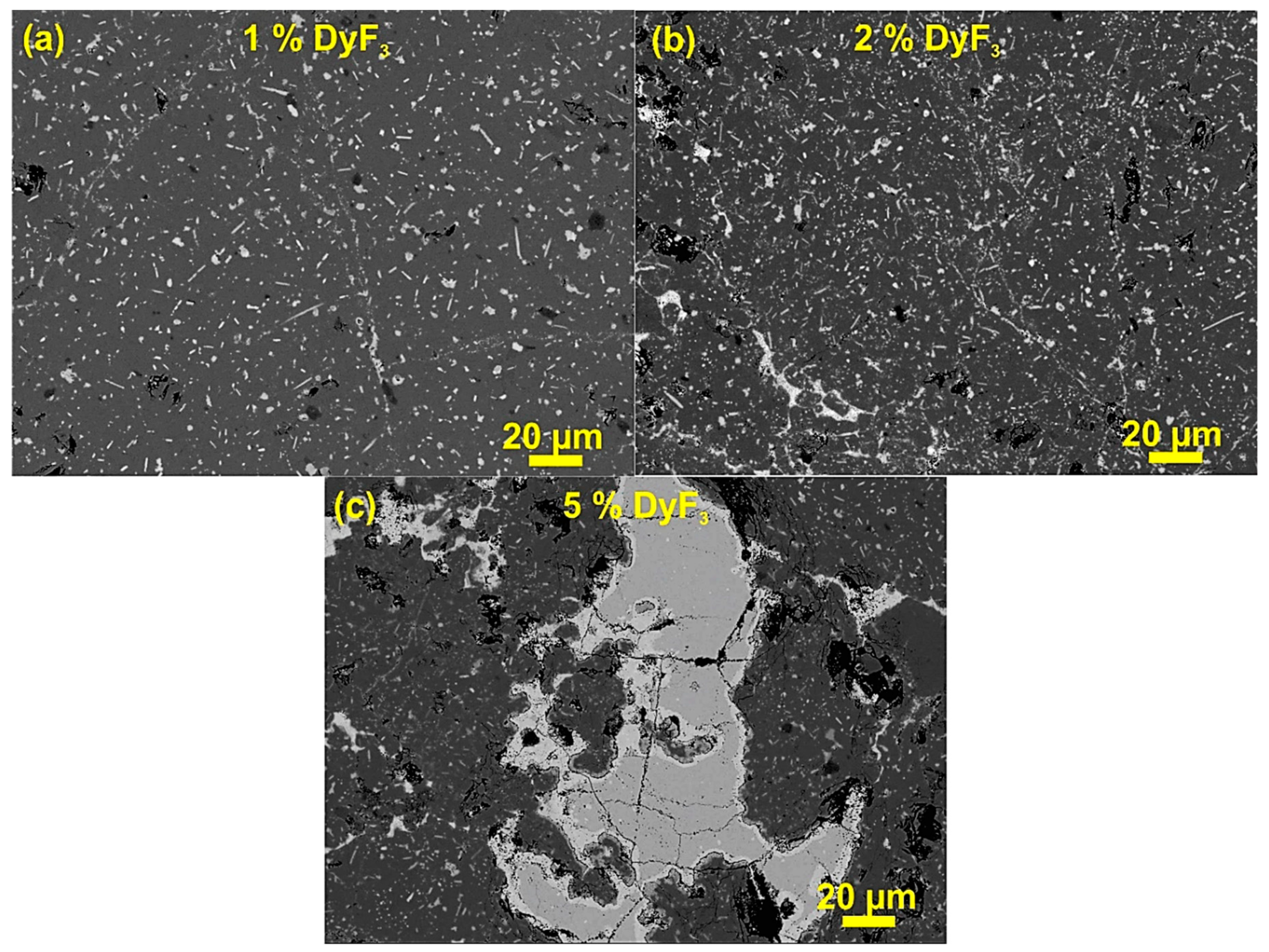
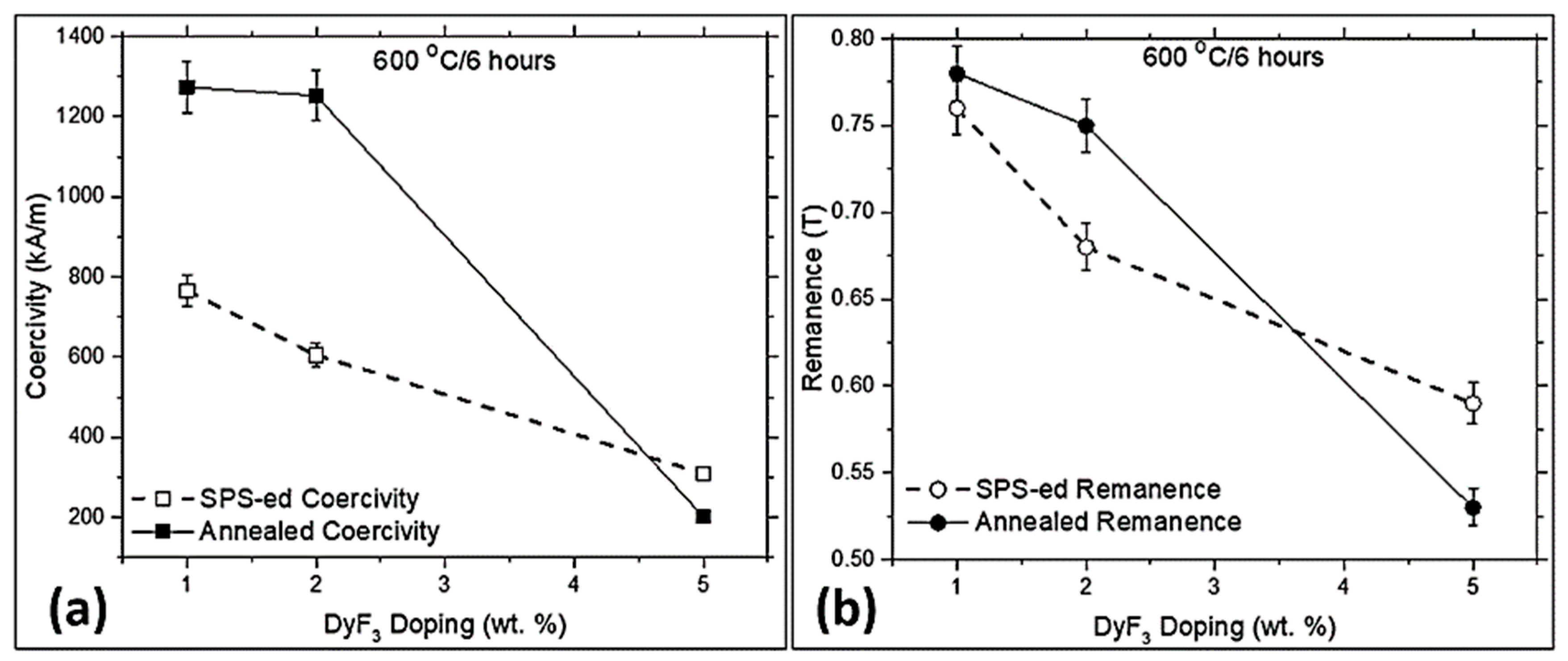
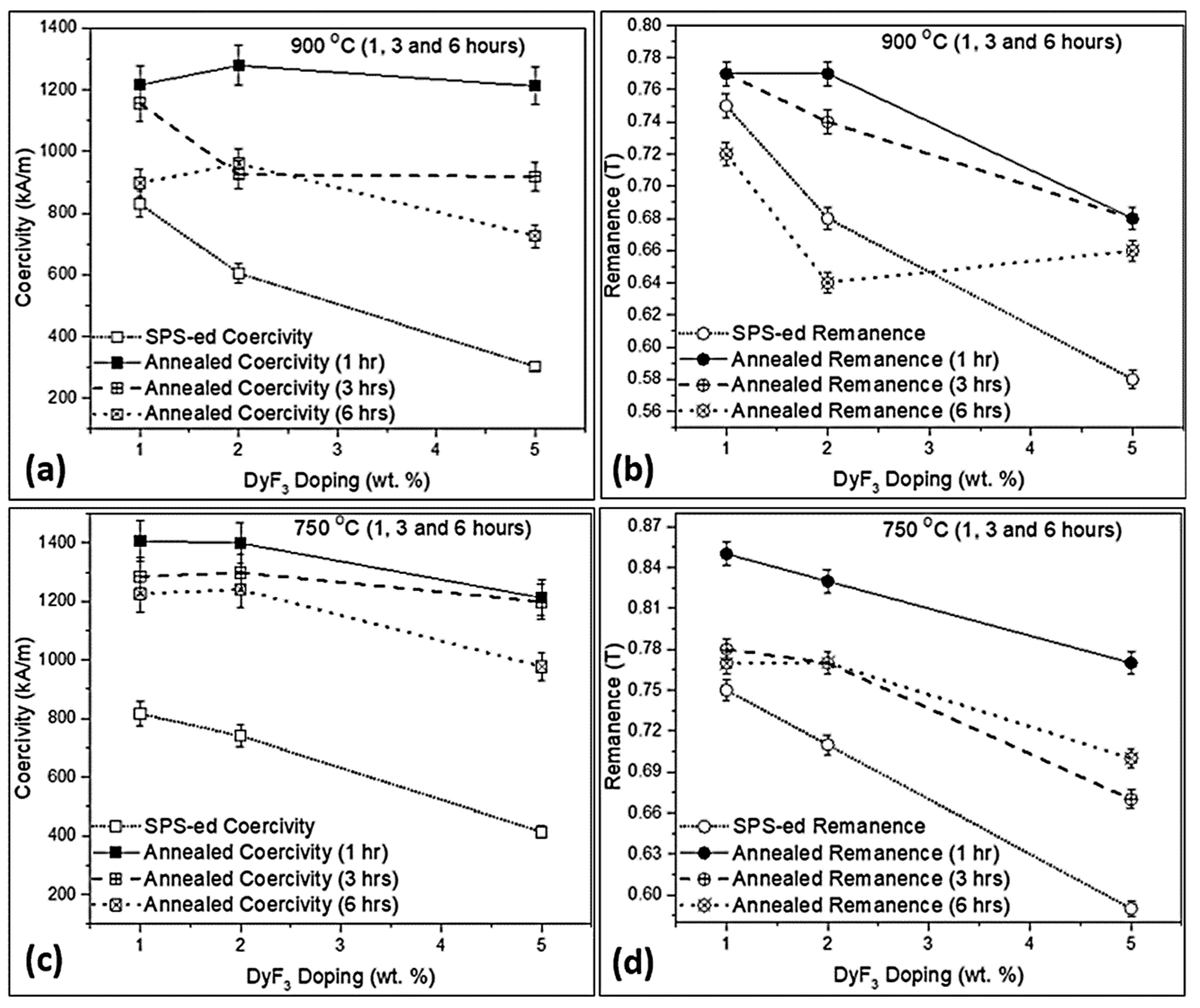
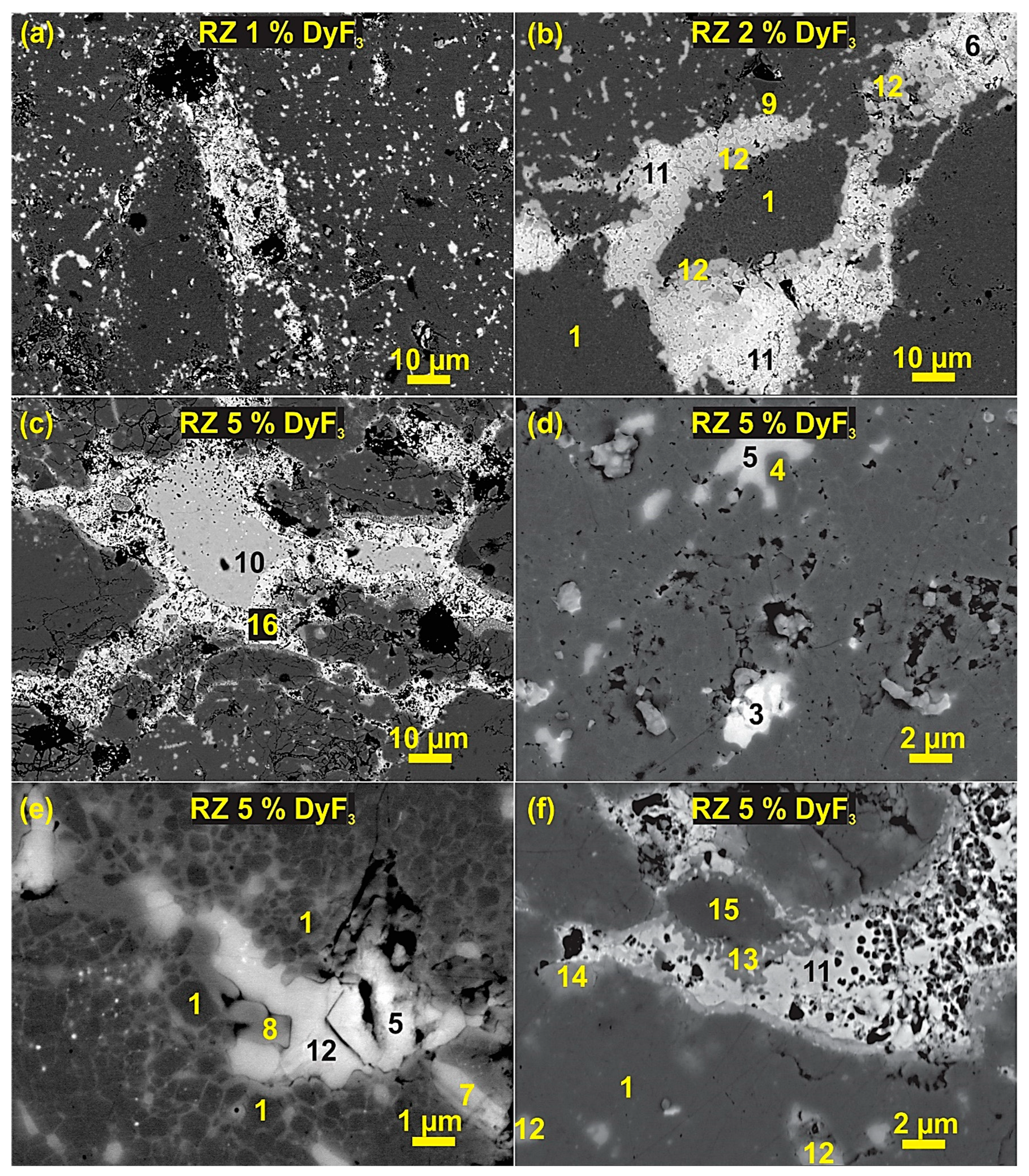
| Phase No. | Phases | Nd (at.%) | Fe (at.%) | F (at.%) | O (at.%) | Nb (at.%) | Al (at.%) | Dy (at.%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Nd2Fe14B Cores | 12 | 85 | - | - | 0.9 | 0.9 | 1.2 |
| DyNdFe14B Shells | 8.1 | 85.5 | - | - | - | - | 5.8 | |
| 2 | Nd-rich NdOx/NdO2 | 24.6 | 29.5 | - | 46.3 | - | - | |
| 3 | Nd2O3 | 34.7 | 1.8 | - | 63.5 | - | - | |
| 4 | NbFe2 Laves | 0.7 | 47.6 | - | - | 51.7 | - | |
| 5 | Nd-O-F | 28.2 | 1.8 | 38.5 | 31.5 | - | - | - |
| 6 | Dy-O-F | 4.5 | 18.8 | 20.9 | 28.9 | - | - | 26.9 |
| 7 | Nd-F4 | 18 | - | 81.1 | - | - | - | 0.9 |
| 8 | Nd-Fe-O-F (interphase) | 10.8 | 45.4 | 28.7 | 9 | - | - | 6.1 |
| 9 | NdFe4B4 | 18.8 | 68.5 | 9.6 | - | - | - | - |
| 10 | Dy-F4 | - | - | 80.8 | - | - | - | 19.2 |
| 11 | Dy-Fe2 (interphase) | 2.4 | 54.6 | 4.6 | 10.4 | - | - | 28 |
| 12 | Nd-O-F2 | 20.7 | 2.4 | 61.3 | 13.7 | - | - | 1.9 |
| 13 | Nd-Fe-F (interphase) | 11.9 | 47.2 | 33.3 | 3.6 | - | 2.3 | 1.7 |
| 14 | Dy-Nd-O-F2 (interphase) | 8.9 | 6.8 | 48.8 | 21.7 | - | - | 13.8 |
| 15 | Nd-Fe-F (interphase) | 13.3 | 39.2 | 39.3 | 3.9 | - | 1.3 | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, A.; Mehmood, M.F.; Samardžija, Z.; Sheridan, R.S.; Awais, M.; Walton, A.; Sturm, S.; Kobe, S.; Žužek Rožman, K. Coercivity Increase of the Recycled HDDR Nd-Fe-B Powders Doped with DyF3 and Processed via Spark Plasma Sintering & the Effect of Thermal Treatments. Materials 2019, 12, 1498. https://doi.org/10.3390/ma12091498
Ikram A, Mehmood MF, Samardžija Z, Sheridan RS, Awais M, Walton A, Sturm S, Kobe S, Žužek Rožman K. Coercivity Increase of the Recycled HDDR Nd-Fe-B Powders Doped with DyF3 and Processed via Spark Plasma Sintering & the Effect of Thermal Treatments. Materials. 2019; 12(9):1498. https://doi.org/10.3390/ma12091498
Chicago/Turabian StyleIkram, Awais, M. Farhan Mehmood, Zoran Samardžija, Richard Stuart Sheridan, Muhammad Awais, Allan Walton, Saso Sturm, Spomenka Kobe, and Kristina Žužek Rožman. 2019. "Coercivity Increase of the Recycled HDDR Nd-Fe-B Powders Doped with DyF3 and Processed via Spark Plasma Sintering & the Effect of Thermal Treatments" Materials 12, no. 9: 1498. https://doi.org/10.3390/ma12091498
APA StyleIkram, A., Mehmood, M. F., Samardžija, Z., Sheridan, R. S., Awais, M., Walton, A., Sturm, S., Kobe, S., & Žužek Rožman, K. (2019). Coercivity Increase of the Recycled HDDR Nd-Fe-B Powders Doped with DyF3 and Processed via Spark Plasma Sintering & the Effect of Thermal Treatments. Materials, 12(9), 1498. https://doi.org/10.3390/ma12091498






