Exterior and Internal Uniform Loading of Pt Nanoparticles on Yolk-Shell La2O3 by Acoustic Levitation Synthesis with Enhanced Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Materials Characterization
2.3. Photocatalytic Experiment
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qin, M.; Lin, K.; Shuai, Q.; Liang, H.; Peng, J.; Mao, C.; Ji, Y.; Wu, H. Facile synthesis of 2D single-phase Ni0.9Zn0.1O and its application in decolorization of dye. J. Mater. Sci. Mater. Electron. 2018, 29, 9740–9744. [Google Scholar] [CrossRef]
- Bao, Y.; Qin, M.; Yu, Y.; Zhang, L.; Wu, H. Facile fabrication of porous NiCo2O4 nanosheets with high adsorption performance toward Congo red. J. Phys. Chem. Solids 2019, 124, 289–295. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, Y.; Zhang, M.; Zhao, X.; Qian, J.; Wu, H. Preparation of ternary Pt-NiO-ZnO hybrids and investigation of its photocatalytic performance toward methyl orange. J. Mater. Sci. Mater. Electron. 2019, 30, 5158–5169. [Google Scholar] [CrossRef]
- Park, H.; Kim, H.; Moonb, G.; Choi, W. Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2. Energy Environ. Sci. 2016, 9, 411–433. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Muhammad, T.; Ma, L.; Huang, Z.; Wang, S.; Wang, L.; Zou, J.; Zhang, X. MOF-derived C-doped ZnO prepared via a two-step calcination for efficient photocatalysis. Appl. Catal. B 2016, 189, 181–191. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Xie, Y.; Fan, Q.; Yu, J.C.; Shu, Q.; Wang, C. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability. Nanoscale 2013, 5, 2142–2151. [Google Scholar] [CrossRef]
- Di Mauro, A.; Zimbone, M.; Scuderi, M.; Nicotra, G.; Fragalà, M.E.; Impellizzeri, G. Effect of Pt Nanoparticles on the Photocatalytic Activity of ZnO Nanofibers. Nanoscale Res. Lett. 2015, 10, 484. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Páez, C.A.; Navío, J.A.; Hidalgo, M.C.; Macías, M. ZnO and Pt-ZnO photocatalysts: Characterization and photocatalytic activity assessing by means of three substrates. Catal. Today 2018, 313, 12–19. [Google Scholar] [CrossRef]
- Lakshmanareddy, N.; Rao, V.N.; Cheralathan, K.K.; Subramaniam, E.P.; Shankar, M.V. Pt/TiO2 nanotube photocatalyst-Effect of synthesis methods on valance state of Pt and its influence on hydrogen production and dye degradation. J. Colloid Interface Sci. 2019, 538, 83–98. [Google Scholar] [CrossRef]
- Zhao, J.; Li, W.; Fan, L.; Quan, Q.; Wang, J.; Xiao, C. Yolk-porous shell nanospheres from siliver-decorated titanium dioxide and silicon dioxide as an enhanced visible-light photocatalyst with guaranteed shielding for organic carrier. J. Colloid Interface Sci. 2019, 534, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, C.; Zhang, C.; Zhao, M.; Zhang, J.; Chen, H.; Zha, Z.; Zhao, T.; Qian, H. Controlled synthesis of upconverting nanoparticles/ZnxCd1−xS yolk-shell nanoparticles for efficient photocatalysis driven by NIR light. Appl. Catal. B 2018, 224, 854–862. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Bian, Z.; Kathiraser, Y.; Kawi, S. Design of highly stable and selective core/yolk-shell nanocatalysts—A review. Appl. Catal. B 2016, 188, 324–341. [Google Scholar] [CrossRef]
- Jin, J.; Wang, C.; Ren, X.; Huang, S.; Wu, M.; Chen, L.; Hasan, T.; Wang, B.; Li, Y.; Su, B. Anchoring ultrafine metallic and oxidized Pt nanoclusters on yolk-shell TiO2 for unprecedentedly high photocatalytic hydrogen production. Nano Energy 2017, 38, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Kong, C.; Liu, K.; Yin, L.; Ma, B.; Zhang, X.; Yang, S. Surfactant-free synthesis of Cu2O yolk-shell cubes decorated with Pt nanoparticles for enhanced H2O2 detection. Chem. Commun. 2018, 54, 8458–8461. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wei, Q.; Yu, X.; Zhang, Y. Metal-Organic Framework-Derived Co3O4/Au Heterostructure as a Catalyst for Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2018, 10, 34068–34076. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z. Noble metal nanoparticle@metal oxide core/yolk-shell nanostructures as catalysts: Recent progress and perspective. Nanoscale 2014, 6, 3995–4011. [Google Scholar] [CrossRef]
- Lee, I.; Joo, J.B.; Yin, Y.; Zaera, F. A Yolk@Shell Nanoarchitecture for Au/TiO2 Catalysts. Angew. Chem. Int. Ed. 2011, 50, 10208–10211. [Google Scholar] [CrossRef]
- Park, J.C.; Song, H. Metal@Silica yolk-shell nanostructures as versatile bifunctional nanocatalysts. Nano Res. 2011, 4, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, L.; Han, D.; Gu, F. CO oxidation on Au@CeO2 yolk-shell nanoparticles with high catalytic stability. Mater. Lett. 2014, 137, 188–191. [Google Scholar] [CrossRef]
- Shim, J.; Hong, Y.J.; Na, H.; Jang, W.; Kang, Y.C.; Roh, H. Highly Active and Stable Pt-Loaded Ce0.75Zr0.25O2 Yolk-Shell Catalyst for Water-Gas Shift Reaction. ACS Appl. Mater. Interfaces 2016, 8, 17239–17244. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Hong, Y.J.; Kang, Y.C.; Lee, J. High performance chemiresistive H2S sensors using Ag-loaded SnO2 yolk-shell nanostructures. RSC Adv. 2014, 4, 16067–16074. [Google Scholar] [CrossRef]
- Hong, Y.J.; Yoon, J.; Lee, J.; Kang, Y.C. One-Pot Synthesis of Pd-Loaded SnO2 Yolk-Shell Nanostructures for Ultraselective Methyl Benzene Sensors. Chem. Eur. J. 2014, 20, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yu, Y.; Lin, K.; Liu, P.; Zheng, C.; Wang, L.; Xu, T.; Wang, Z.; Wu, H. Easy hydrothermal synthesis of multi-shelled La2O3 hollow spheres for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 1232–1237. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Liu, J.L.; Qin, M.; Kou, K.C.; Wu, G.L.; Wu, H.J. Effective Cocatalyst Pt/PtO Nanodots on La2O3 Microspheres for Degradation of Methyl Orange. J. Nanosci. Nanotechnol. 2020, 20, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qu, S.; Zang, D.; Wang, L.; Wu, H. Fast Synthesis of Pt Nanocrystals and Pt/Microporous La2O3 Materials Using Acoustic Levitation. Nanoscale Res. Lett. 2018, 13, 50. [Google Scholar] [CrossRef] [Green Version]
- Cao, A.; Lu, R.; Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2010, 12, 13499–13510. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.Q.; Kleitz, F.; Chen, Z.G.; Yang, T.; Strounina, E.; Lu, G.Q.M.; Qiao, S.Z. Yolk-Shell Hybrid Materials with a Periodic Mesoporous Organosilica Shell: Ideal Nanoreactors for Selective Alcohol Oxidation. Adv. Funct. Mater. 2012, 22, 591–599. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q.; Yao, L.; Sun, K.; Wei, M.; Guo, E. Preparation and characterization of ultrathin Pt/CeO2/Bi2WO6 nanobelts with enhanced photoelectrochemical properties. Dyes Pigment. 2018, 149, 612–619. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y.C.; Ye, D. Effect of reduction treatment on structural properties of TiO2 supported Pt nanoparticles and their catalytic activity for formaldehyde oxidation. J. Mater. Chem. 2011, 21, 9647–9652. [Google Scholar] [CrossRef]
- Xing, J.; Jiang, H.B.; Chen, J.F.; Li, Y.H.; Wu, L.; Yang, S.; Zheng, L.R.; Wang, H.F.; Hu, P.; Zhao, H.J.; et al. Active sites on hydrogen evolution photocatalyst. J. Mater. Chem. A 2013, 1, 15258–15264. [Google Scholar] [CrossRef]

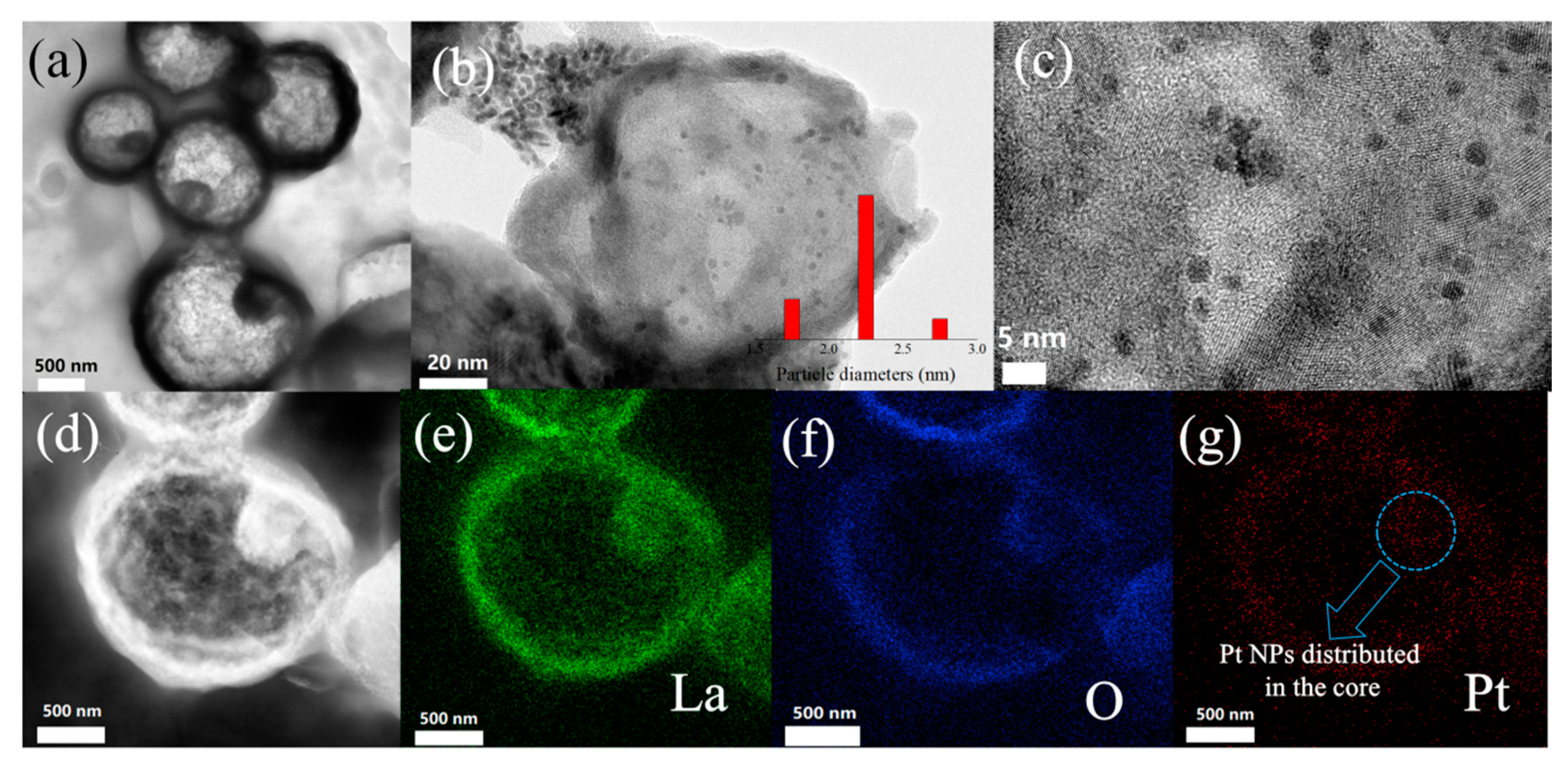
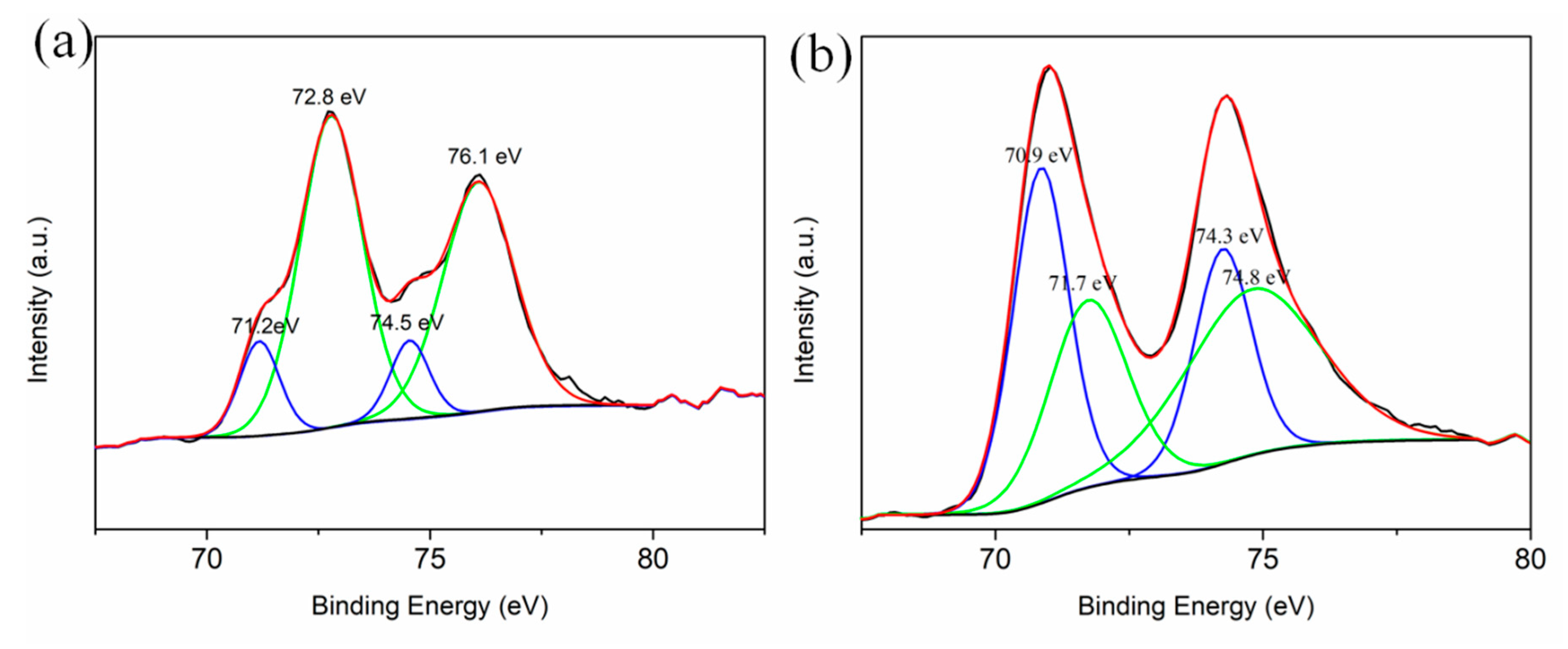
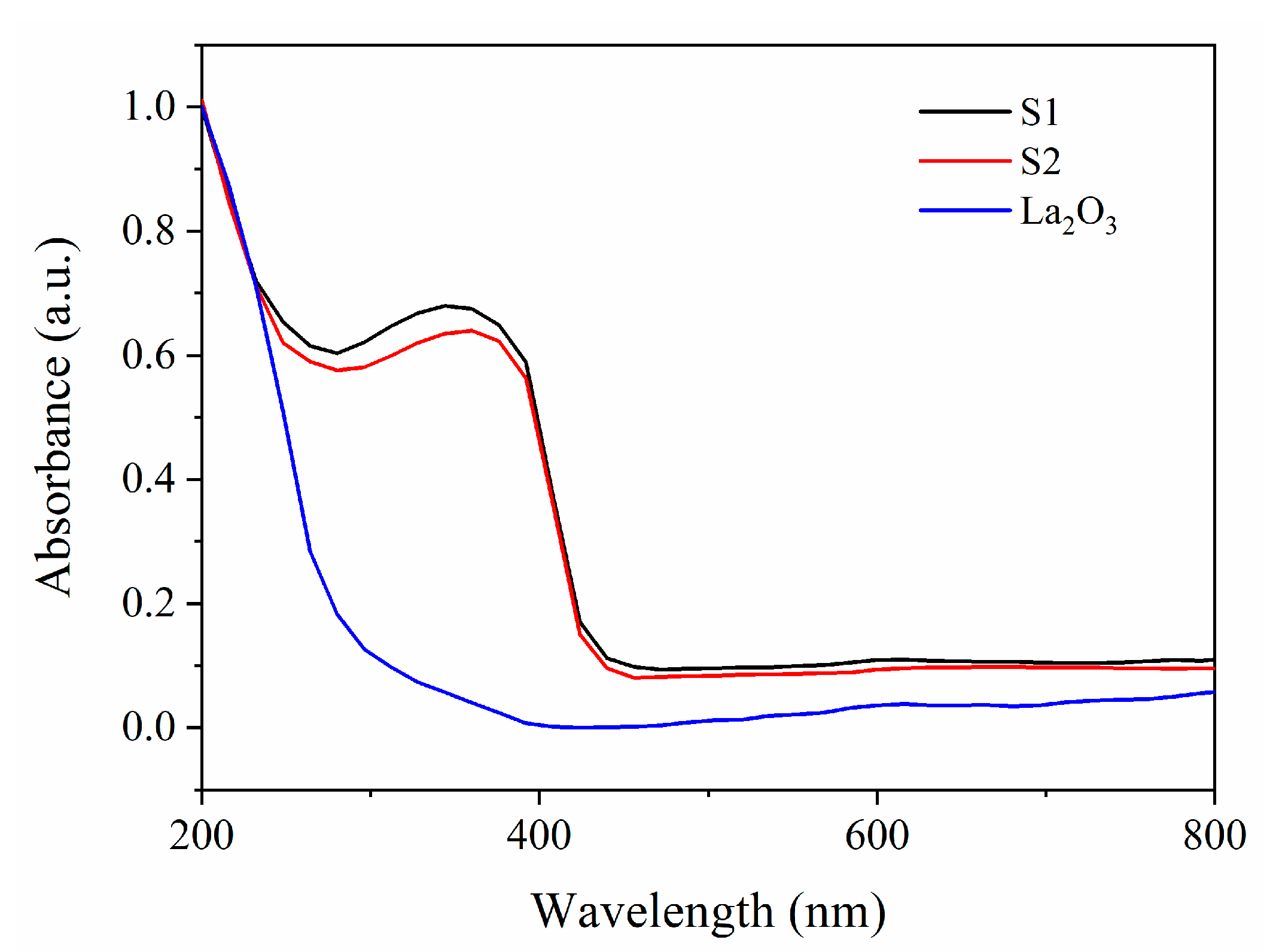
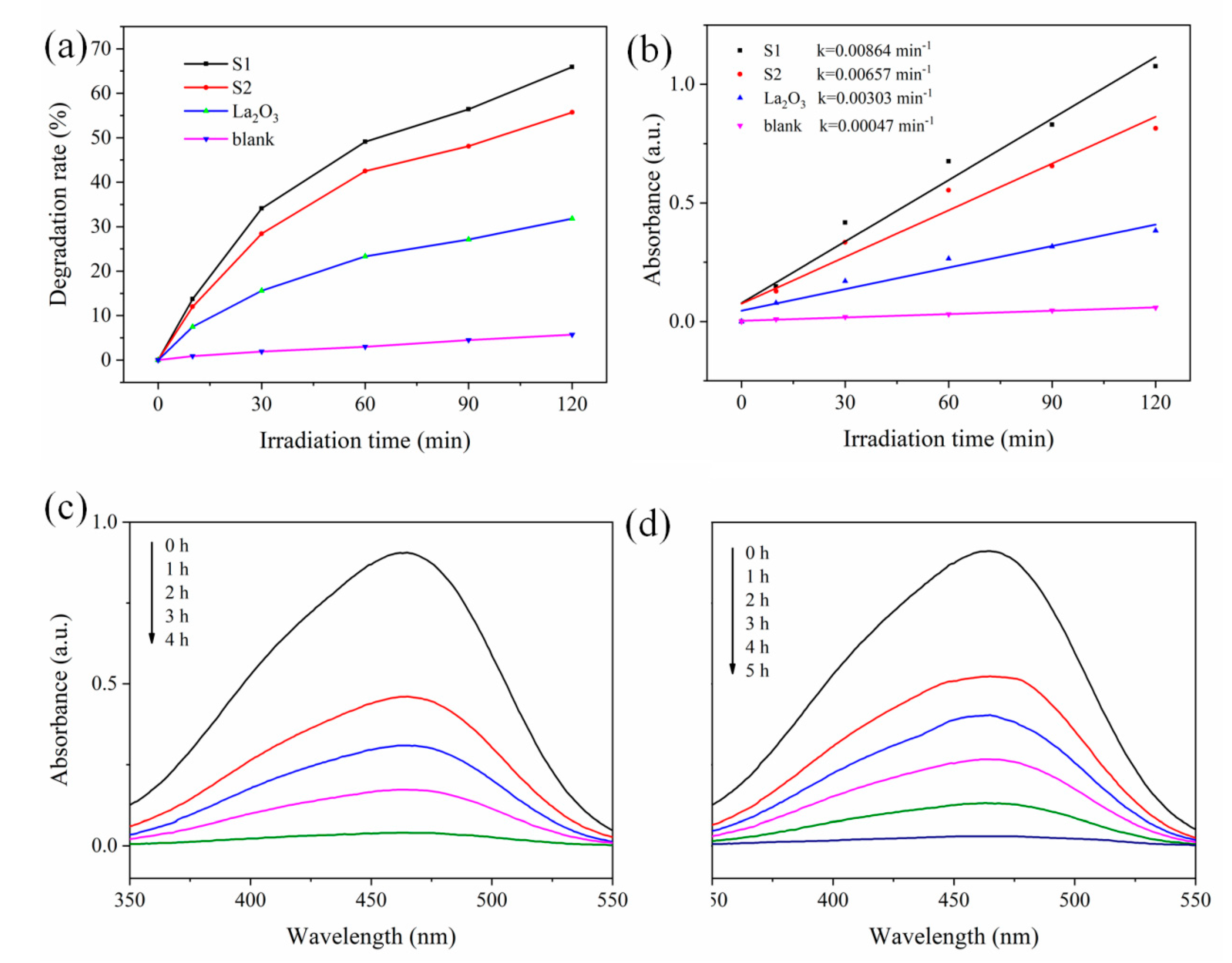
| Samples | Pt with Zero Valence State (%) | Pt with Oxidation State (%) |
|---|---|---|
| S1 | 28.37 | 71.63 |
| S2 | 56.25 | 43.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, M.; Chang, Q.; Yu, Y.; Wu, H. Exterior and Internal Uniform Loading of Pt Nanoparticles on Yolk-Shell La2O3 by Acoustic Levitation Synthesis with Enhanced Photocatalytic Performance. Materials 2020, 13, 107. https://doi.org/10.3390/ma13010107
Qin M, Chang Q, Yu Y, Wu H. Exterior and Internal Uniform Loading of Pt Nanoparticles on Yolk-Shell La2O3 by Acoustic Levitation Synthesis with Enhanced Photocatalytic Performance. Materials. 2020; 13(1):107. https://doi.org/10.3390/ma13010107
Chicago/Turabian StyleQin, Ming, Qing Chang, Yinkai Yu, and Hongjing Wu. 2020. "Exterior and Internal Uniform Loading of Pt Nanoparticles on Yolk-Shell La2O3 by Acoustic Levitation Synthesis with Enhanced Photocatalytic Performance" Materials 13, no. 1: 107. https://doi.org/10.3390/ma13010107






