Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions
Abstract
:1. Introduction
2. Low Dimension Carbon-Based Materials
2.1. Graphene
2.2. Graphene Oxide (GO)/Reduced Graphene Oxide (rGO)
2.3. Graphitic Carbon Nitride
2.4. Graphene Quantum Dots/Graphene Quantum Sheets
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 0D | Zero-Dimensional |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| BVO | BiVO4 |
| CB | Conduction Band |
| c-GQD | Inter-Connected Graphene Quantum Dots |
| CN | Carbon Nitride |
| CNT | Carbon Nanotube |
| CV | Cyclic Voltammetry |
| EC | Electrochemistry/Electrochemical |
| EDS | Energy Dispersive X-ray Spectroscopy |
| EHP | Electron-Hole Pair |
| FTO | Fluorine-doped Tin Oxide |
| g-C3N4 | Graphitic Carbon Nitride |
| GO | Graphene Oxide |
| GO QD | Graphene Oxide Quantum Dot |
| GQD | Graphene Quantum Dot |
| GQS | Graphene Quantum Sheet |
| Gr | Graphene |
| HAADF-STEM | High-angle Annular Dark-field Scanning Transmission Electron Microscopy |
| HER | Hydrogen Evolution Reaction |
| HRTEM | High-resolution Transmission Electron Microscopy |
| ITO | Indium-doped Tin Oxide |
| LDH | Layered Double Hydroxide |
| LSV | Linear Sweep Voltammetry |
| MNG | Melamine Nanogeodes |
| N-GQD | N-doped Graphene Quantum Dot |
| N-GQS | N-doped Graphene Quantum Sheet |
| NGR | N-doped Graphene |
| NP | Nanoparticle |
| OER | Oxygen Evolution Reaction |
| PEC | Photoelectrochemisty / Photoelectrochemical |
| rGO | Reduced Graphene Oxide |
| SEM | Scanning Electron Microscopy |
| SiNW | Silicon Nanowire |
| TCN | Tubular Carbon Nitride |
| TEOA | Triethanolamine |
| TNTA | TiO2 Nanotube Arrays |
| TVD | Thermal Vapor Deposition |
| VB | Valance Band |
| XRD | X-ray Diffraction |
References
- Sim, Y.; John, J.; Surendran, S.; Moon, B.; Sim, U. Efficient photoelectrochemical water splitting reaction using electrodeposited Co3Se4 catalyst. Appl. Sci. 2019, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- An, T.-Y.; Surendran, S.; Kim, H.; Choe, W.-S.; Kim, J.K.; Sim, U. A polydopamine-mediated biomimetic facile synthesis of molybdenum carbide-phosphide nanodots encapsulated in carbon shell for electrochemical hydrogen evolution reaction with long-term durability. Compos. Part B Eng. 2019, 175, 107071. [Google Scholar] [CrossRef]
- Kale, V.S.; Sim, U.; Yang, J.; Jin, K.; Chae, S.I.; Chang, W.J.; Sinha, A.K.; Ha, H.; Hwang, C.-C.; An, J.; et al. Sulfur-modified graphitic carbon nitride nanostructures as an efficient electrocatalyst for water oxidation. Small 2017, 13, 1603893. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Sim, U.; Kim, D.J.; Ahn, H.-Y.; An, J.; Ha, H.; Choi, K.S.; Jeon, C.; Lee, J.; Nam, K.T.; et al. Hierarchical carbon–silicon nanowire heterostructures for the hydrogen evolution reaction. Nanoscale 2018, 10, 13936–13941. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Sim, U.; Jin, K.; Oh, S.; Jeong, D.; Moon, J.; Oh, J.; Nam, K.T. Hydrogen production by electrolysis and photoelectrochemical system. In Handbook of Clean Energy Systems; Wiley: Hoboken, NJ, USA, 2015; pp. 1–42. [Google Scholar]
- Sim, Y.; John, J.; Moon, J.; Sim, U. Photo-assisted hydrogen evolution with reduced graphene oxide catalyst on silicon nanowire photocathode. Appl. Sci. 2018, 8, 2046. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Albero, J.; Mateo, D.; García, H. Graphene-based materials as efficient photocatalysts for water splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Lei, D.; Wong, K.-Y. Two-dimensional layered nanomaterials for visible-light-driven photocatalytic water splitting. Mater. Today Energy 2018, 10, 352–367. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Syu, J.-M.; Cheng, C.; Chang, T.-H.; Teng, H. Graphite oxide as a photocatalyst for hydrogen production from water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Wang, X.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Electronic and structural engineering of carbon-based metal-free electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1803625. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Albero, J.; Garcia, H.; Shalom, M. A water-splitting carbon nitride photoelectrochemical cell with efficient charge separation and remarkably low onset potential. Angew. Chem. 2018, 130, 16033–16037. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Chen, Y.P. Polycrystalline graphene and other two-dimensional materials. Nat. Nanotechnol. 2014, 9, 755–767. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced graphene oxide as a solid-state electron mediator in z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Cao, M.; Shi, W.; Wang, M.; Shen, Y. A new strategy of preparing uniform graphitic carbon nitride films for photoelectrochemical application. Carbon 2017, 117, 343–350. [Google Scholar] [CrossRef]
- Volokh, M.; Peng, G.; Barrio, J.; Shalom, M. Carbon nitride materials for water splitting photoelectrochemical cells. Angew. Chem. Int. Ed. 2019, 58, 6138–6151. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, F.; Zhang, H.; Jiang, B.; Hu, S.; Wu, B.; Wang, Y.; Fu, H. Graphene quantum-dot-modified hexagonal tubular carbon nitride for visible-light photocatalytic hydrogen evolution. Chem. Cat. Chem. 2018, 10, 1330–1335. [Google Scholar] [CrossRef]
- Moon, J.; An, J.; Sim, U.; Cho, S.-P.; Kang, J.H.; Chung, C.; Seo, J.-H.; Lee, J.; Nam, K.T.; Hong, B.H. One-step synthesis of n-doped graphene quantum sheets from monolayer graphene by nitrogen plasma. Adv. Mater. 2014, 26, 3501–3505. [Google Scholar] [CrossRef] [Green Version]
- Sim, U.; Moon, J.; An, J.; Kang, J.H.; Jerng, S.E.; Moon, J.; Cho, S.-P.; Hong, B.H.; Nam, K.T. N-doped graphene quantum sheets on silicon nanowire photocathodes for hydrogen production. Energy Environ. Sci. 2015, 8, 1329–1338. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, K.; Guo, B.; Liu, Q.; Fang, L.; Gong, J.R. Graphene-based materials for hydrogen generation from light-driven water splitting. Adv. Mater. 2013, 25, 3820–3839. [Google Scholar] [CrossRef]
- Hasani, A.; Tekalgne, M.; Van Le, Q.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: Hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Sa, R.; Ma, Z.; Li, Q.; Wu, K. Interfacial electronic structure and charge transfer of hybrid graphene quantum dot and graphitic carbon nitride nanocomposites: Insights into high efficiency for photocatalytic solar water splitting. Phys. Chem. Chem. Phys. 2016, 18, 1050–1058. [Google Scholar]
- Apell, S.P.; Hanson, G.W.; Hägglund, C. High Optical Absorption in Grapheme. 2012. Available online: https://arxiv.org/abs/1201.3071 (accessed on 24 December 2019).
- Bunch, J.S. Mechanical and Electrical Properties of Graphene Sheets; Cornell University Ithaca: Ithaca, NY, USA, 2008. [Google Scholar]
- Li, M.; Zhang, L.; Xu, Q.; Niu, J.; Xia, Z. N-doped graphene as catalysts for oxygen reduction and oxygen evolution reactions: Theoretical considerations. J. Catal. 2014, 314, 66–72. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Sun, Z.; Cao, T.; Zhang, Z.; Wang, E.; Liu, Z.; Liu, K. Interfacial engineering in graphene bandgap. Chem. Soc. Rev. 2018, 47, 3059–3099. [Google Scholar] [CrossRef] [PubMed]
- Raziq, F.; Qu, Y.; Zhang, X.; Humayun, M.; Wu, J.; Zada, A.; Yu, H.-T.; Sun, X.; Jing, L. Enhanced cocatalyst-free visible-light activities for photocatalytic fuel production of g-C3N4 by trapping holes and transferring electrons. J. Phys. Chem. C 2015, 120, 98–107. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Zhang, T.; Ma, J.; Liu, S.; Zhuang, X.; Yuan, C.; Feng, X. Efficient electrochemical and photoelectrochemical water splitting by a 3D nanostructured carbon supported on flexible exfoliated graphene foil. Adv. Mater. 2017, 29, 1604480. [Google Scholar] [CrossRef] [PubMed]
- Sim, U.; Moon, J.; Lee, J.; An, J.; Ahn, H.-Y.; Kim, D.J.; Jo, I.; Jeon, C.; Han, S.; Hong, B.H.; et al. Double-layer graphene outperforming monolayer as catalyst on silicon photocathode for hydrogen production. ACS Appl. Mater. Interfaces 2017, 9, 3570–3580. [Google Scholar] [CrossRef]
- Mou, Z.; Wu, Y.; Sun, J.; Yang, P.; Du, Y.; Lu, C. TiO2 Nanoparticles-functionalized n-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 13798–13806. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Zhou, Y.; Lu, J.; Zhou, K.; Yang, L.; Tang, Z.; Li, L.; Chen, S. N-doped carbon-wrapped cobalt nanoparticles on n-doped graphene nanosheets for high-efficiency hydrogen production. Chem. Mater. 2015, 27, 2026–2032. [Google Scholar] [CrossRef]
- Sim, U.; Yang, T.-Y.; Moon, J.; An, J.; Hwang, J.; Seo, J.-H.; Lee, J.; Kim, K.Y.; Lee, J.; Han, S.; et al. N-doped monolayer graphene catalyst on silicon photocathode for hydrogen production. Energy Environ. Sci. 2013, 6, 3658. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhou, S.-X.; Zhang, C.; Chang, H.-X.; Chen, Y.; Fu, W.-F. Synergetic effect of Cu and graphene as cocatalyst on TiO2 for enhanced photocatalytic hydrogen evolution from solar water splitting. J. Mater. Chem. 2012, 22, 18542. [Google Scholar] [CrossRef]
- Yoon, K.-Y.; Lee, J.-S.; Kim, K.; Bak, C.H.; Kim, S.-I.; Kim, J.-B.; Jang, J.-H. Hematite-based photoelectrochemical water splitting supported by inverse opal structures of graphene. ACS Appl. Mater. Interfaces 2014, 6, 22634–22639. [Google Scholar] [CrossRef]
- Joy, J.; Sekar, A.; Vijayaraghavan, S.; Kumar, P.; Pillai, V.K.; Alwarappan, S. Nickel-incorporated, nitrogen-doped graphene nanoribbons as efficient electrocatalysts for oxygen evolution reaction. J. Electrochem. Soc. 2018, 165, H141–H146. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, K.; Hou, D.; Liu, X.; Li, G.; Sang, Y.; Liu, H.; Li, L.; Chen, S. Three-dimensional hierarchical frameworks based on MoS2 nanosheets self-assembled on graphene oxide for efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2014, 6, 21534–21540. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Shang, X.; Han, G.-Q.; Dong, B.; Liu, Y.-R.; Li, X.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. MoSx supported graphene oxides with different degree of oxidation as efficient electrocatalysts for hydrogen evolution. Carbon 2016, 100, 236–242. [Google Scholar] [CrossRef]
- Sehrawat, P.; Islam, S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar]
- Zhang, Y.; Du, J.; Wang, Z.; Luo, M.; Tian, Y.; Fujita, T.; Xue, Q.; Chen, M. Three-dimensional nanoporous heterojunction of monolayer MoS2@rGO for photoenhanced hydrogen evolution reaction. ACS Appl. Energy Mater. 2018, 1, 2183–2191. [Google Scholar] [CrossRef]
- Mahvelati-Shamsabadi, T.; Goharshadi, E.K. ZnS nanospheres/reduced graphene oxide photoanode for highly efficient solar water oxidation. Sol. Energy 2018, 161, 226–234. [Google Scholar] [CrossRef]
- Ghorbani, M.; Abdizadeh, H.; Taheri, M.; Golobostanfard, M.R. Enhanced photoelectrochemical water splitting in hierarchical porous ZnO/Reduced graphene oxide nanocomposite synthesized by sol-gel method. Int. J. Hydrogen Energy 2018, 43, 7754–7763. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, M.; Sangpour, P.; Yousefzadeh, S.; Bagheri, M. Elevated temperature annealed α-Fe2O3/reduced graphene oxide nanocomposite photoanode for photoelectrochemical water oxidation. J. Environ. Chem. Eng. 2019, 7, 102999. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Su, W.-N.; Dubale, A.A.; Pan, C.-J.; Chen, H.-M.; Ayele, D.W.; Lee, J.-F.; Hwang, B.-J. Efficient photoelectrochemical water splitting using three dimensional urchin-like hematite nanostructure modified with reduced graphene oxide. J. Power Sources 2015, 287, 119–128. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible light-driven α-Fe2O3 nanorod/graphene/BiV1–xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Shao, M.; Xu, S.; Fu, Y.; Zhang, R.; Wei, M.; Evans, D.G.; Duan, X. TiO2/graphene/NiFe-layered double hydroxide nanorod array photoanodes for efficient photoelectrochemical water splitting. Energy Environ. Sci. 2016, 9, 2633–2643. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jin, B.; Zou, G.; Zhang, K.; Hu, X.; Park, J.H. Rationally designed copper-modified polymeric carbon nitride as a photocathode for solar water splitting. ChemSusChem 2019, 12, 866–872. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, J.; Wang, K.; Qiu, Y.; Liang, Q.; Chen, Z. Efficient photoelectrochemical water splitting by g-C3N4/TiO2 nanotube array heterostructures. Nano Micro Lett. 2018, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Wang, T.; Gao, B.; Xie, X.; Zhang, S.; Meng, X.; Gong, H.; Guo, Y.; Huang, X.; He, J. Layered double hydroxides decorated graphic carbon nitride film as efficient photoanodes for photoelectrochemical water splitting. Catal. Today 2019, 335, 423–428. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Z.P.; Moehwald, H.; Fiechter, S.; van de Krol, R.; Wen, L.; Jiang, L.; Antonietti, M. Microcontact-printing-assisted access of graphitic carbon nitride films with favorable textures toward photoelectrochemical application. Adv. Mater. 2015, 27, 712–718. [Google Scholar] [CrossRef]
- Peng, G.; Xing, L.; Barrio, J.; Volokh, M.; Shalom, M. Frontispiz: A general synthesis of porous carbon nitride films with tunable surface area and photophysical properties. Angew. Chem. 2018, 130, 1186–1192. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Jailani, M.F.A.M.; Khalid, M.N.; Noh, M.F.M.; Aadenan, A.; Nasir, S.N.S.; Sagu, J.S.; Teridi, M.A.M. The influences of post-annealing temperatures on fabrication graphitic carbon nitride, (g-C3N4) thin film. Appl. Surf. Sci. 2019, 489, 92–100. [Google Scholar] [CrossRef]
- Sima, M.; Vasile, E.; Sima, A.; Preda, N.; Logofatu, C. Graphitic carbon nitride based photoanodes prepared by spray coating method. Int. J. Hydrogen Energy 2019, 44, 24430–24440. [Google Scholar] [CrossRef]
- Bian, J.; Li, Q.; Huang, C.; Li, J.; Guo, Y.; Zaw, M.; Zhang, R.-Q. Thermal vapor condensation of uniform graphitic carbon nitride films with remarkable photocurrent density for photoelectrochemical applications. Nano Energy 2015, 15, 353–361. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Li, J.; Zhang, J.; Guo, Z. Novel framework g-C3N4 film as efficient photoanode for photoelectrochemical water splitting. Appl. Catal. B Environ. 2017, 209, 657–662. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Gao, B.; Gong, H.; Xue, H.; Guo, H.; Song, L.; Xia, W.; Huang, X.; He, J. Preparation of the TiO2/graphic carbon nitride core–shell array as a photoanode for efficient photoelectrochemical water splitting. Langmuir 2016, 32, 13322–13332. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride nanosheet–carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. 2014, 126, 7409–7413. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Teng, C.-Y.; Chen, S.-J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Guo, C.X.; Dong, Y.; Bin Yang, H.; Li, C.M. Graphene quantum dots as a green sensitizer to functionalize ZnO nanowire arrays on F-Doped SnO2 glass for enhanced photoelectrochemical water splitting. Adv. Energy Mater. 2013, 3, 997–1003. [Google Scholar] [CrossRef]
- Bian, S.; Zhou, C.; Li, P.; Xi, F.; Liu, J.; Dong, X. Graphene quantum dots decorated titania nanosheets heterojunction: Efficient charge separation and enhanced visible-light photocatalytic performance. ChemCatChem 2017, 9, 3349–3357. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.-B.; Chen, S.; Chen, P.; Xiao, F.-X. Insight into the charge transport correlation in Au x clusters and graphene quantum dots deposited on TiO2 nanotubes for photoelectrochemical oxygen evolution. J. Mater. Chem. A 2018, 6, 11154–11162. [Google Scholar] [CrossRef]
- Chan, D.K.L.; Cheung, P.L.; Yu, J.C. A visible-light-driven composite photocatalyst of TiO2 nanotube arrays and graphene quantum dots. Beilstein J. Nanotechnol. 2014, 5, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Malik, B.; Pattanayak, D.K.; Pitchai, R.; Pillai, V.K. Unraveling the hydrogen evolution reaction active sites in n-functionalized interconnected graphene quantum dots. ChemistrySelect 2017, 2, 4511–4515. [Google Scholar] [CrossRef]
- Luo, P.; Jiang, L.; Zhang, W.; Guan, X. Graphene quantum dots/Au hybrid nanoparticles as electrocatalyst for hydrogen evolution reaction. Chem. Phys. Lett. 2015, 641, 29–32. [Google Scholar] [CrossRef]
- Yang, K.D.; Ha, Y.; Sim, U.; An, J.; Lee, C.W.; Jin, K.; Kim, Y.; Park, J.; Hong, J.S.; Lee, J.H.; et al. Graphene quantum sheet catalyzed silicon photocathode for selective CO2 conversion to CO. Adv. Funct. Mater. 2016, 26, 233–242. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L.; Zhu, J.; Bian, X.; Zhu, L.; Scanlon, M.D.; Girault, H.; Liu, B. MoS 2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv. Funct. Mater. 2013, 23, 5326–5333. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.-J.; Kong, C.; Li, X.; Sun, X.-Y.; Xie, W.-J.; Oh, W.-C. Synthesis and characterization of MoS2/Graphene-TiO2 ternary photocatalysts for high-efficiency hydrogen production under visible light. J. Korean Ceram. Soc. 2019, 56, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ma, Y.; Hu, X.; Liu, E.; Fan, J. Enhanced photocatalytic H2 production over dual-cocatalyst-modified g-C3N4 heterojunctions. Chin. J. Catal. 2019, 40, 434–445. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, M.; Gao, J.; Lv, P.; Liu, X.; Li, C.; Yan, Y. Fabrication of nitrogen-doped graphene quantum dots-Cu2O catalysts for enhanced photocatalytic hydrogen evolution. Nano 2018, 13, 1850099. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, P.; Wang, C.; Zhang, X.; Zhang, C. Silicon nanowires/reduced graphene oxide composites for enhanced photoelectrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 1961–1966. [Google Scholar] [CrossRef]
- Dubale, A.A.; Su, W.-N.; Tamirat, A.G.; Pan, C.-J.; Aragaw, B.A.; Chen, H.-M.; Chen, C.-H.; Hwang, B.-J. The synergetic effect of graphene on Cu2O nanowire arrays as a highly efficient hydrogen evolution photocathode in water splitting. J. Mater. Chem. A 2014, 2, 18383–18397. [Google Scholar] [CrossRef]
- Shah, A.K.; Sahu, T.K.; Banik, A.; Gogoi, D.; Peela, N.R.; Qureshi, M. Reduced graphene oxide modified CuBi2O4 as an efficient and noble metal free photocathode for superior photoelectrochemical hydrogen production. Sustain. Energy Fuels 2019, 3, 1554–1561. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Menati, M. Fabrication, characterization and photoelectrochemical properties of cuprous oxide-reduced graphene oxide photocatalysts for hydrogen generation. J. Mater. Sci. Mater. Electron. 2018, 29, 4136–4146. [Google Scholar] [CrossRef]
- Kang, S.; Jang, J.; Pawar, R.C.; Ahn, S.; Lee, C.S. Direct coating of a g-C3N4 layer onto one-dimensional TiO2 nanocluster/nanorod films for photoactive applications. Dalton Trans. 2018, 47, 7237–7244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Li, H.; Wei, X.; Feng, J.; Liu, K.; Dang, Y.; Zhou, A. Efficient and stable photoelectrochemical seawater splitting with TiO2@g-C3N4 nanorod arrays decorated by Co-Pi. J. Phys. Chem. C 2015, 119, 20283–20292. [Google Scholar] [CrossRef]
- Alam, K.M.; Kumar, P.; Kar, P.; Thakur, U.K.; Zeng, S.; Cui, K.; Shankar, K. Enhanced charge separation in g-C3N4–BiOI heterostructures for visible light driven photoelectrochemical water splitting. Nanoscale Adv. 2019, 1, 1460–1471. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Volokh, M.; Tzadikov, J.; Sun, J.; Shalom, M. Carbon nitride/reduced graphene oxide film with enhanced electron diffusion length: An efficient photo-electrochemical cell for hydrogen generation. Adv. Energy Mater. 2018, 8, 1800566. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B.-K. Efficient promotion of charge separation with reduced graphene oxide (rGO) in BiVO4/rGO photoanode for greatly enhanced photoelectrochemical water splitting. Sol. Energy Mater. Sol. Cells 2018, 185, 325–332. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Hur, S.H.; Kim, E.J.; Rajagopalan, B.; Babu, K.F.; Senthilkumar, V.; Chung, J.S.; Choi, W.M.; Kim, Y.S. Highly-ordered maghemite/reduced graphene oxide nanocomposites for high-performance photoelectrochemical water splitting. RSC Adv. 2015, 5, 29159–29166. [Google Scholar] [CrossRef]
- Majumder, T.; Debnath, K.; Dhar, S.; Hmar, J.J.L.; Mondal, S.P. Nitrogen-doped graphene quantum dot-decorated ZnO nanorods for improved electrochemical solar energy conversion. Energy Technol. 2016, 4, 950–958. [Google Scholar] [CrossRef]

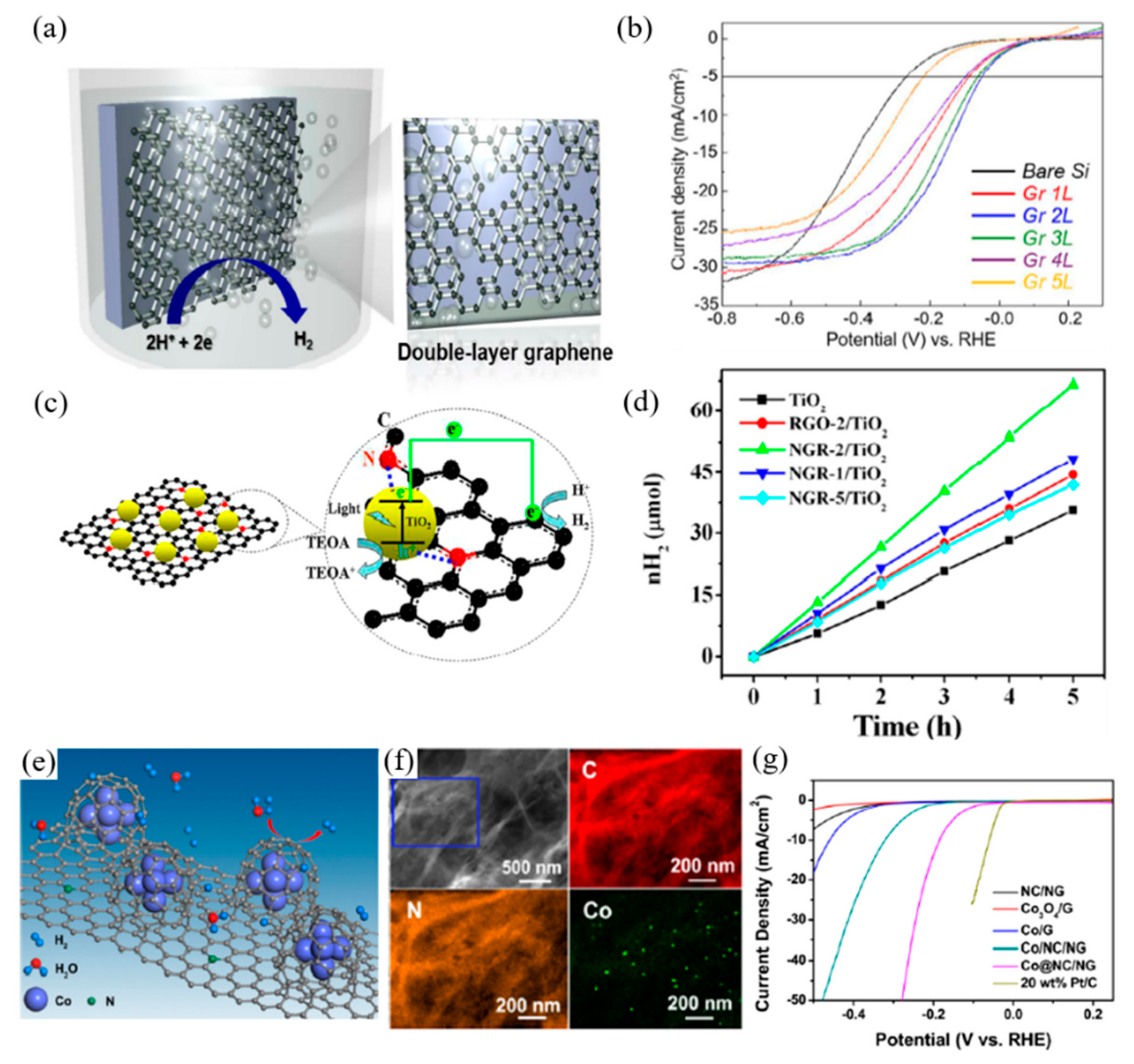



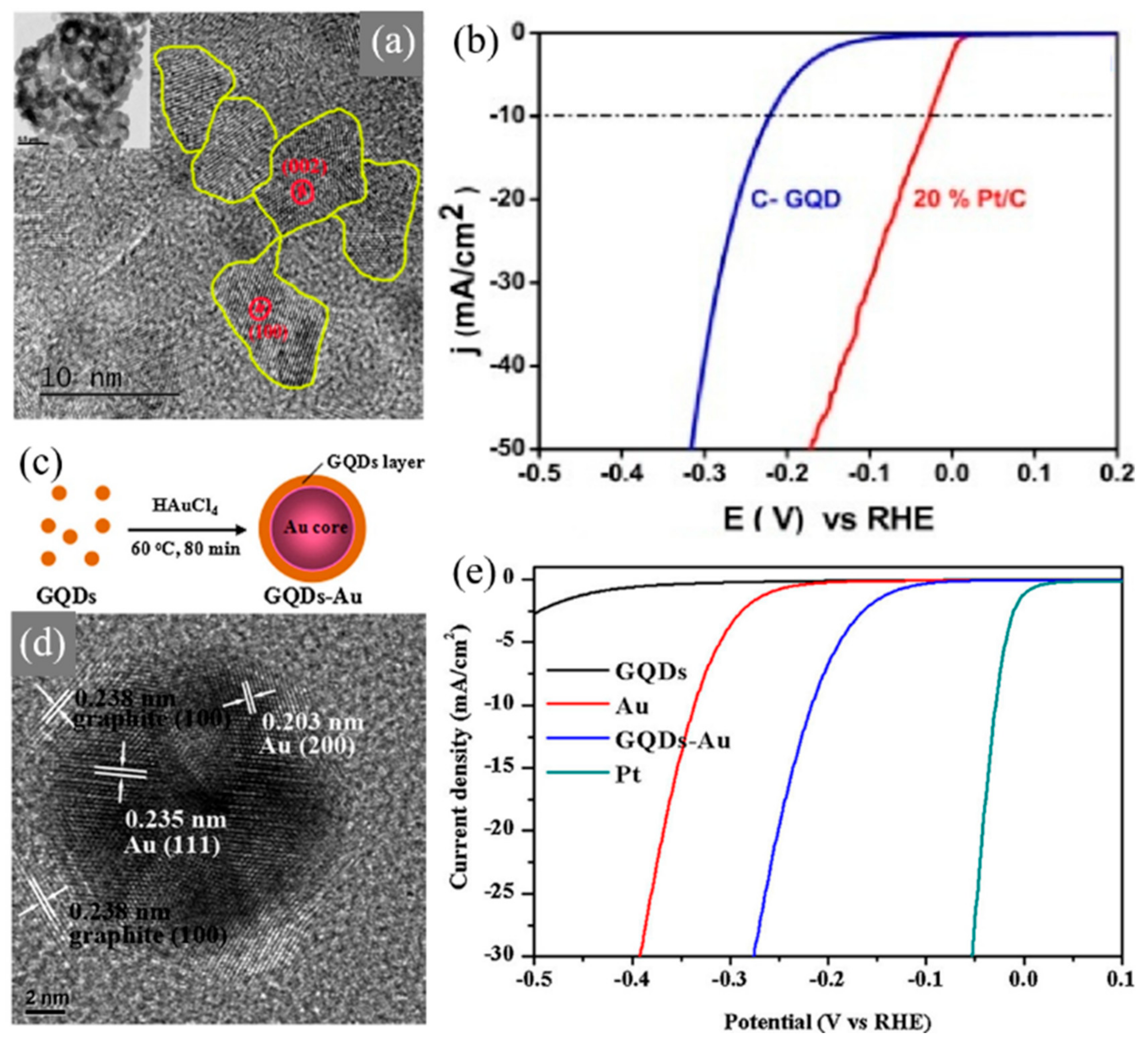

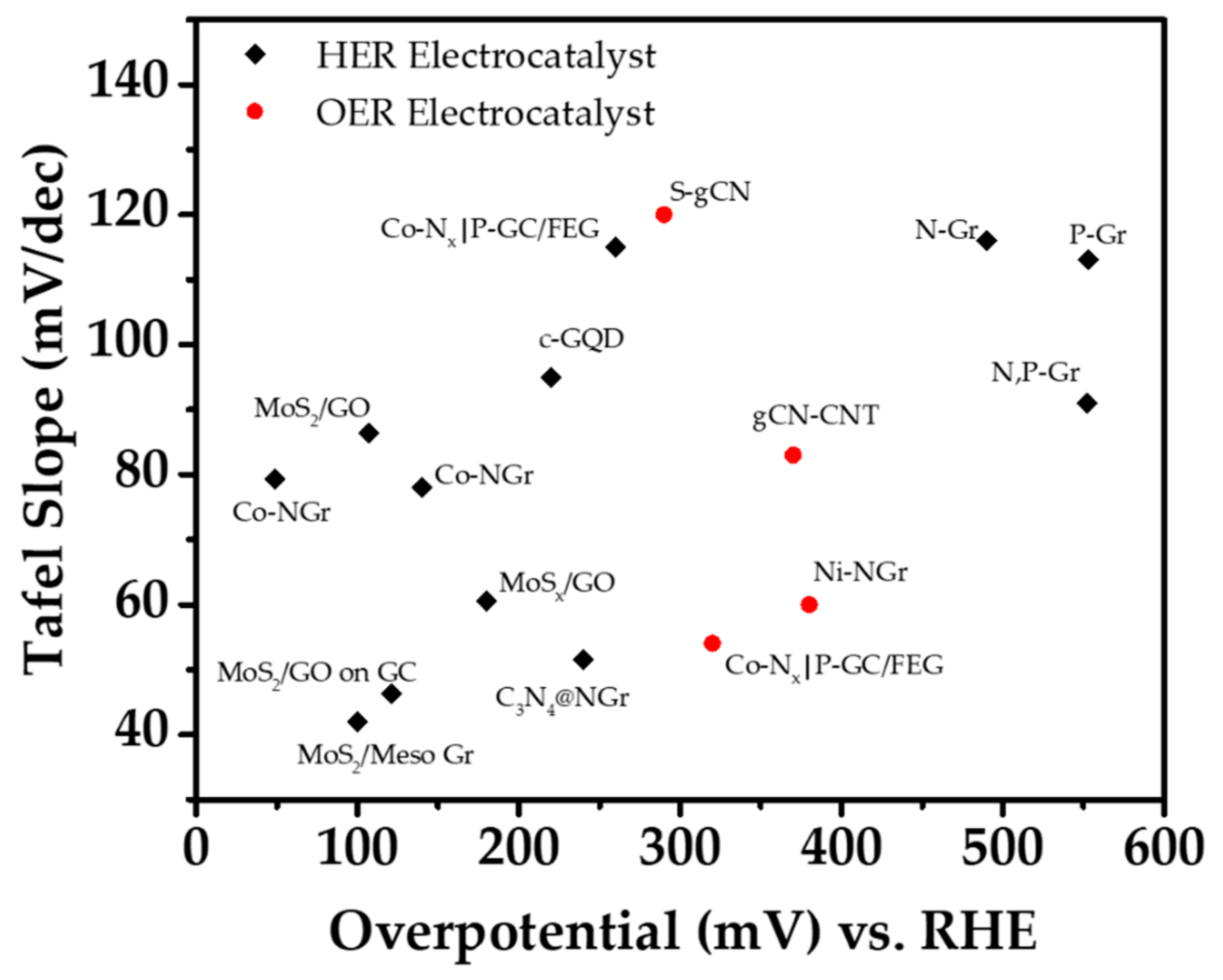
| Reaction | Material | Overpotential (mV vs. RHE) | Tafel Slope (mV/dec) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| HER | N-Gr | 490 | 116 | 0.5M H2SO4 | [76] |
| P-Gr | 553 | 113 | |||
| N, P-Gr | 552 | 91 | |||
| Co-NGR | 49 | 79.3 | 0.5M H2SO4 | [44] | |
| C3N4@NG | 240 | 51.5 | 0.5M H2SO4 | [77] | |
| MoS2/Meso graphene | 100 | 42 | 0.5M H2SO4 | [78] | |
| Co-Nx|P-GC/FEG | 260 | 115 | 1M KOH | [37] | |
| MoSx/GO | 180 | 60.5 | 0.5M H2SO4 | [47] | |
| MoS2/GO | 107 | 86.3 | 0.5M H2SO4 | [46] | |
| MoS2/GO on Glassy Carbon | 121 | 46.3 | 0.5M H2SO4 | ||
| c-GQD | 220 | 95 | 0.5M H2SO4 | [73] | |
| GQD/Au | 140 | 78 | 0.5M H2SO4 | [74] | |
| OER | Co-Nx|P-GC/FEG | 320 | 54 | 1M KOH | [37] |
| Ni-NGR | 380 | 60 | 1M KOH | [43] | |
| S-g-C3N4 | 290 | 120 | 1M (KOH + NaClO4) | [3] | |
| g-C3N4/CNT | 370 | 83 | 0.1M KOH | [67] |
| Material | H2 Generation Rate | Electrolyte | Light Source | Ref. |
|---|---|---|---|---|
| Cu/Gr/TiO2 | 63.75 mmoL/g·h | 0.1M NaClO4 + 10 vol.% methanol | 300 W Hg Lamp | [40] |
| NGr/TiO2 | 13.72 μmoL/h | TEOA 10 vol.% | 150W Xe Lamp | [42] |
| MoS2/Graphene-TiO2 | 1989 μmoL/g·h | 20 vol.% methanol | 300W Xe Lamp 545 mW/cm2 | [79] |
| GO | 5.67 mmoL/g·h | 20 vol.% methanol | 400W Hg Lamp | [12] |
| TiO2/B-g-C3N4 | 150 μmoL/g·h | 20 vol.% methanol | 300 W Xe Lamp 420 nm filter | [36] |
| NiS/Ag/g-C3N4 | 9.728 mmoL/g·h | TEOA 10 vol.% | 300 W Xe Lamp 46.31 mW/cm2 | [80] |
| P-TCN/GQDs | 112.1 μmoL/h | 20 vol.% methanol | 300 W Xe Lamp 420 nm filter | [26] |
| N-GO QD | 0.45 μmol/g h | Water | 300 W Xe Lamp 420 nm < λ < 800 nm | [68] |
| NGQDs-Cu2O | 22.6 μmol/g h | 20 vol.% methanol | 300 W Xe Lamp 420 nm filter | [81] |
| Material | Onset Potential (@ −1 mA/cm2) V vs. RHE | Over Potential (@ −10 mA/cm2) V vs. RHE | Electrolyte | Light Source | Ref. |
| Gr-Si | 0.01 | −0.21 | 1M HClO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [38] |
| NGr-Si | 0.12 | −0.04 | |||
| Pt-NGr-Si | 0.35 | 0.25 | |||
| double-layer Gr-Si | 0.05 | −0.11 | 1M HClO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [39] |
| Plasma Double-layer Gr-Si | 0.15 | 0.01 | |||
| SiNW/rGO | 0.08 * | −0.13 * | H2SO4 + 0.5M K2SO4 | 300W Xe Lamp 100 mW/cm2 | [82] |
| MoS2/rGO | −0.048 * | −0.141 * | 0.5M H2SO4 | AM1.5 100 mW/cm2 | [49] |
| rGO-SiNW | 0.326 | 0.239 | 1M HClO4 | 100W Xe Lamp AM1.5 100 mW/cm2 | [7] |
| GQS-bare Si | 0.12 | 0.01 (@ −5mA/cm2) | 1M HClO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [27] |
| GQS-porous Si | 0.16 | 0.08 (@ −5mA/cm2) | 1M HClO4 | ||
| N-GQSs/planar Si | 0.13 | −0.04 | 1M HClO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [28] |
| N-GQSs/ SiNW | 0.26 | 0.16 | 1M HClO4 | ||
| Material | Photocurrent Density (mA/cm2) | Measured Potential | Electrolyte | Light Source | Ref. |
| Cu-CN-W | 200 μA/cm2 | 0.42 V vs. RHE | 0.2M Na2SO4 | 300W Xe Lamp AM1.5 420 nm filter | [57] |
| Gr/Cu2O/Cu mesh | 4.8 | 0 V vs. RHE | 1M Na2SO4 + 0.1M Potassium Phosphate | AM1.5 100 mW/cm2 | [83] |
| CuBi2O4/rGO | 0.94 | 0 V vs. RHE | 0.5M Na2SO4 | 300W Halogen Lamp 100 mW/cm2 | [84] |
| rGO/Cu2O/Cu foil | 2.3 | 0 V vs. RHE | 0.5M Na2SO4 | 50W Halogen Tungsten Lamp 85 mW/cm2 | [85] |
| Material | Photocurrent Density (mA/cm2) | Measured Potential | Electrolyte | Light Source | Ref. |
|---|---|---|---|---|---|
| g-C3N4 | 89 μA/cm2 | 1.1 V vs. RHE | 0.1M Na2SO4 | Xe Lamp AM1.5 100 mW/cm2 | [65] |
| g-C3N4 | 0.12 | 1.55 V vs. RHE | 0.1M Na2SO4 + 0.01M Na2S | Xe lamp AM1.5 100 mW/cm2 | [64] |
| g-C3N4, dicyanamide | 63 μA/cm2 | 1.23 V vs. RHE | 0.1M Na2SO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [23] |
| g-C3N4, melamine | 52 μA/cm2 | ||||
| g-C3N4, cyanamide | 39 μA/cm2 | ||||
| g-C3N4 | 20.73 μA/cm2 | 1.23 V vs. Ag/AgCl | 0.5M Na2SO4 | Xe Lamp 100 mW/cm2 | [62] |
| g-C3N4 | 45 μA/cm2 | 0.86 V vs. RHE | 0.2M Na2SO4 | 500 W Xe Lamp 420 nm filter | [60] |
| g-C3N4/NiCo-LDH | 11.8 μA/cm2 | 0.6 V vs. SCE | 0.2M Na2SO4 | 200 W Xe lamp 100 mW/cm2 420 nm filter | [59] |
| TiO2/g-C3N4 | 3.6 μA/cm2 | 1.23 V vs. RHE | 0.5M Na2SO4 | 300 W Xe Lamp AM1.5 100 mW/cm2 | [63] |
| TiO2/g-C3N4 Core Shell array | 80.9 μA/cm2 | 0.6 V vs. SCE | 0.2M Na2SO4 | 200 W Xe Lamp 20 mW/cm2 420 nm filter | [66] |
| TiO2-CN | 29.4 μA/cm2 | 1.23 V vs. RHE | 0.5M Na2SO4 | 300W Xe Lamp AM 1.5 420 nm filter | [86] |
| TiO2@ g-C3N4@CoPi | 1.6 | 1.23 V vs. RHE | 0.1M Na2SO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [87] |
| g-C3N4/BiOI | 0.7 | 0.8 V vs. Ag/AgCl | 0.1M Na2SO4 | AM1.5 100 mW/cm2 | [88] |
| g-C3N4/TNTA | 0.86 | 1.23 V vs. RHE | 0.1M Na2SO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [58] |
| Co-Nx|P-GC/FEG/Fe2O3 | 2.15 | 1.23 V vs. RHE | 1M KOH | AM1.5 100 mW/cm2 | [37] |
| CN/rGO | 72 μA/cm2 | 1.23 V vs. RHE | 0.1M KOH | AM1.5 100 mW/cm2 | [89] |
| 660 μA/cm2 | 1.23 V vs. RHE | 0.1M KOH + 10 vol.% TEOA | |||
| TiO2/rGO/NiFe-LDH | 1.74 | 0.6 V vs. SCE | 0.5M Na2SO4 | 150W Xe Lamp 100 mW/cm2 | [55] |
| Fe2O3-rGO | 1.06 | 1.23 V vs. RHE | 1M NaOH | 300W Xe Lamp AM1.5 100 mW/cm2 | [53] |
| BVO/rGO | 554.44 μA/cm2 | 1.2 V vs. Ag/AgCl | 0.1M Na2SO4 | 300W Xe Lamp AM1.5 100 mW/cm2 | [90] |
| rGO/γ-Fe2O3 | 6.74 | 1.8 V vs. RHE | 1M NaOH | 360 nm UV light | [91] |
| α-Fe2O3/Gr/BiV1−xMoxO4 | 1.97 | 1.0 V vs. Ag/AgCl | 0.01M Na2SO4 | 150W Xe Lamp AM1.5 64 mW/cm2 420 nm filter | [54] |
| Aux/GQDs/NP-TNTAs | 1.1 * | 0 V vs. Ag/AgCl | 0.5M Na2SO4 | 300 W Xe Lamp AM1.5 100 mW/cm2 | [71] |
| GQD@ZnO | 0.34 | 0.6 V vs. Pt | 0.5M Na2SO4 | 150 W Xe Lamp 100 mW/cm2 | [69] |
| N-GQD-ZnO | 2.45 | 1 V vs. Ag/AgCl | 0.1M Na2SO4 | 270W Xe Lamp | [92] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials 2020, 13, 114. https://doi.org/10.3390/ma13010114
Lim Y, Lee D-K, Kim SM, Park W, Cho SY, Sim U. Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials. 2020; 13(1):114. https://doi.org/10.3390/ma13010114
Chicago/Turabian StyleLim, Yoongu, Dong-Kyu Lee, Seong Min Kim, Woosung Park, Sung Yong Cho, and Uk Sim. 2020. "Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions" Materials 13, no. 1: 114. https://doi.org/10.3390/ma13010114
APA StyleLim, Y., Lee, D.-K., Kim, S. M., Park, W., Cho, S. Y., & Sim, U. (2020). Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials, 13(1), 114. https://doi.org/10.3390/ma13010114






