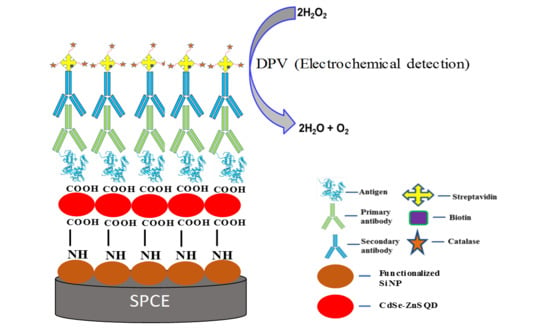
Surface Enhanced CdSe/ZnS QD/SiNP Electrochemical Immunosensor for the Detection of Mycobacterium Tuberculosis by Combination of CFP10-ESAT6 for Better Diagnostic Specificity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Preparation of CdSe/ZnS QD/SiNPs/SPCE
2.4. Immunoassay Procedures
3. Results and Discussion
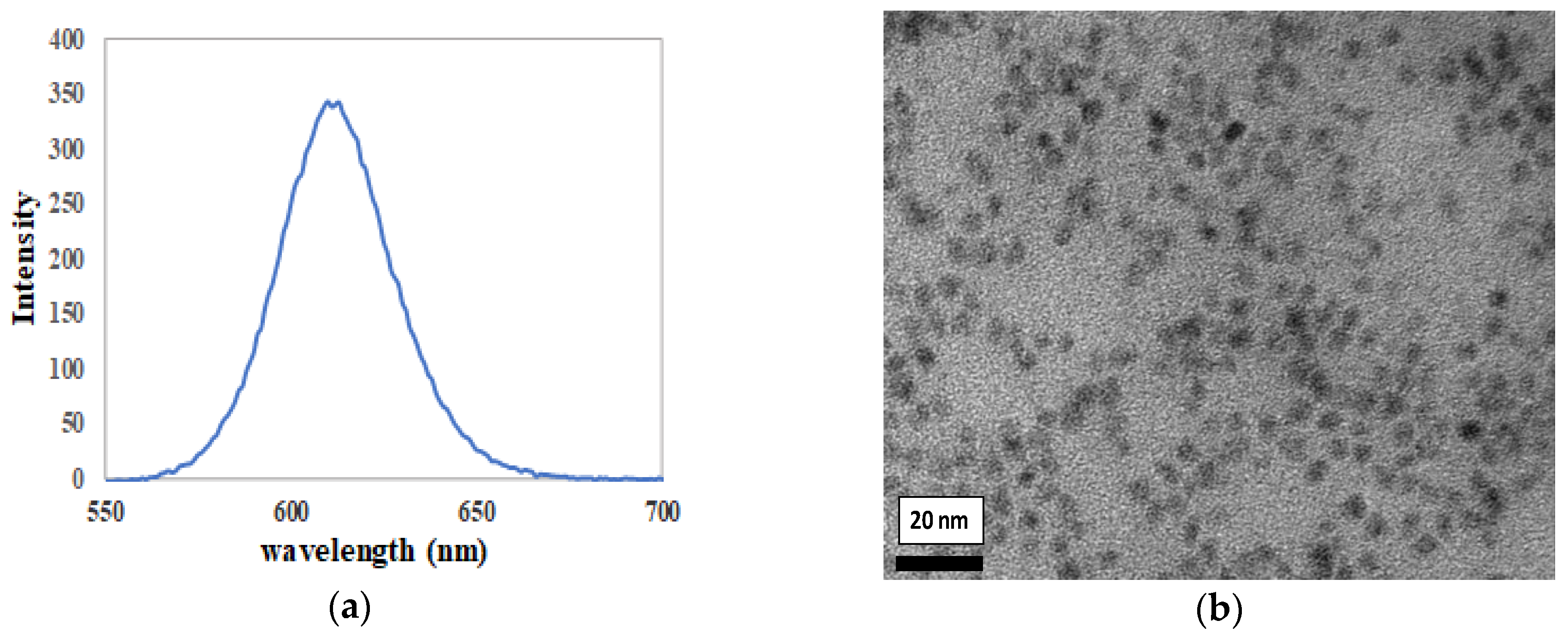
3.1. Functionalized Silica Nanoparticles
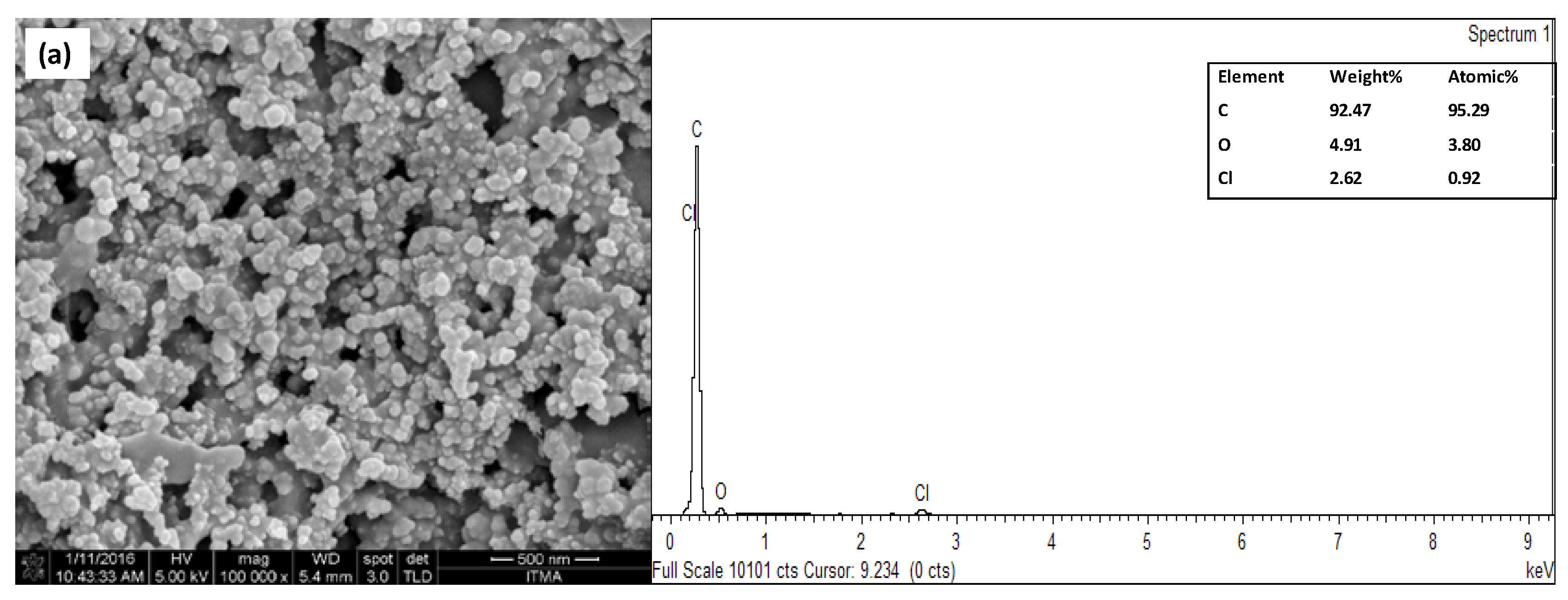
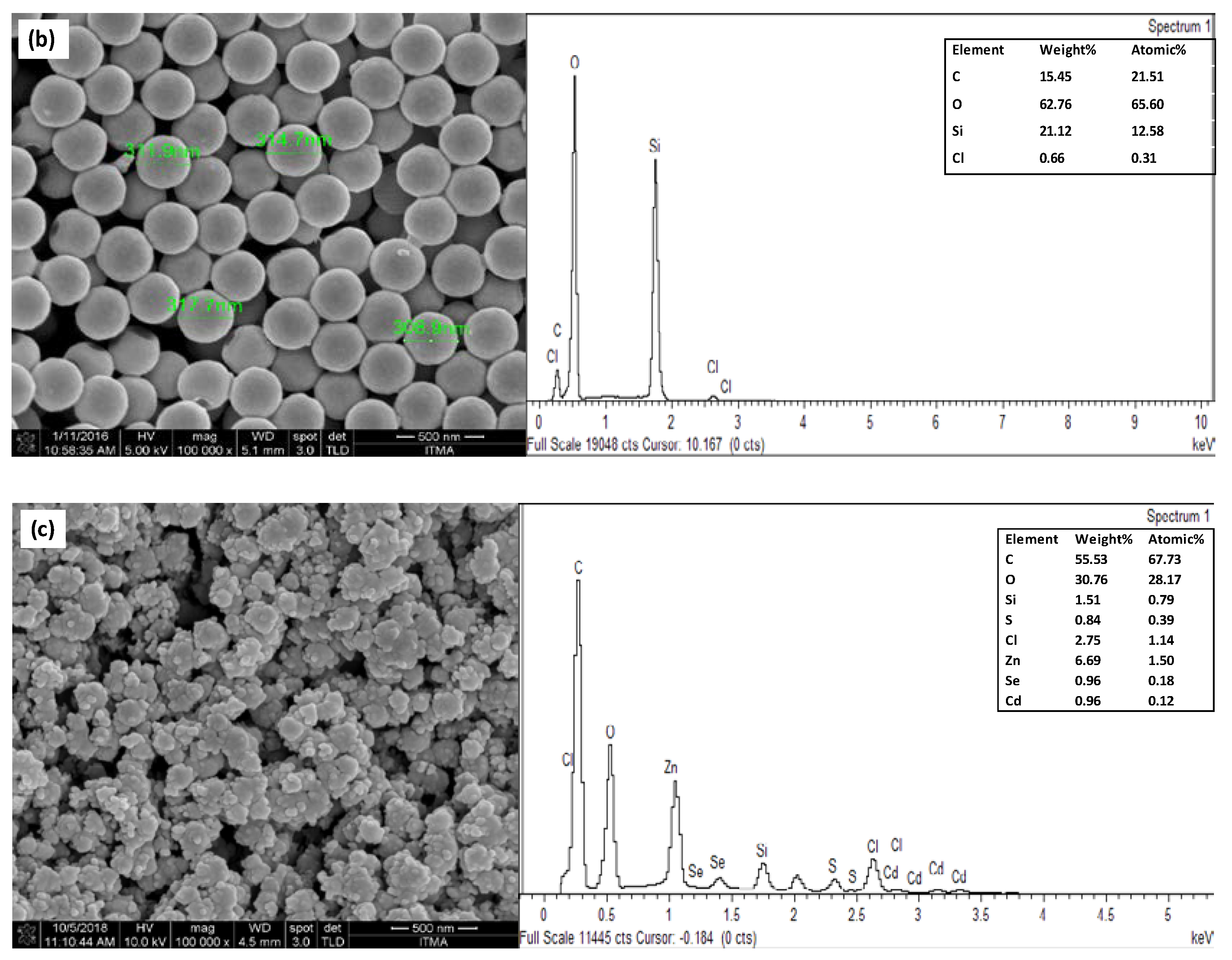
3.2. Surface Characterization of Modified Electrode
3.3. Electrochemical Characterization of SiNPs/SPCE and CdSe/ZnS QD/SiNPs/SPCE
3.4. Immunoreaction for CFP10–ESAT6 Detection using DPV
3.5. Selectivity, Sensitivity, and Reproducibility Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McNerney, R.; Maeurer, M.; Abubakar, I.; Marais, B.; McHugh, T.D.; Ford, N.; Zumla, A. Tuberculosis diagnostics and biomarkers: Needs, challenges, recent advances, and opportunities. J. Infect. Dis. 2012, 205, 1–12. [Google Scholar] [CrossRef] [PubMed]
- William, T.; Parameswaran, U.; Lee, W.K.; Yeo, T.W.; Anstey, N.M.; Ralph, A.P. Pulmonary tuberculosis in outpatients in Sabah, Malaysia: Advanced disease but low incidence of HIV co-infection. BMC Infect. Dis. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dande, P.; Samant, P. Acquaintance to Artificial Neural Networks and use of artificial intelligence as a diagnostic tool for tuberculosis: A review. Tuberculosis 2018, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ayub, M.; Nasir, M.; Khattak, K. Prevalence of sputum smear positive pulmonary tuberculosis at Dargai, District Malakand, Pakistan: A four year retrospective study. Egypt. J. Chest Dis. Tuberc. 2016, 65, 461–464. [Google Scholar] [CrossRef]
- Suraiya, S.; Musa, M.; Suppian, R.J.A.H. Serological diagnosis for active tuberculosis in Malaysian population: Comparison of four protein candidat. Asian Pac. J. Trop. Dis. 2012, 2, s312–s315. [Google Scholar] [CrossRef]
- Tama, L.; Hansted, E.; Vitkauskien, A.; Miliauskas, S.; Naud, A.; Brigita, Š. ScienceDirect use of interferon-gamma release assay and tuberculin skin test in diagnosing tuberculosis in Lithuanian adults: A comparative analysis. Medicina 2017, 53, 159–165. [Google Scholar]
- Tammam, S.N.; Khalil, M.A.F.; Abdul Gawad, E.; Althani, A.; Zaghloul, H.; Azzazy, H.M.E. Chitosan gold nanoparticles for detection of amplified nucleic acids isolated from sputum. Carbohydr. Polym. 2017, 164, 57–63. [Google Scholar] [CrossRef]
- Jaramillo, M.; Montagut, Y.J.; Montoya, A.; Robledo, J.; Marin, P.A.; Betancur, J.E.; Torres, R.A. Design of a piezoelectric immunosensor for tuberculosis biomarker detection. Pan Am. Health Care Exch. 2017, 1–7. [Google Scholar] [CrossRef]
- Arora, J.; Kumar, G.; Verma, A.K.; Bhalla, M.; Sarin, R.; Myneedu, V.P. Utility of MPT64 antigen detection for rapid confirmation of mycobacterium tuberculosis complex. J. Glob. Infect. Dis. 2015, 7, 66–69. [Google Scholar]
- Diouani, M.F.; Ouerghi, O.; Refai, A.; Belgacem, K.; Tlili, C.; Laouini, D.; Essafi, M. Detection of ESAT-6 by a label free miniature immuno-electrochemical biosensor as a diagnostic tool for tuberculosis. Mater. Sci. Eng. C 2017, 74, 465–470. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Balaji, K.N. The PE and PPE proteins of mycobacterium tuberculosis. Tuberculosis 2011, 91, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, P.; Chaisriratanakul, W.; Bunjongpru, W.; Sripumkhai, W.; Srisuwan, A.; Hruanun, C.; Promptmas, C. A silicon nitride ISFET based immunosensor for Ag85B detection of tuberculosis. Analyst 2016, 141, 5767–5775. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.N.; Chen, J.P.; Chen, D.L. Serodiagnosis efficacy and immunogenicity of the fusion protein of mycobacterium tuberculosis composed of the 10-kilodalton culture filtrate protein, ESAT-6, and the extracellular domain fragment of PPE68. Clin. Vaccine Immunol. 2012, 19, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Welin, A.; Björnsdottir, H.; Winther, M.; Christenson, K.; Oprea, T.; Karlsson, A.; Dahlgren, C. CFP-10 from mycobacterium tuberculosis selectively activates human neutrophils through a pertussis toxin-sensitive chemotactic receptor. Infect. Immun. 2015, 83, 205–213. [Google Scholar] [CrossRef]
- Renshaw, P.S.; Lightbody, K.L.; Veverka, V.; Muskett, F.W.; Kelly, G.; Frenkiel, T.A.; Carr, M.D. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and. EMBO J. 2005, 24, 2491–2498. [Google Scholar] [CrossRef]
- Hong, S.C.; Lee, J.; Shin, H.C.; Kim, C.M.; Park, J.Y.; Koh, K.; Lee, J. Clinical immunosensing of tuberculosis CFP-10 in patient urine by surface plasmon resonance spectroscopy. Sens. Actuators B Chem. 2011, 160, 1434–1438. [Google Scholar] [CrossRef]
- Soo, P.C.; Horng, Y.T.; Chen, A.T.; Yang, S.C.; Chang, K.C.; Lee, J.J.; Peng, W.P. Validation of nanodiamond-extracted CFP-10 antigen as a biomarker in clinical isolates of Mycobacterium tuberculosis complex in broth culture media. Tuberculosis 2015, 95, 620–624. [Google Scholar] [CrossRef]
- Moina, C.; Ybarra, G. Fundamentals and applications of immunosensors. In Advances in Immunoassay Technology; Norman, H.L.C., Ed.; IntechOpen: London, UK, 2012; pp. 65–80. [Google Scholar]
- Güner, A.; Çevik, E.; Mehmet, S. An electrochemical immunosensor for sensitive detection of Escherichia coli O157: H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chesm. 2017, 229, 358–365. [Google Scholar] [CrossRef]
- Mouli, C.; Tiwari, I.; Nand, V.; Sood, K.N.; Sumana, G.; Dhar, B. Chemical Highly sensitive electrochemical immunosensor based on graphene-wrapped copper oxide-cysteine hierarchical structure for detection of pathogenic bacteria. Sens. Actuators B. Chem. 2017, 238, 1060–1069. [Google Scholar]
- Liu, L.; Zhao, Z.; Cai, M.; Jiang, X.; Kang, Y.; Dai, Q.; Xie, G. Electrochemical determination of 16s ribosomal RNA of mycobacterium tuberculosis using magnetite on silica with DNA-functionalized gold nanoparticles AU—Sheng, Shangchun. Anal. Lett. 2016, 49, 1379–1387. [Google Scholar]
- Bhardwaj, N.; Bhardwaj, S.K.; Nayak, M.K.; Mehta, J.; Deep, A. Fluorescent nanobiosensors for the targeted detection of foodborne bacteria. Trends Anal. Chem. 2017, 61, 7. [Google Scholar] [CrossRef]
- Su, X.; Li, Y. A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157: H7. Biosens. Bioelectron. 2004, 19, 563–574. [Google Scholar] [CrossRef]
- Hong, S.; Choi, S.; Do, H.; Hong, S. Biosensors and bioelectronics development of QCM biosensor to detect a marine derived pathogenic bacteria Edwardsiella tarda using a novel immobilisation method. Biosens. Bioelectron. 2009, 24, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Lee, J.; Kim, J.; Kang, M.; Paik, J.K.; Ku, S.; Kim, D. Current technologies of electrochemical immunosensors: Perspective on signal amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef]
- Kaushik, A.; Yndart, A.; Kumar, S.; Jayant, R.D.; Vashist, A.; Brown, A.N.; Nair, M. A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Nature 2018, 8, 3–7. [Google Scholar] [CrossRef]
- Alvarez-toral, A.; Fernández, B.; Malherbe, J.; Claverie, F.; Pecheyran, C.; Pereiro, R. Synthesis of amino-functionalized silica nanoparticles for preparation of new laboratory standards. Spectrochim. Acta Part B At. Spectrosc. 2017, 138, 1–7. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Picco, S.A.; Larissa, B.C.; Kaliandra, A.G.; d João, H.Z.; dos Santos, J.K.; Mateus, B.C.L. Tailored silica nanoparticles surface to increase drug load and enhance bactericidal response. J. Braz. Chem. Soc. 2017, 28, 1715–1724. [Google Scholar] [CrossRef]
- Lu, H.T. Synthesis and characterization of amino functionalized. Colloid J. 2013, 75, 311–312. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Amy, S.R.; Chabal, Y.J. Attachment of 3- (aminopropyl) triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef]
- Beganskiene, A.; Sirutkaitis, V.; Kurtinaitiene, M.; Juskenas, R.; Kareiva, A. FTIR, TEM and NMR iinvestigations of stöber silica nanoparticles. Mater. Sci. 2004, 10, 287–290. [Google Scholar]
- Rahman, S.A.; Ariffin, N.; Yusof, N.A.; Abdullah, J. Thiolate-Capped CdSe/ZnS core-shell quantum dots for the sensitive detection of glucose. Sensors 2017, 17, 1–12. [Google Scholar]
- Benvidi, A.; Rajabzadeh, N.; Zahedi, H.M.; Mazloum-ardakani, M.; Heidari, M.M.; Hosseinzadeh, L. Simple and label-free detection of DNA hybridization on a modified graphene nanosheets electrode. Talanta 2015, 15, 1–25. [Google Scholar] [CrossRef]
- Amelia, M.; Lincheneau, C.; Credi, A. Electrochemical properties of CdSe and CdTe quantum dots. Chem. Soc. Rev. 2012, 41, 5728–5743. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Barclay, J.E.; Butt, J.N.; Lomonossoff, G.P.; Evans, D.J. Redox-Active ferrocene-modified Cowpea mosaic virus nanoparticles. Dalton Trans. 2010, 39, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Rahman, M.S.; Ahmed, E.; Bakshi, P.K.; Shaikh, A.A. A cyclic voltammetric study of the redox reaction of Cu ( II ) in presence of ascorbic acid in different pH media. Dhaka. Univ. J. Sci. 2013, 61, 161–166. [Google Scholar] [CrossRef]
- Kirkman, H.N.; Gaetani, G.F. Catalase: A tetrameric enzyme with four tightly bound molecules of NADPH. Proc. Natl. Acad. Sci. USA 1984, 81, 4343–4347. [Google Scholar] [CrossRef]
- Prakash, K.; Prajapati, S.; Ahmad, A.; Jain, S.K.; Bhakuni, V. Unique oligomeric intermediates of bovine liver catalase. Protein Sci. A Publ. Protein Soc. 2002, 11, 46–57. [Google Scholar] [CrossRef]
- Rashtbari, S.; Dehghan, G.; Yekta, R.; Jouyban, A. Investigation of the binding mechanism and inhibition of bovine liver catalase by quercetin: Multi-spectroscopic and computational study. Bioimpacts: Bi 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Vetrano, A.M.; Heck, D.E.; Mariano, T.M.; Mishin, V.; Laskin, D.L.; Laskin, J.D. Characterization of the oxidase activity in mammalian catalase. J. Biol. Chem. 2005, 280, 35372–35381. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Zayats, M.; Willner, I. Photoelectrochemical and optical applications of semiconductor quantum dots for bioanalysis. Adv. Biochem. Engin. Biotechnol. 2008, 109, 255–283. [Google Scholar]
- Rahman, S.F.A.; Yusof, N.A.; Hashim, U.; Hushiarian, R.; Nuzaihan, M.; Hamidon, M.N. Enhanced sensing of dengue virus DNA detection using O2 plasma treated-silicon nanowire based electrical biosensor. Anal. Chim. Acta 2016, 942, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Bäumler, S.; Block, A.; Felsenstein, F.G.; Wenzel, G. Determination of detection and quantification limits for SNP allele frequency estimation in DNA pools using real time PCR. Nucl. Acids Res. 2004, 32, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, Z.; Liu, Z.; Xiu, B.; Yang, X.; Zhao, P.; Zhang, X.; Duan, C.; Que, H.; Zhang, H.; Feng, X. A multiple-antigen detection assay for tuberculosis diagnosis based on broadly reactive polyclonal antibodies. Iran. J. Basic Med. Sci. 2017, 20, 360–367. [Google Scholar]
- Li, N.; Huang, X.; Sun, D.; Yu, W.; Tan, W.; Luo, Z.; Chen, Z. Dual-Aptamer-Based voltammetric biosensor for the Mycobacterium tuberculosis antigen MPT64 by using a gold electrode modified with a peroxidase loaded composite consisting of gold nanoparticles and a Zr (IV)/terephthalate metal-organic framework. Microchim. Acta 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Tufa, L.T.; Oh, S.; Tran, V.T.; Kim, J.; Jeong, K.-J.; Park, T.J.; Lee, J. Electrochemical immunosensor using nanotriplex of graphene quantum dots, Fe3O4, and Ag nanoparticles for tuberculosis. Electrochim. Acta 2018, 290, 369–377. [Google Scholar] [CrossRef]
- Wang, X. Fabrication of electrochemical immunosensor for interferon-γ determination and its application of tuberculosis diagnosis. Int. J. Electrochem. Sci. 2017, 12, 7262–7271. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Shi, S.; Yan, Y.; Zhang, M. Detection of interferon-gamma for latent tuberculosis diagnosis using an immunosensor based on CdS quantum dots coupled to magnetic beads as labels. Int. J. Electrochem. Sci. 2015, 10, 2580–2593. [Google Scholar]
- Li, L.; Yuan, Y.; Chen, Y.; Zhang, P.; Bai, Y.; Bai, L. Aptamer based voltammetric biosensor for mycobacterium tuberculosis antigen ESAT-6 using a nanohybrid material composed of reduced graphene oxide and a metal-organic framework. Microchim. Acta 2018, 2, 2–10. [Google Scholar] [CrossRef]
- Azmi, M.U.Z.; Yusof, N.A.; Kusnin, N.; Abdullah, J.; Suraiya, S.; Ong, P.S.; Mohamad Fathil, M.F. Sandwich electrochemical immunosensor for early detection of tuberculosis based on graphene/polyaniline-modified screen-printed gold electrode. Sensors 2018, 18, 3926. [Google Scholar] [CrossRef]
- Van Pinxteren, L.A.; Ravn, P.; Agger, E.M.; Pollock, J.; Andersen, P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin. Diagn. Lab. Immunol. 2000, 7, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.; Hingley-Wilson, S.M.; Chen, B.; Chen, M.; Dai, A.Z.; Morin, P.M. The primary mechanism of attenuation of bacillus Calmette-Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 2003, 100, 12420–12425. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; De Jonge, M.I.; Majlessi, L.; Leclerc, C.; Nilges, M.; Cole, S.T.; Brosch, R. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. J. Biol. Chem. 2005, 280, 33953–33959. [Google Scholar] [CrossRef] [PubMed]





| Method | Linear Range | LOD | Tuberculosis Biomarker | References |
|---|---|---|---|---|
| DPV | 0.00002–1 ng/mL | 1.0 × 10−7 g/mL | MPT64 | [46] |
| DPV | 5–500,000 ng/mL | 3.3 × 10−10 g/mL | CFP10 | [47] |
| EIS | 0.0001–0.1 ng/mL | 1.2 × 10−4 g/mL | IFN-ɣ | [48] |
| SWASV | 0.001–0.5 ng/mL | 3.4 × 10−4 g/mL | IFN-ɣ | [49] |
| EIS | 0.0001–0.02 ng/mL | 3.3 × 10−5 g/mL | ESAT6 | [50] |
| DPV | 20–100 ng/mL | 1.5 × 10−8 g/mL | CFP10 | [51] |
| SWV | 10–1000 ng/mL | 7.0 × 10−9 g/mL | ESAT6 | [10] |
| DPV | 20–100 ng/mL | 1.5 × 10−10 g/mL | CFP10-ESAT6 | This work |
| Replicate | Peak Current (ng/mL) |
|---|---|
| 1 | 8.14 |
| 2 | 8.22 |
| 3 | 8.36 |
| 4 | 8.42 |
| 5 | 8.18 |
| Mean | 8.264 |
| SD | 0.1203 |
| RSD | 1.4561 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Bakhori, N.; Yusof, N.A.; Abdullah, J.; Wasoh, H.; Ab Rahman, S.K.; Abd Rahman, S.F. Surface Enhanced CdSe/ZnS QD/SiNP Electrochemical Immunosensor for the Detection of Mycobacterium Tuberculosis by Combination of CFP10-ESAT6 for Better Diagnostic Specificity. Materials 2020, 13, 149. https://doi.org/10.3390/ma13010149
Mohd Bakhori N, Yusof NA, Abdullah J, Wasoh H, Ab Rahman SK, Abd Rahman SF. Surface Enhanced CdSe/ZnS QD/SiNP Electrochemical Immunosensor for the Detection of Mycobacterium Tuberculosis by Combination of CFP10-ESAT6 for Better Diagnostic Specificity. Materials. 2020; 13(1):149. https://doi.org/10.3390/ma13010149
Chicago/Turabian StyleMohd Bakhori, Noremylia, Nor Azah Yusof, Jaafar Abdullah, Helmi Wasoh, Siti Khadijah Ab Rahman, and Siti Fatimah Abd Rahman. 2020. "Surface Enhanced CdSe/ZnS QD/SiNP Electrochemical Immunosensor for the Detection of Mycobacterium Tuberculosis by Combination of CFP10-ESAT6 for Better Diagnostic Specificity" Materials 13, no. 1: 149. https://doi.org/10.3390/ma13010149
APA StyleMohd Bakhori, N., Yusof, N. A., Abdullah, J., Wasoh, H., Ab Rahman, S. K., & Abd Rahman, S. F. (2020). Surface Enhanced CdSe/ZnS QD/SiNP Electrochemical Immunosensor for the Detection of Mycobacterium Tuberculosis by Combination of CFP10-ESAT6 for Better Diagnostic Specificity. Materials, 13(1), 149. https://doi.org/10.3390/ma13010149