Influence of Different Stabilization Systems and Multiple Ultraviolet A (UVA) Aging/Recycling Steps on Physicochemical, Mechanical, Colorimetric, and Thermal-Oxidative Properties of ABS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
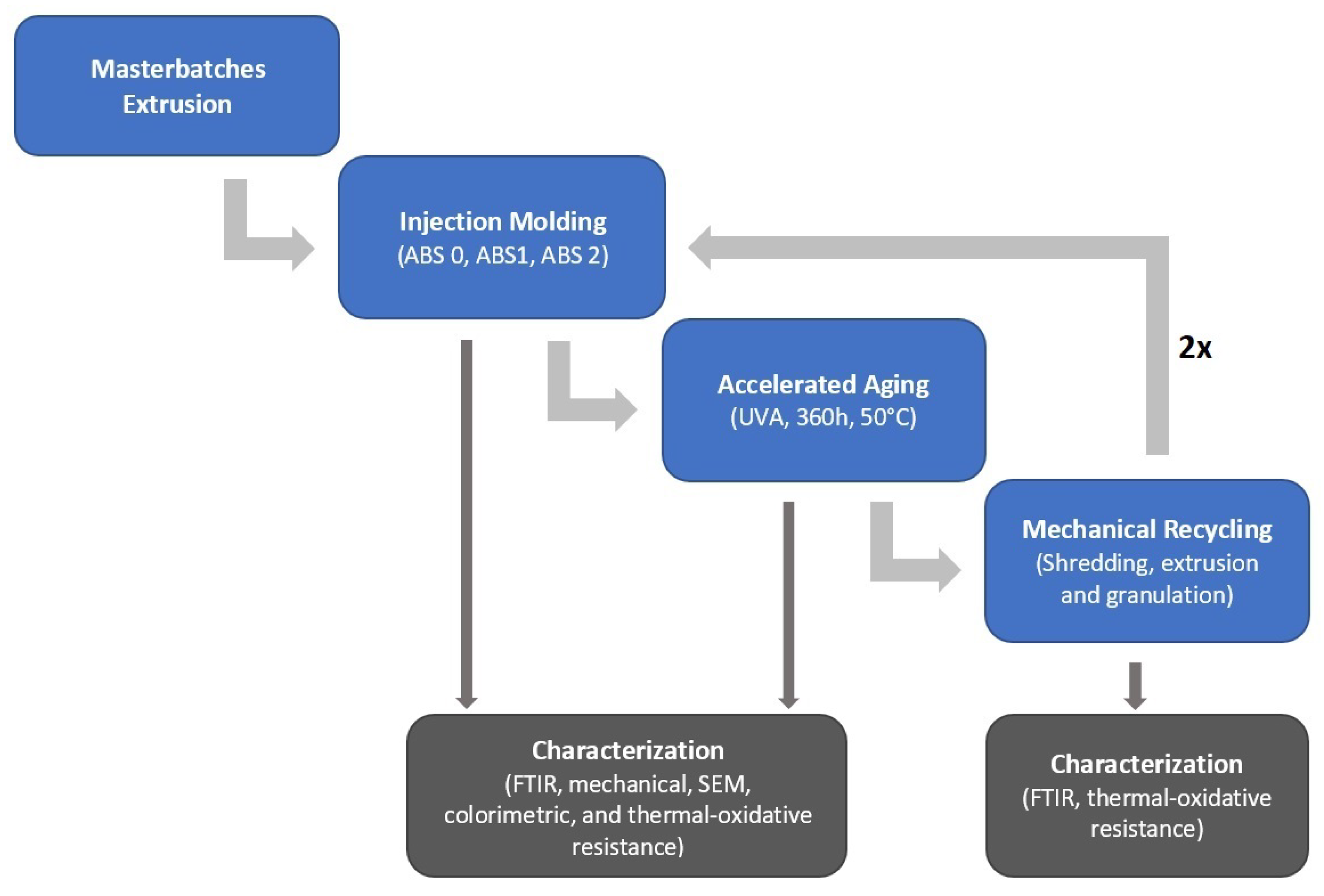
2.2.1. Extrusion-Based Manufacturing of Stabilization Masterbatches for First Injection Molding
2.2.2. Injection Molding
2.2.3. Accelerated Aging
2.2.4. Mechanical Recycling
2.3. Characterization
2.3.1. Chemical Characterization
2.3.2. Mechanical Properties
2.3.3. Morphological Characterization
2.3.4. Colorimetric Characterization
2.3.5. Thermal-Oxidative Resistance
3. Results and Discussion
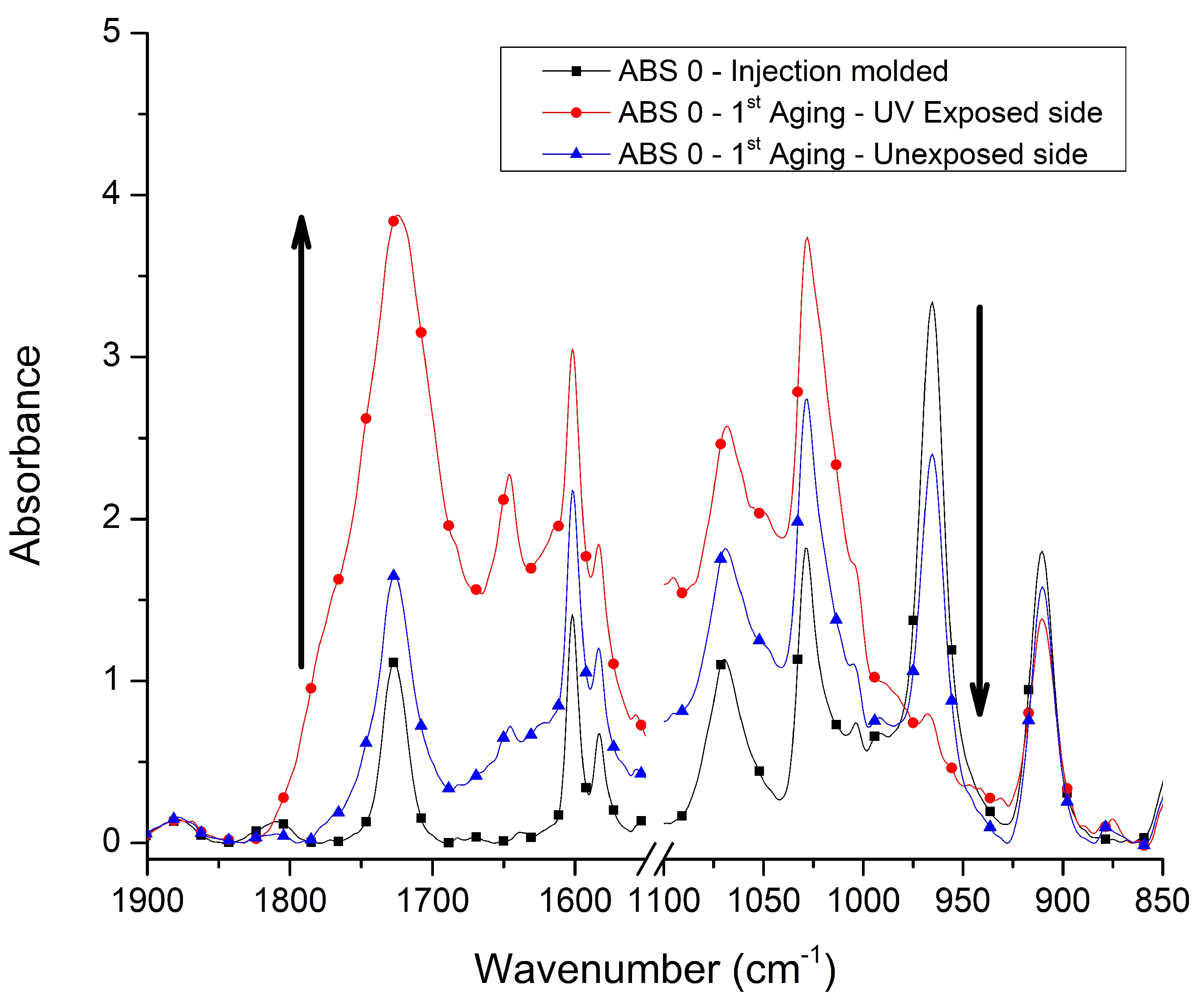
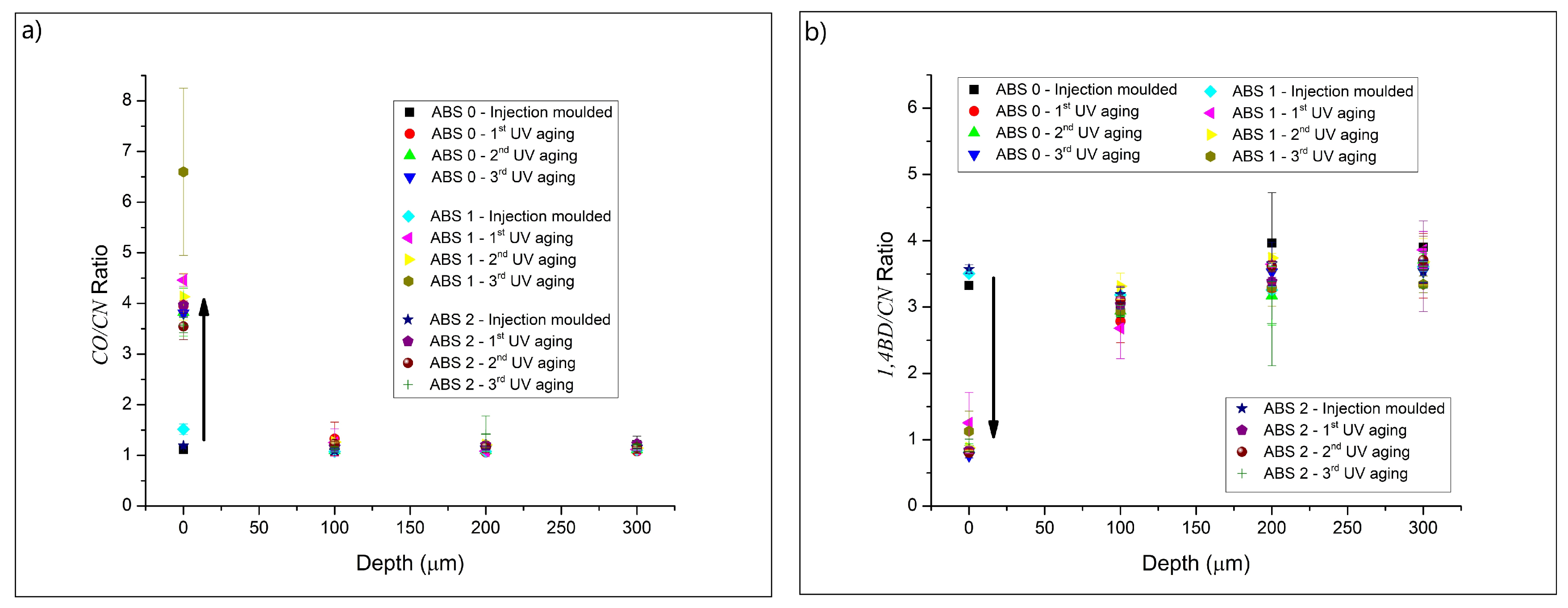
3.1. Chemical Characterization
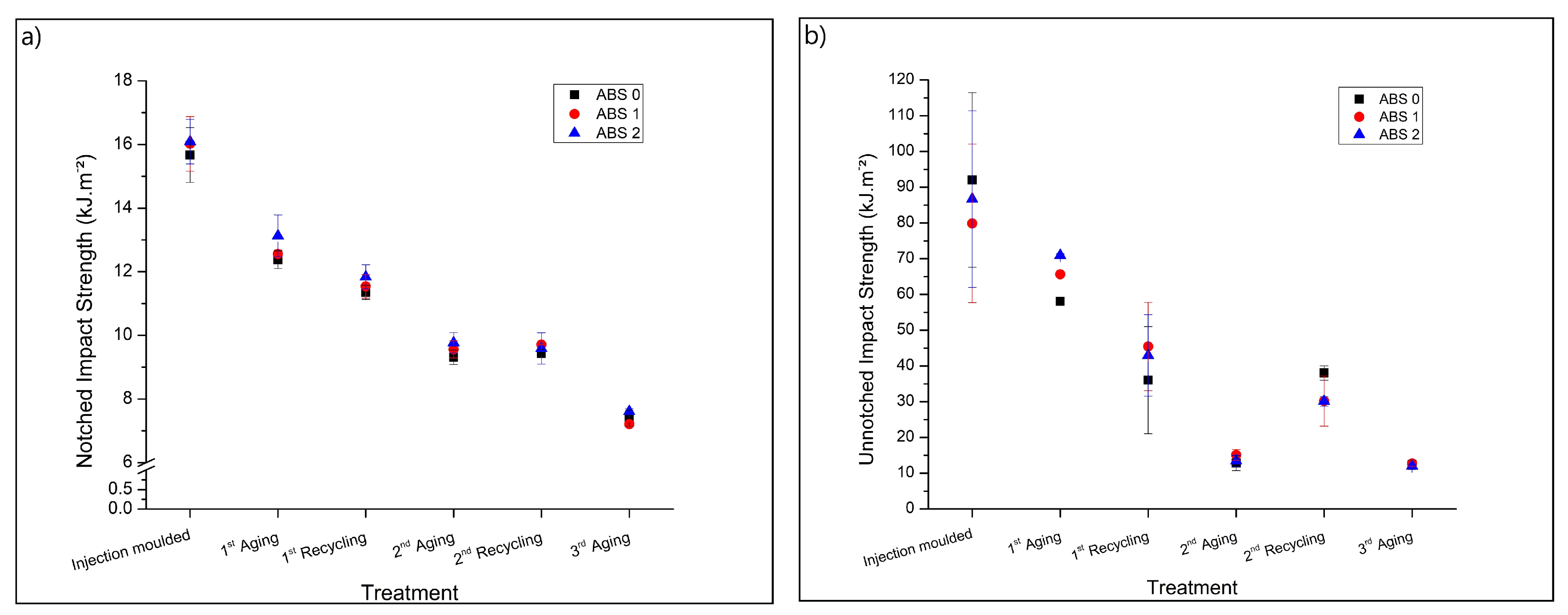
3.2. Mechanical Properties
3.2.1. Impact Strength
3.2.2. Tensile Properties
3.3. Morphological Characterization
3.4. Colorimetric Characterization
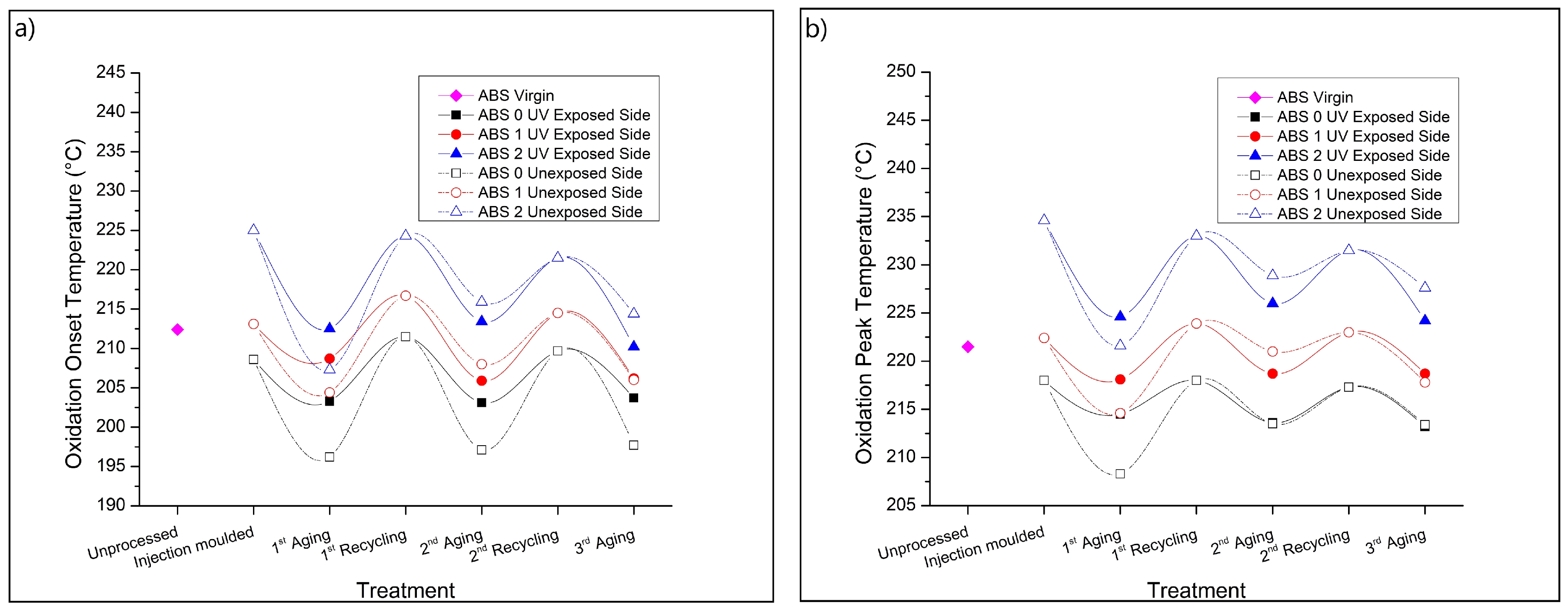
3.5. Thermal-Oxidative Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scaffaro, R.; Botta, L.; Di Benedetto, G. Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Bokria, J.G.; Schlick, S. Spatial effects in the photodegradation of poly(acrylonitrile–butadiene–styrene): A study by ATR-FTIR. Polymer 2002, 43, 3239–3246. [Google Scholar] [CrossRef]
- Tracy, J.; D’hooge, D.R.; Bosco, N.; Delgado, C.; Dauskardt, R. Evaluating and predicting molecular mechanisms of adhesive degradation during field and accelerated aging of photovoltaic modules. Prog. Photovolt. Res. Appl. 2018, 26, 981–993. [Google Scholar] [CrossRef]
- Yu, L.; Yan, X.; Fortin, G. Effects of weathering aging on mechanical and thermal properties of injection molded glass fiber reinforced polypropylene composites. J. Polym. Res. 2018, 25, 247. [Google Scholar] [CrossRef]
- Scott, G.; Tahan, M. Comparison of the photo-oxidative behaviour of some polybutadiene-based polyblends. Eur. Polym. J. 1977, 13, 981–987. [Google Scholar] [CrossRef]
- Adeniyi, J. Clarification and discussion of chemical transformations involved in thermal and photo-oxidative degradation of ABS. Eur. Polym. J. 1984, 20, 291–299. [Google Scholar] [CrossRef]
- Jouan, X.; Gardette, J. Photo-oxidation of ABS: Part 2—Origin of the photodiscoloration on irradiation at long wavelengths. Polym. Degrad. Stab. 1992, 36, 91–96. [Google Scholar] [CrossRef]
- Davis, P.; Tiganis, B.; Burn, L. The effect of photo-oxidative degradation on fracture in ABS pipe resins. Polym. Degrad. Stab. 2004, 84, 233–242. [Google Scholar] [CrossRef]
- Piton, M.; Rivaton, A. Photo-oxidation of ABS at long wavelengths (λ > 300 nm). Polym. Degrad. Stab. 1997, 55, 147–157. [Google Scholar] [CrossRef]
- Scott, G. Atmospheric oxidation and antioxidants. In Color in Food: Technological and Psychophysical Aspects; Scott, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; Chapter 5; pp. 279–326. [Google Scholar]
- Zweifel, H. Stabilization of Polymeric Materials; Springer: Berlin, Germany, 1998. [Google Scholar]
- Pérez, J.; Vilas, J.; Laza, J.; Arnáiz, S.; Mijangos, F.; Bilbao, E.; León, L. Effect of reprocessing and accelerated weathering on ABS properties. J. Polym. Environ. 2010, 18, 71–78. [Google Scholar] [CrossRef]
- Boldizar, A.; Möller, K. Degradation of ABS during repeated processing and accelerated ageing. Polym. Degrad. Stab. 2003, 81, 359–366. [Google Scholar] [CrossRef]
- Santos, R.; Botelho, G.; Machado, A. Artificial and natural weathering of ABS. J. Appl. Polym. Sci. 2010, 116, 2005–2014. [Google Scholar] [CrossRef]
- ISO-527-1. Plastics—Determination of Tensile Properties—Part 1: General Principles; Technical Report; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO-4892-3. Plastics—Methods of Exposure to Laboratory Light Sources— Part 3: Fluorescent UV Lamps; Technical Report; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Jouan, X.; Gardette, J.L. Photooxidation of ABS at long-wavelengths. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 685–696. [Google Scholar] [CrossRef]
- Celina, M.; Wise, J.; Ottesen, D.; Gillen, K.; Clough, R. Oxidation profiles of thermally aged nitrile rubber. Polym. Degrad. Stab. 1998, 60, 493–504. [Google Scholar] [CrossRef]
- Fiorio, R.; D’hooge, D.R.; Ragaert, K.; Cardon, L. A Statistical Analysis on the Effect of Antioxidants on the Thermal-Oxidative Stability of Commercial Mass-and Emulsion-Polymerized ABS. Polymers 2019, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- ISO-180. Plastics—Determination of Izod Impact Strength; Technical Report; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Hirschler, R. Whiteness, yellowness, and browning in food colorimetry. In Color in Food: Technological and Psychophysical Aspects; Caivano, J., Buera, M., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Chapter 10; pp. 93–104. [Google Scholar]
- ASTM-E2009. Standard Test Methods for Oxidation Onset Temperature of Hydrocarbons by Differential Scanning Calorimetry; Technical Report; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Adeniyi, J.; Kolawole, E. Thermal and photo-degradation of unstabilized ABS. Eur. Polym. J. 1984, 20, 43–47. [Google Scholar] [CrossRef]
- Wyzgoski, M.G. Effects of oven aging on ABS, poly (acrylonitrile–butadiene–styrene). Polym. Eng. Sci. 1976, 16, 265–269. [Google Scholar] [CrossRef]
- Karahaliou, E.K.; Tarantili, P. Stability of ABS compounds subjected to repeated cycles of extrusion processing. Polym. Eng. Sci. 2009, 49, 2269–2275. [Google Scholar] [CrossRef]
- Iannuzzi, G.; Mattsson, B.; Rigdahl, M. Color changes due to thermal ageing and artificial weathering of pigmented and textured ABS. Polym. Eng. Sci. 2013, 53, 1687–1695. [Google Scholar] [CrossRef]
- Chiu, H.T.; Huang, J.K.; Kuo, M.T.; Huang, J.H. Characterisation of PC/ABS blend during 20 reprocessing cycles and subsequent functionality recovery by virgin additives. J. Polym. Res. 2018, 25, 124. [Google Scholar] [CrossRef]
- Rahimi, M.; Esfahanian, M.; Moradi, M. Effect of reprocessing on shrinkage and mechanical properties of ABS and investigating the proper blend of virgin and recycled ABS in injection molding. J. Mater. Process.Technol. 2014, 214, 2359–2365. [Google Scholar] [CrossRef]
- Carter, R., III; McCallum, J. Photoacoustic infrared spectroscopy for evaluation of an ABS as an automotive interior material. Polym. Degrad. Stab. 1994, 45, 1–10. [Google Scholar] [CrossRef]
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Polym. Degrad. Stab. 2018, 154, 203–210. [Google Scholar] [CrossRef]
- Camacho, W.; Karlsson, S. Assessment of thermal and thermo-oxidative stability of multi-extruded recycled PP, HDPE and a blend thereof. Polym. Degrad. Stab. 2002, 78, 385–391. [Google Scholar] [CrossRef]



| Sample Type | Masterbatch | Irganox 1076 (m%) | Irganox 245 (m%) | Irgafos 168 (m%) |
|---|---|---|---|---|
| ABS 0 | - | - | - | - |
| ABS 1 | 1 | 0.2 | - | - |
| ABS 2 | 2 | 0.2 | 0.2 | 0.2 |
| Absorbance (cm) | Group |
|---|---|
| 2237 | Nitrile (CN) |
| 1730 | Carbonyl (CO) |
| 966 | 1,4-Butadiene (1,4BD) |
| 911 | 1,2-Butadiene (1,2BD) |
| Treatment | Tensile Strength (MPa) | ||
|---|---|---|---|
| ABS 0 | ABS 1 | ABS 2 | |
| Injection molded | 32.2 ± 0.2 | 31.8 ± 0.1 | 31.9 ± 0.1 |
| 1st Aging | 28.8 ± 2.3 | 27.4 ± 1.4 | 26.5 ± 1.2 |
| 1st Recycling | 30.9 ± 0.1 | 30.6 ± 0.1 | 31.0 ± 0.1 |
| 2nd Aging | 29.8 ± 0.1 | 28.7 ± 0.7 | 29.5 ± 0.3 |
| 2nd Recycling | 30.8 ± 0.1 | 30.2 ± 0.3 | 30.4 ± 0.5 |
| 3rd Aging | 32.0 ± 0.1 | 31.0 ± 0.9 | 30.8 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorio, R.; Villanueva Díez, S.; Sánchez, A.; D’hooge, D.R.; Cardon, L. Influence of Different Stabilization Systems and Multiple Ultraviolet A (UVA) Aging/Recycling Steps on Physicochemical, Mechanical, Colorimetric, and Thermal-Oxidative Properties of ABS. Materials 2020, 13, 212. https://doi.org/10.3390/ma13010212
Fiorio R, Villanueva Díez S, Sánchez A, D’hooge DR, Cardon L. Influence of Different Stabilization Systems and Multiple Ultraviolet A (UVA) Aging/Recycling Steps on Physicochemical, Mechanical, Colorimetric, and Thermal-Oxidative Properties of ABS. Materials. 2020; 13(1):212. https://doi.org/10.3390/ma13010212
Chicago/Turabian StyleFiorio, Rudinei, Sara Villanueva Díez, Alberto Sánchez, Dagmar R. D’hooge, and Ludwig Cardon. 2020. "Influence of Different Stabilization Systems and Multiple Ultraviolet A (UVA) Aging/Recycling Steps on Physicochemical, Mechanical, Colorimetric, and Thermal-Oxidative Properties of ABS" Materials 13, no. 1: 212. https://doi.org/10.3390/ma13010212
APA StyleFiorio, R., Villanueva Díez, S., Sánchez, A., D’hooge, D. R., & Cardon, L. (2020). Influence of Different Stabilization Systems and Multiple Ultraviolet A (UVA) Aging/Recycling Steps on Physicochemical, Mechanical, Colorimetric, and Thermal-Oxidative Properties of ABS. Materials, 13(1), 212. https://doi.org/10.3390/ma13010212








