Synthesis of ZIF-8/Fly Ash Composite for Adsorption of Cu2+, Zn2+ and Ni2+ from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZIF-8/FA
2.3. Characterization of Materials
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Morphology
3.2. Grain Size Distribution
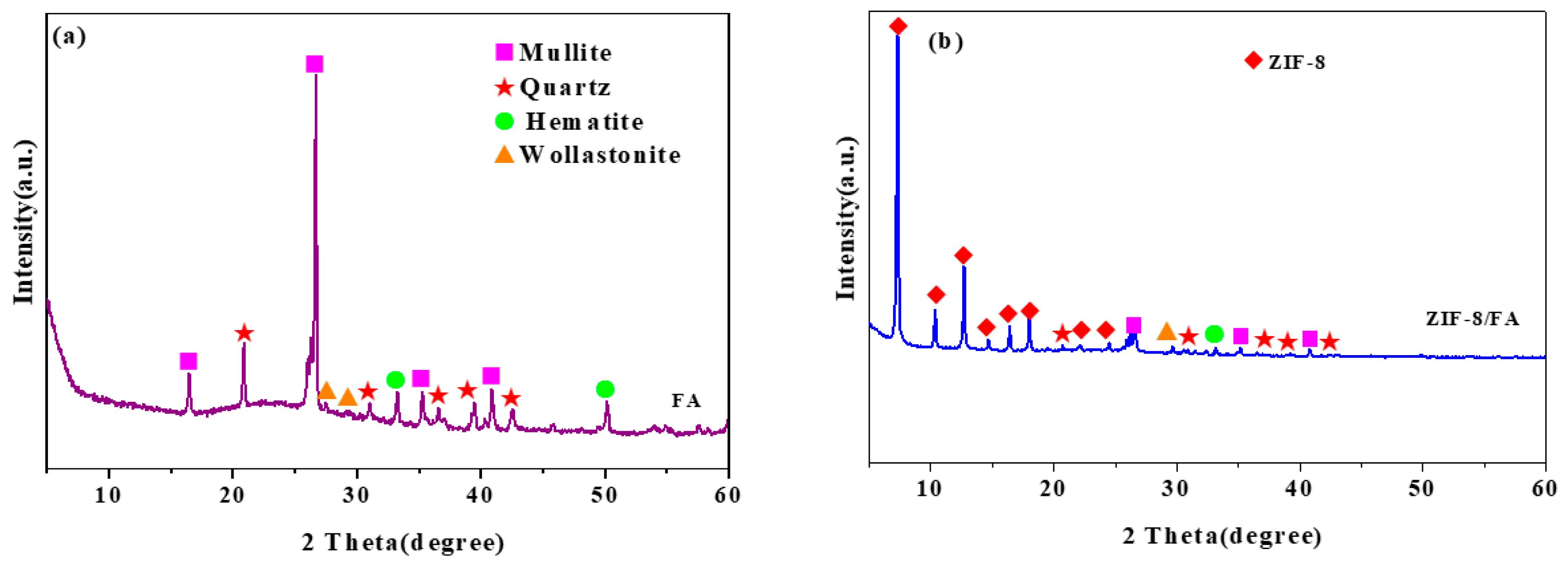
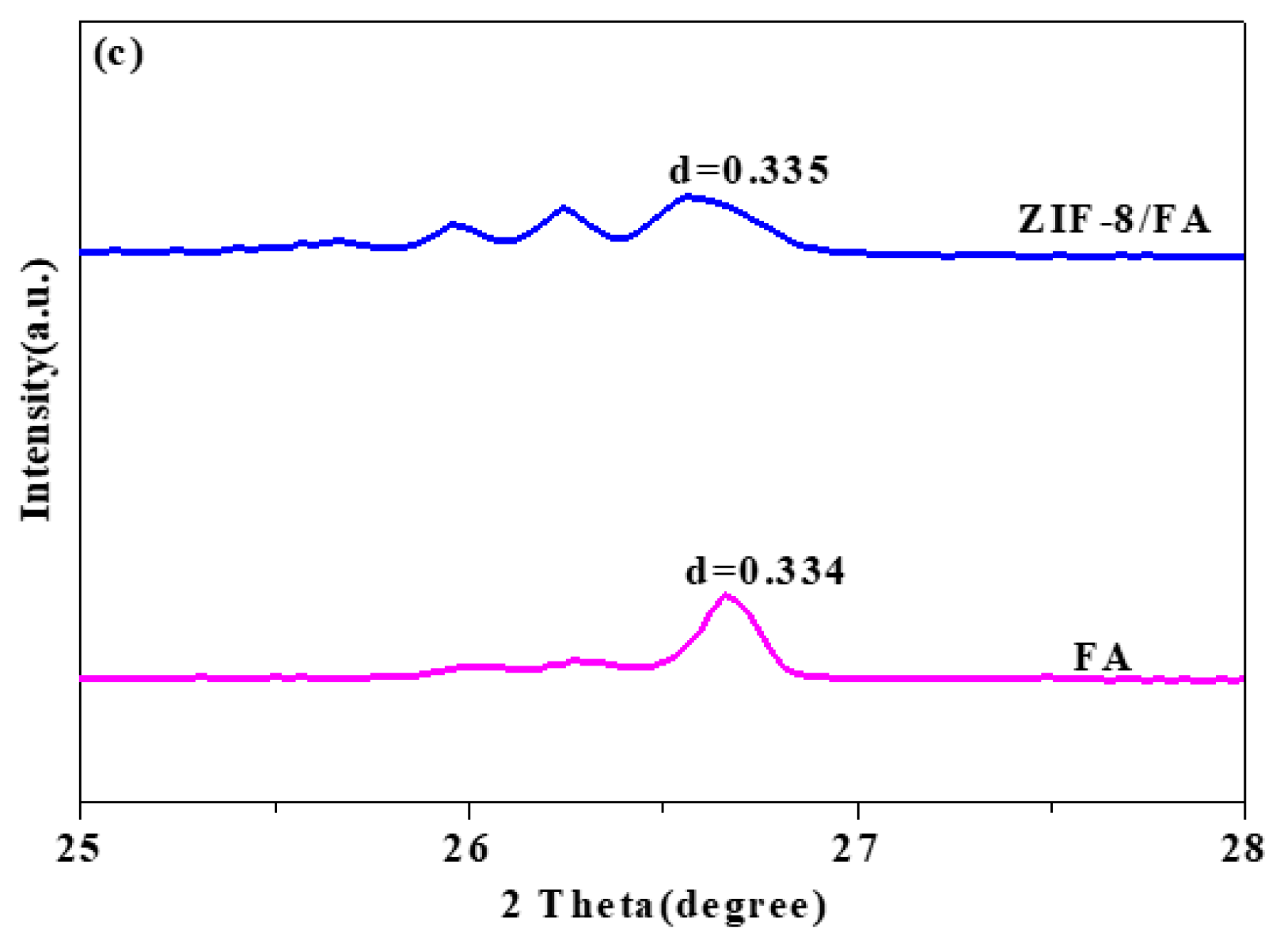
3.3. XRD Analysis
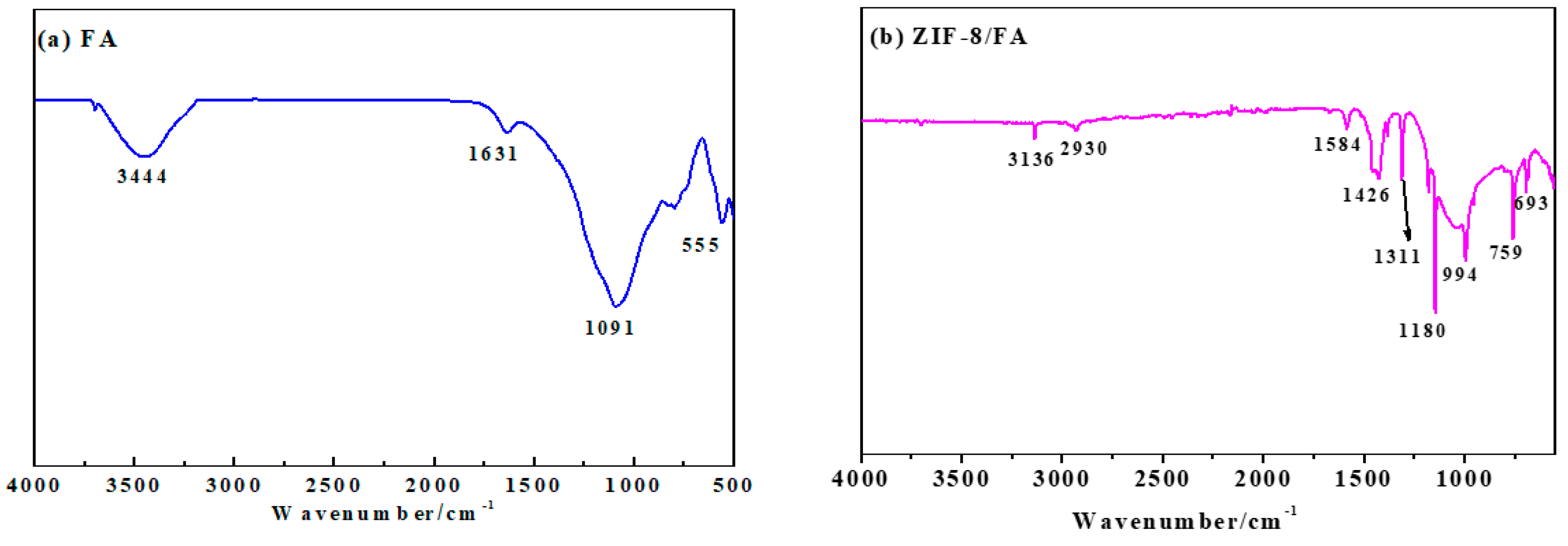
3.4. FTIR Analysis
3.5. Specific Surface Area and Pore Size
3.6. Adsorption Ability
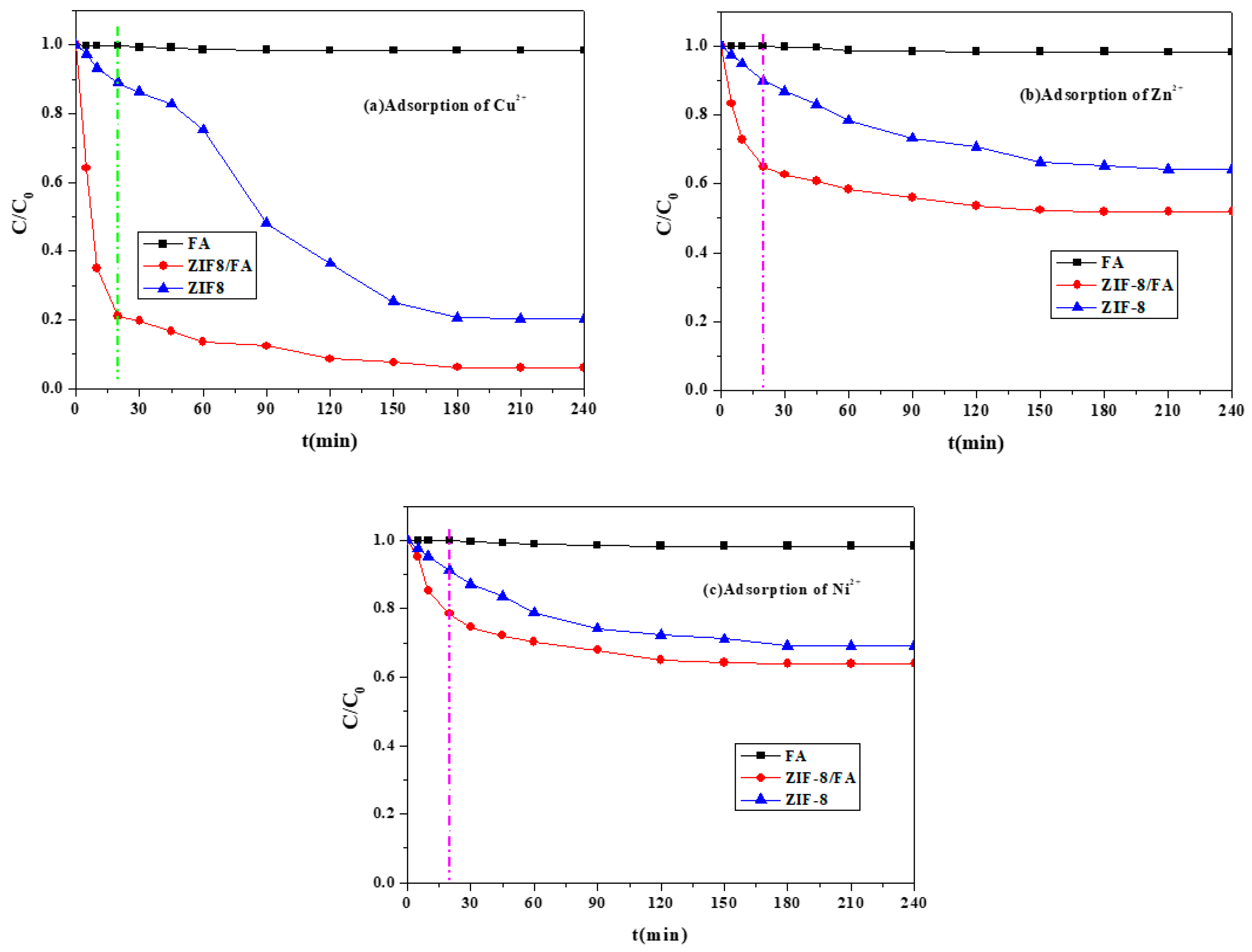
3.6.1. Comparison of FA, ZIF-8/FA, and ZIF-8
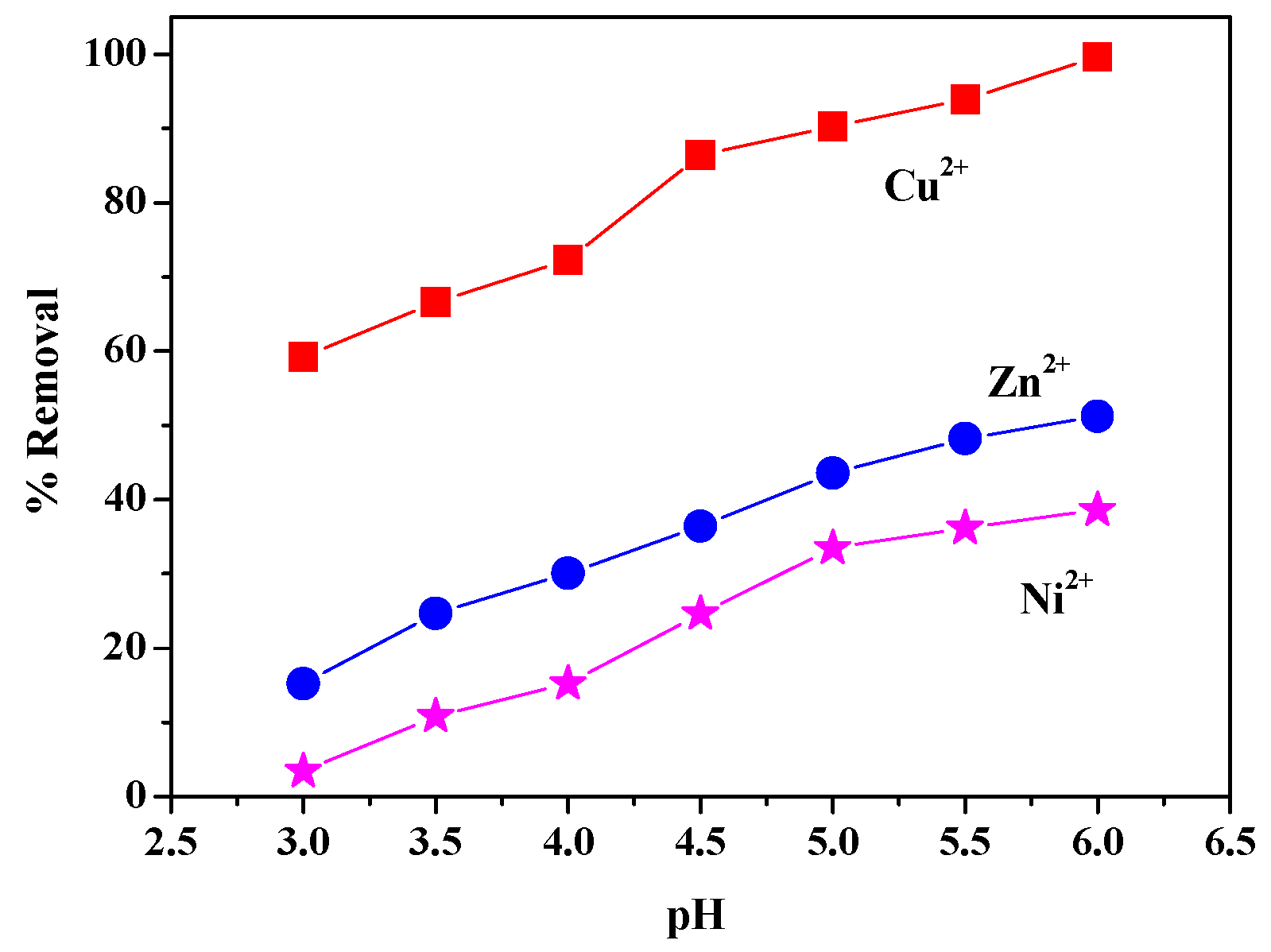
3.6.2. pH Value
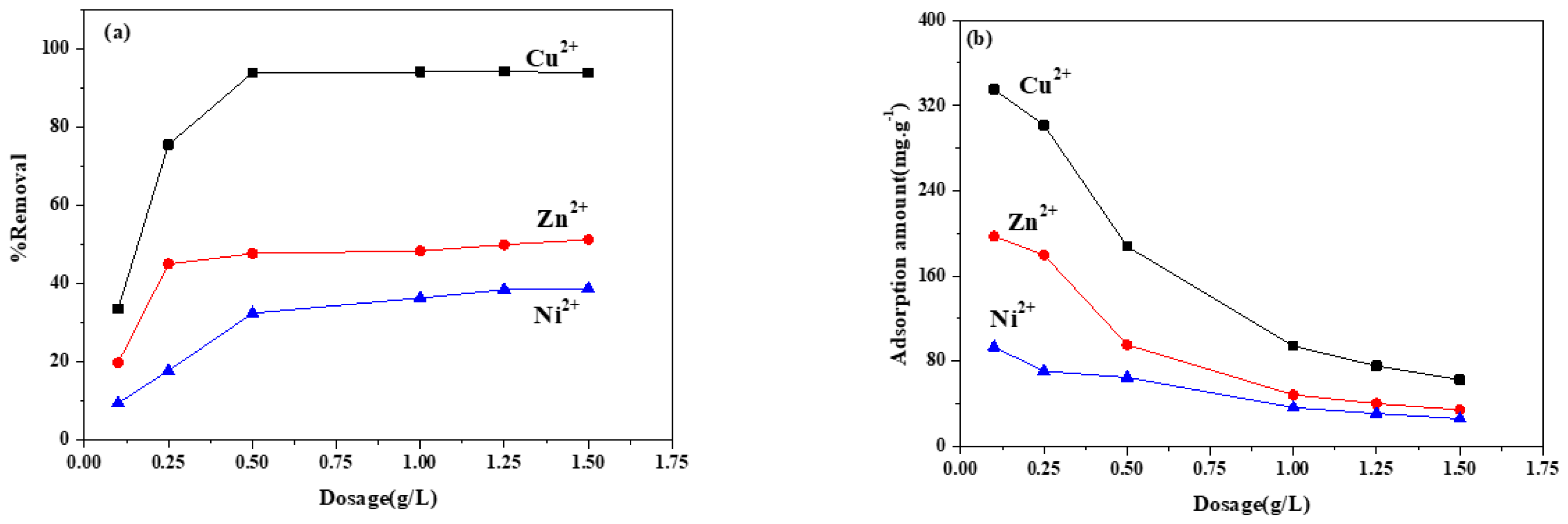
3.6.3. ZIF-8/FA Concentration
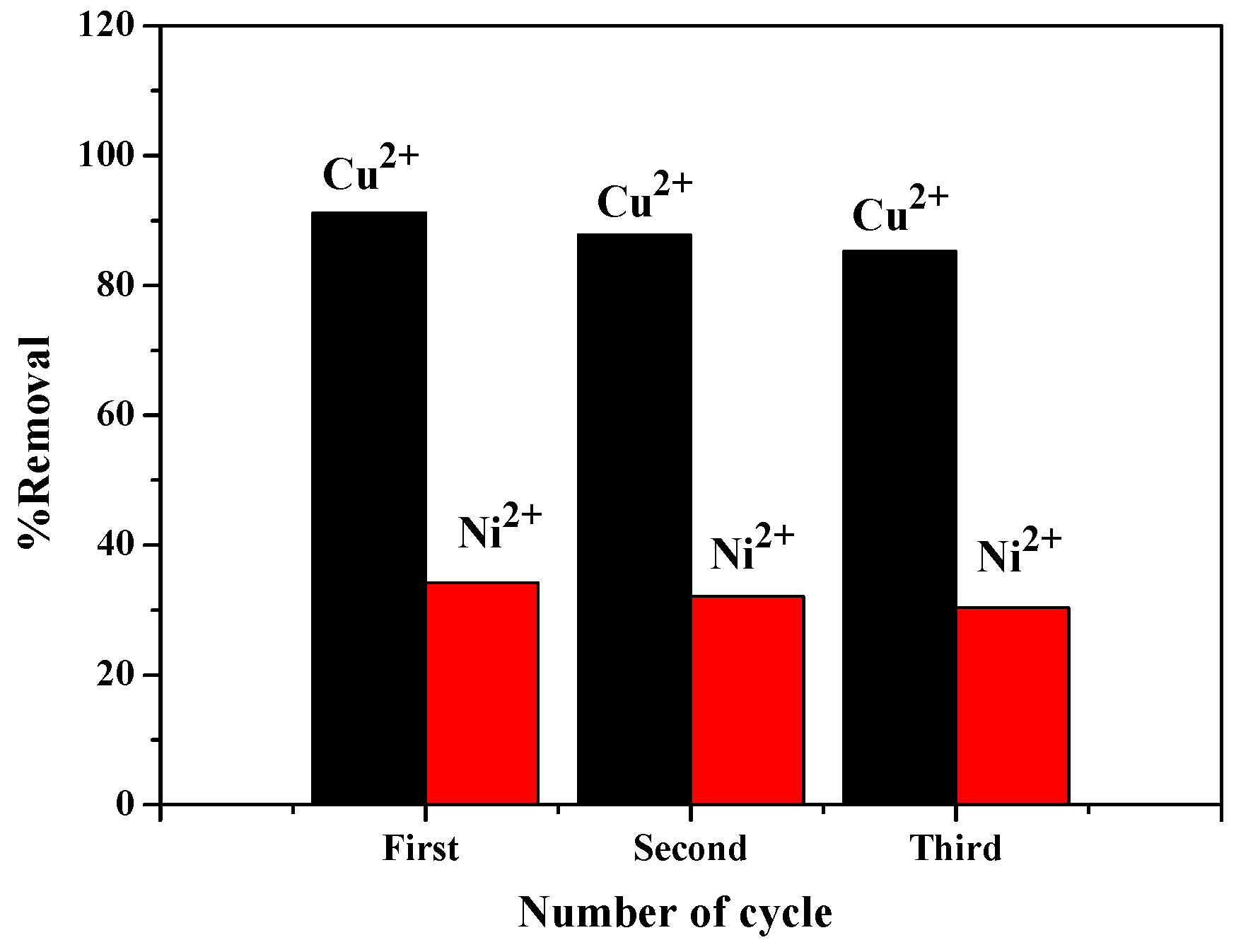
3.7. Recycling Study of ZIF-8/FA Composites
3.8. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Hadadian, M.; Goharshadi, E.K.; Fard, M.M.; Ahmadzadeh, H. Synergistic effect of graphene nanosheets and zinc oxide nanoparticles for effective adsorption of Ni (II) ions from aqueous solutions. Appl. Phys. A. 2018, 124, 239. [Google Scholar] [CrossRef]
- Wang, S.B.; Li, L.; Zhu, Z.H. Solid-state conversion of fly ash to effective adsorbents for Cu removal from wastewater. J. Hazard. Mater. 2007, 139, 254–259. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper(II) and nickel(II) from aqueous media using the complexation ultra filtration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef]
- Fu, F.L.; Chen, R.M.; Xiong, Y. Application of a novel strategy-coordination polymerization precipitation to the treatment of Cu2+-containing wastewaters. Sep. Purif. Technol. 2006, 52, 388–393. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) present in aqueous solutions by aluminium electrocoagulation. J. Hazard. Mater. 2008, 152, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Jin, X.Y.; Lu, X.Q.; Chen, Z.L. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-Based Solar Photocatalysts for the Efficient Decontamination of Persistent Organic Pollutants and Hexavalent Chromium in Wastewater. Catalysts 2019, 9, 974. [Google Scholar] [CrossRef] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Sudilovskiy, P.S.; Kagramanov, G.G.; Kolesnikov, V.A. Use of RO and NF for treatment of copper containing wastewaters in combination with flotation. Desalination 2008, 221, 192–201. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.; Furukawa, H.; Wang, B.; Glover, T.G.; Yaghi, O.M. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc. Natl. Acad. Sci. USA 2009, 106, 20637–20640. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Peng, X.; Cheng, X.; Li, X.; Cao, D. CNT@Cu3(BTC)2 and Metal-Organic Frameworks for Separation of CO2/CH4 Mixture. J. Phys. Chem. C 2011, 115, 19864–19871. [Google Scholar] [CrossRef]
- Kim, M.; Cahill, J.F.; Fei, H.; Prather, K. Postsynthetic ligand and cation exchange in robust metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 18082–18088. [Google Scholar] [CrossRef]
- Shultz, A.M.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A catalytically active, permanently microporous MOF with metalloporphyrin struts. J. Am. Chem. Soc. 2009, 131, 4204–4205. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; CÔté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zeng, X.F.; Guo, L.L.; Lan, J.H.; Zhang, L.L.; Cao, D.P. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Hu, M.Q.; Lou, H.; Yan, X.L.; Hu, X.Y.; Feng, R.; Zhou, M. In-situ fabrication of ZIF-8 decorated layered double oxides for adsorption and photocatalytic degradation of methylene blue. Microporous Mesoporous Mater. 2018, 271, 68–72. [Google Scholar] [CrossRef]
- Wang, S.B.; Wu, H.W. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard. Mater. 2006, 136, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Sayari, A.; Hamoudi, S.; Yang, Y. Applications of Pore-Expanded Mesoporous Silica. 1. Removal of Heavy Metal Cations and Organic Pollutants from Wastewater. Chem. Mater. 2005, 17, 212–216. [Google Scholar] [CrossRef]
- Bibby, A.; Mercier, L. Mercury(II) Ion Adsorption Behavior in Thiol-Functionalized Mesoporous Silica Microspheres. Chem. Mater. 2002, 14, 1591–1597. [Google Scholar] [CrossRef]
- Yang, Y.F.; Gai, G.S.; Cai, Z.F.; Chen, Q.R. Surface modification of purified fly ash and application in polymer. J. Hazard. Mater. 2006, 133, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Wang, J.; Bai, L.Q.; Yang, R.Q.; Wang, H.F. Preparation and Characterization of Fly Ash Coated with Zinc Oxide Nanocomposites. Materials 2019, 12, 3550. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole(PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415, 577–586. [Google Scholar] [CrossRef]
- Jafari, S.; Ghorbani-shahna, F.; Bahrami, A.; Kazemian, H. Effects of Post Synthesis Activation and Relative Humidity on Adsorption Performance of ZIF-8 for Capturing Toluene from a Gas Phase in a Continuous Mode. Appl. Sci. 2018, 8, 310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.F.; Zhao, M.; Yang, Y.; Lin, Y.S. Hydrolysis and condensation of ZIF-8 in water. Microporous Mesoporous Mater. 2019, 288. [Google Scholar] [CrossRef]
- Zhao, X.W.; Hu, B.; Ye, J.J.; Jia, Q. Preparation, Characterization, and Application of Graphene−Zinc Oxide Composites (G−ZnO) for the Adsorption of Cu(II), Pb(II), and Cr(III). J. Chem. Eng. Data 2013, 58, 2395–2401. [Google Scholar] [CrossRef]
- Alejandro, G.A.; Virginia, H.M.; Adrian, B.P.; Miguel, A.M.M.; Didilia, I.M.C. Improving the adsorption of heavy metals from water using commercial carbons modified with egg shell wastes. Ind. Eng. Chem. Res. 2011, 50, 9354–9362. [Google Scholar]
- Mosayebi, E.; Azizian, S. Study of copper ion adsorption from aqueous solution with different nanostructured and microstructured zinc oxides and zinc hydroxide loaded on activated carbon cloth. J. Mol. Liq. 2016, 214, 384–389. [Google Scholar] [CrossRef]
- Qian, S.H.; Zhang, S.J.; Huang, Z.; Xiao, M.; Huang, F. Preconcentration of ultra-trace copper in water samples with nano-meter size TiO2 colloid and determination by GFAAS with slurry sampling. Microchim. Acta 2009, 166, 251–254. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, J.T.; Zhang, Z.Y.; Wang, B.L.; Sun, H.W. Adsorption of Cd(II), Ni(II), and Zn(II) by tourmaline at acidic conditions: Kinetics, Thermodynamics and Mechanisms. Ind. Eng. Chem. Res. 2012, 51, 4397–4406. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Perumal, S.V.; Vijayaraghavan, K. Equilibrium isotherm studies for the multicomponent adsorption of lead, zinc and cadmium onto Indonesian peat. Ind. Eng. Chem. Res. 2009, 48, 2093–2099. [Google Scholar] [CrossRef]
- Sajid, A. Synthesis and Adsorption Properties of Zeolitic Imidazolate Framework-8(ZIF-8); Beijing University of Chemical Technology: Beijing, China, 2017. [Google Scholar]
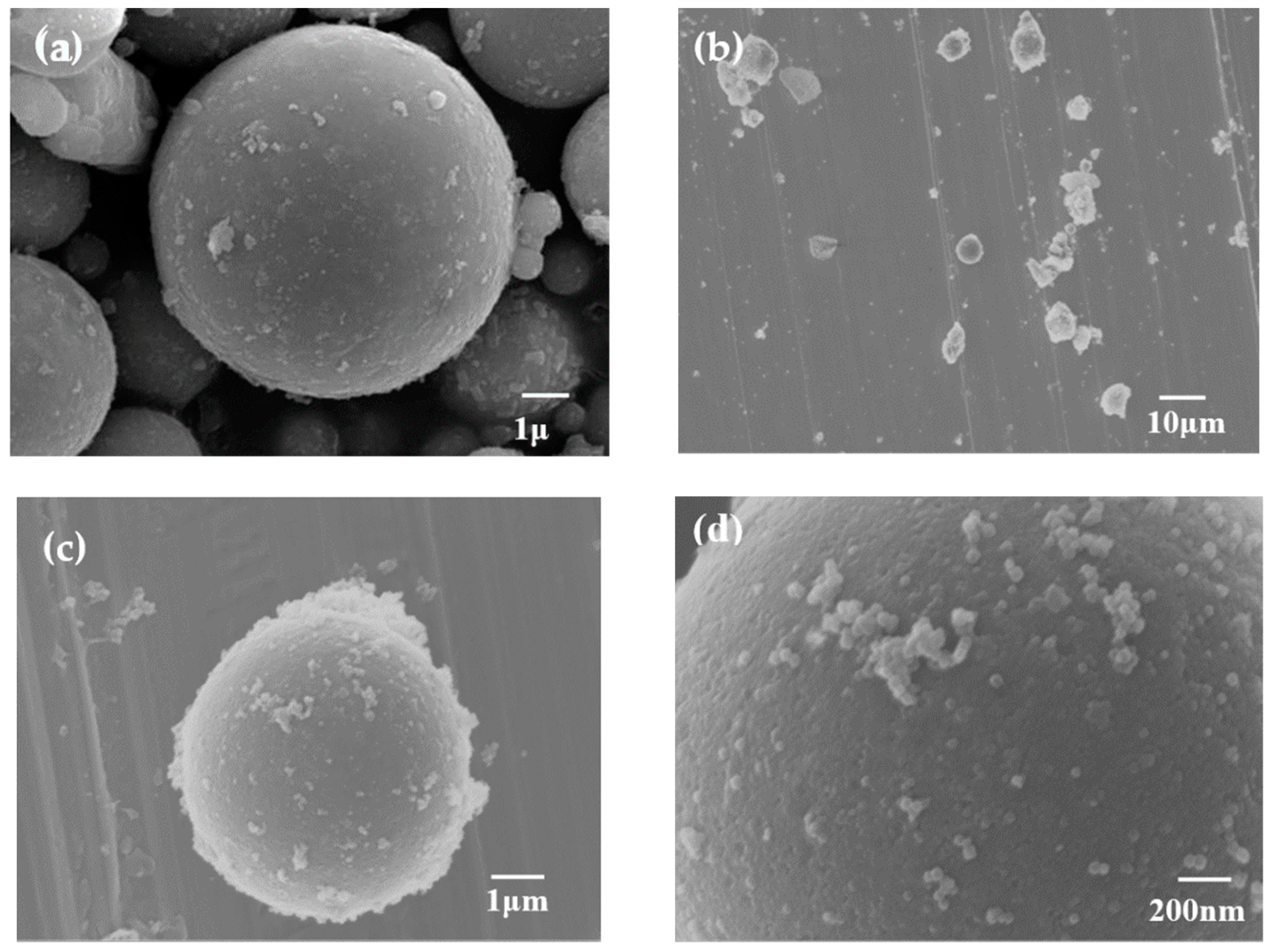
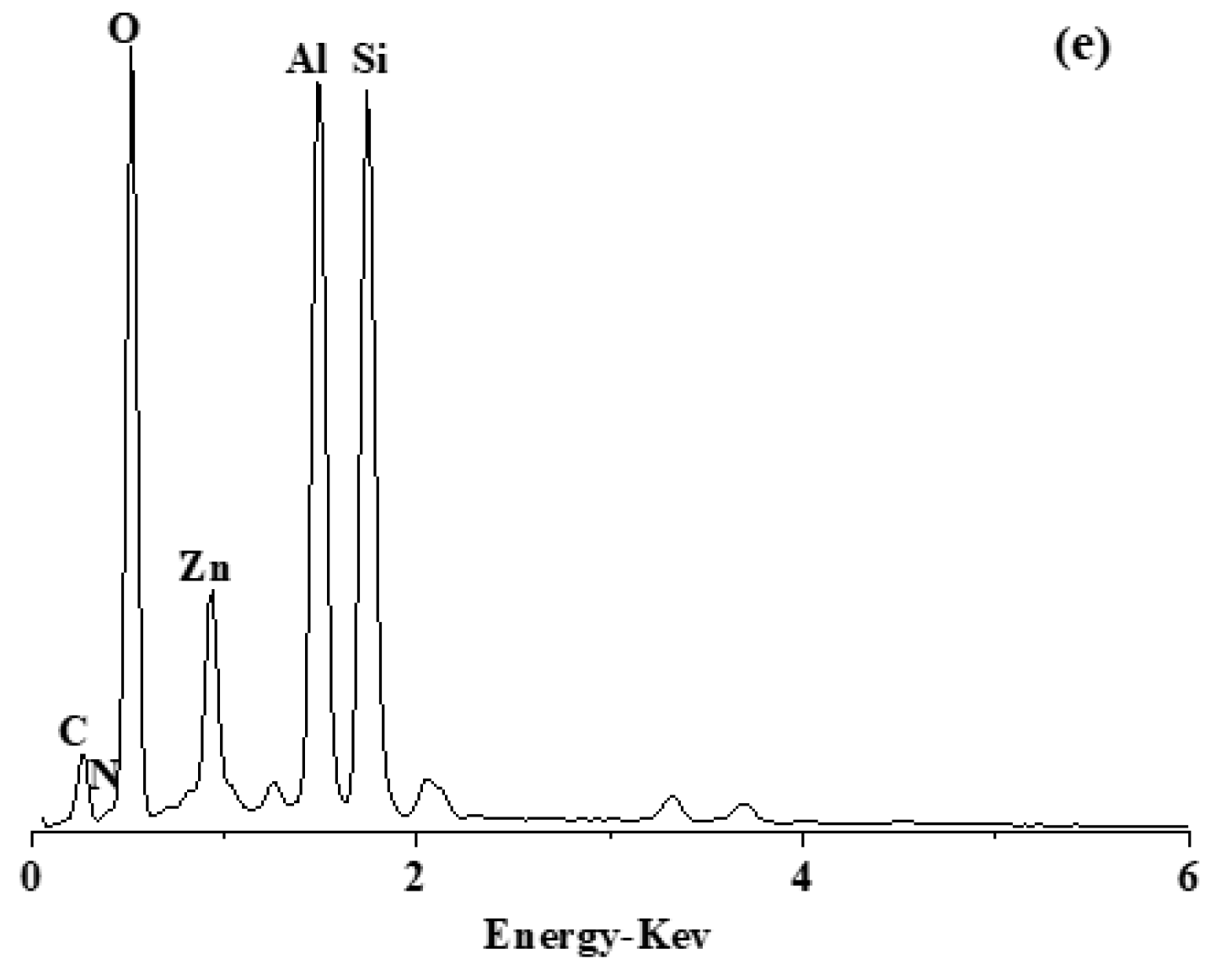


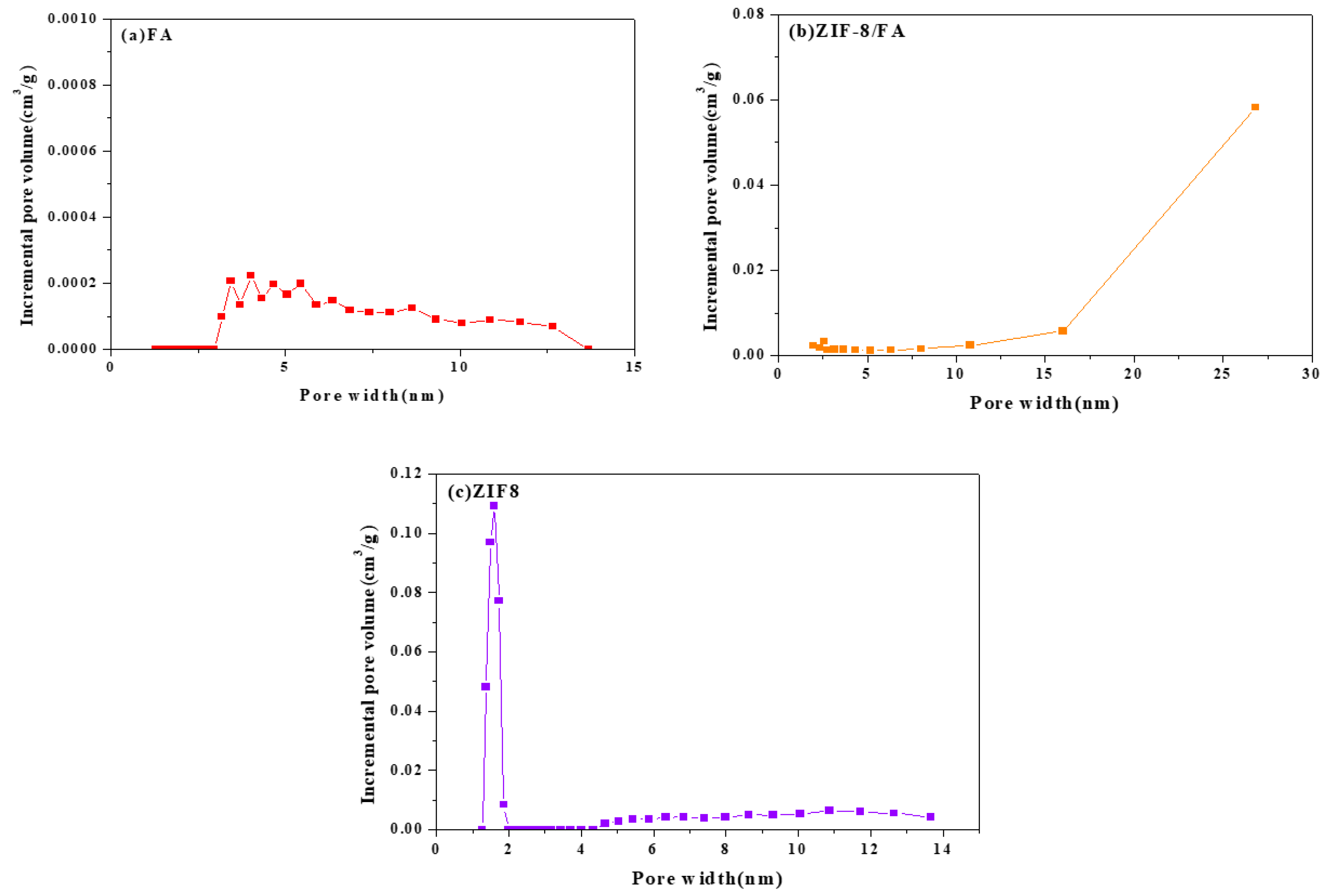
| Sample | SBET (m2g−1) | Vt (cm3g−1) | Vmicro (cm3g−1) | Vmeso (cm3g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| FA | 1.8 | 0.0025 | 0 | 0.0025 | 4.21 |
| ZIF-8/FA | 249.5 | 0.08 | 0.002 | 0.078 | 11.29 |
| ZIF-8 | 1594.8 | 0.73 | 0.67 | 0.06 | 4.22 |
| Adsorbent Type | Saturated Adsorption Capacities (mg.g−1) | References | ||
|---|---|---|---|---|
| Cu2+ | Ni2+ | Zn2+ | ||
| G-ZnO composite | 37.54 | [31] | ||
| Modified Carbons | 9.81 | 10.7 | [32] | |
| ZnO/activated carbon cloth | 1300 | [33] | ||
| Nanometer-size TiO2 | 26.5 | [34] | ||
| Tourmaline | 30.67 | 37.88 | [35] | |
| Indonesian peat | 32.07 | [36] | ||
| Fly ash derived geopolymer | 90 | [3] | ||
| ZnO-Gr | 66.7 | [2] | ||
| ZIF-8/FA composite | 335 | 93 | 197 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yang, R.; Wang, H. Synthesis of ZIF-8/Fly Ash Composite for Adsorption of Cu2+, Zn2+ and Ni2+ from Aqueous Solutions. Materials 2020, 13, 214. https://doi.org/10.3390/ma13010214
Wang C, Yang R, Wang H. Synthesis of ZIF-8/Fly Ash Composite for Adsorption of Cu2+, Zn2+ and Ni2+ from Aqueous Solutions. Materials. 2020; 13(1):214. https://doi.org/10.3390/ma13010214
Chicago/Turabian StyleWang, Caili, Runquan Yang, and Huaifa Wang. 2020. "Synthesis of ZIF-8/Fly Ash Composite for Adsorption of Cu2+, Zn2+ and Ni2+ from Aqueous Solutions" Materials 13, no. 1: 214. https://doi.org/10.3390/ma13010214





