Estimating the Internal Oxidation of Ni-Al Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
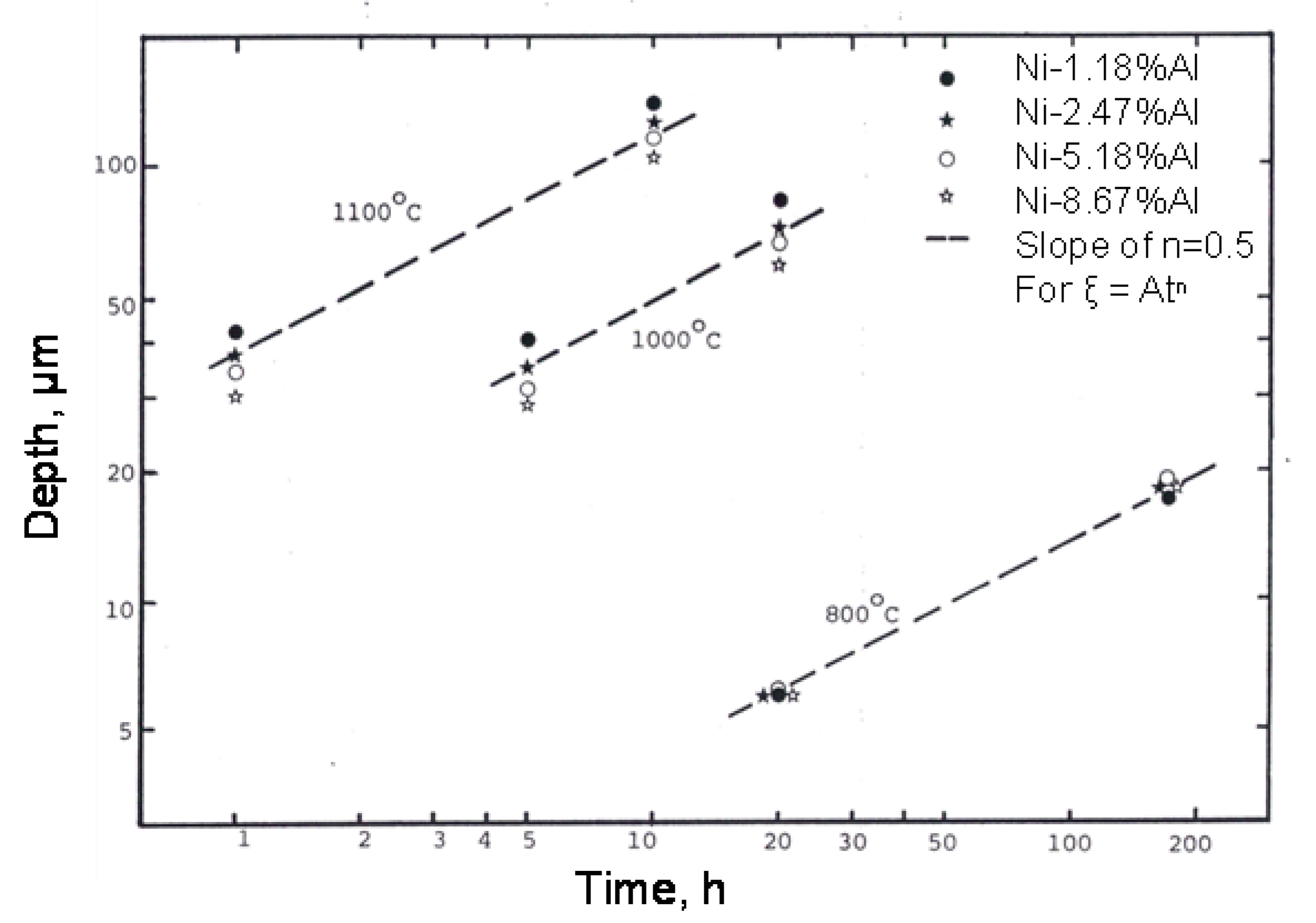
3.1. Internal Oxidation Depth
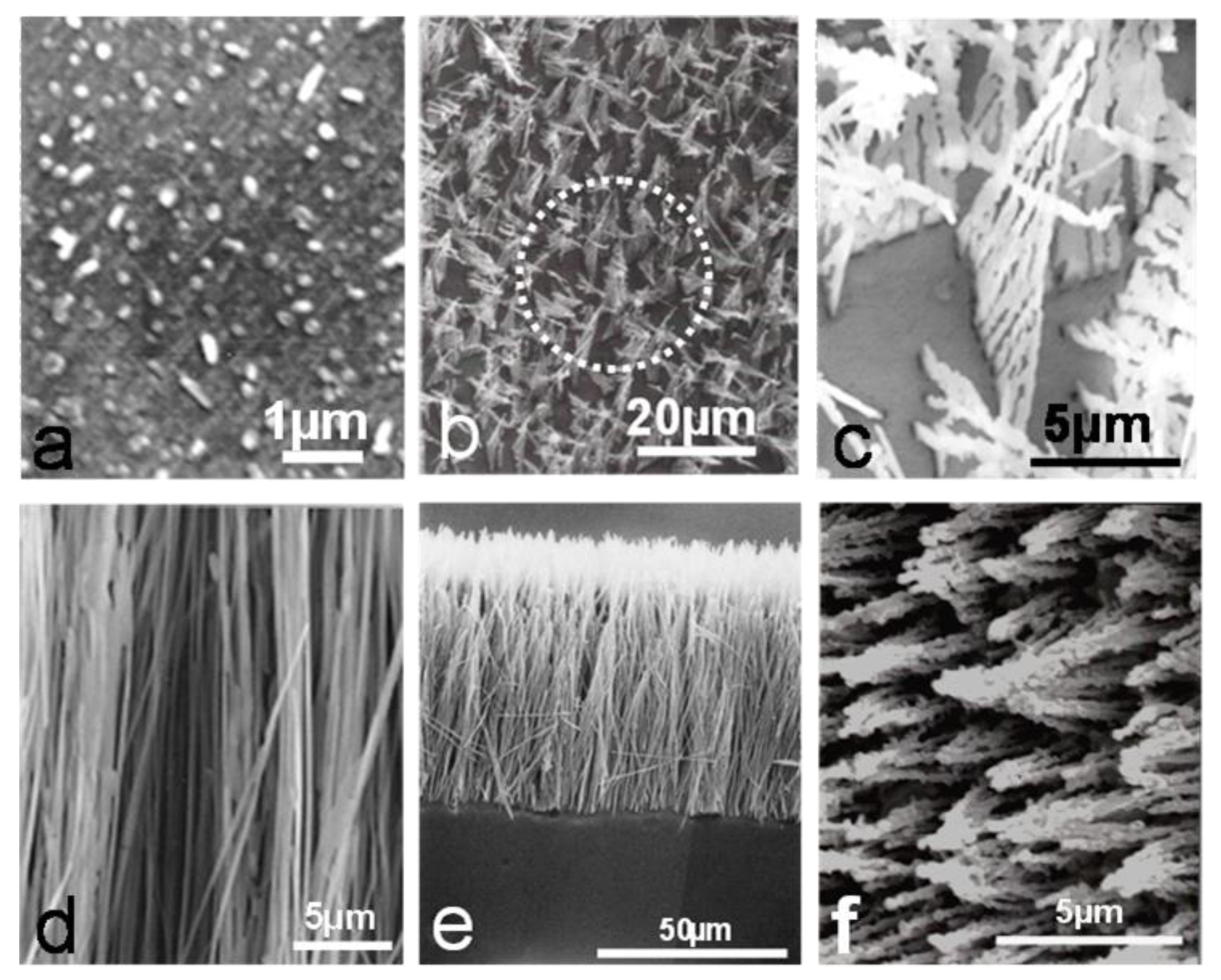
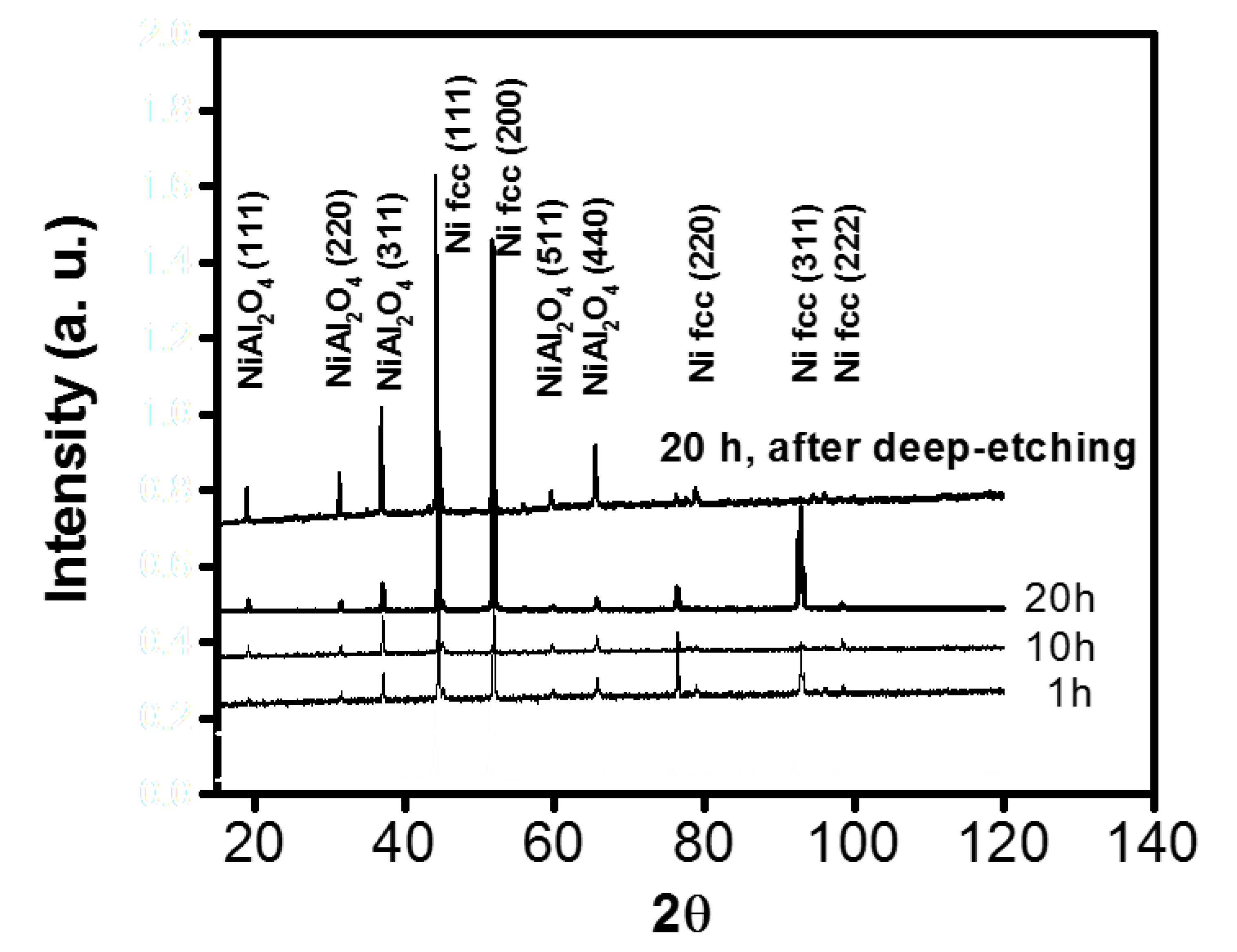
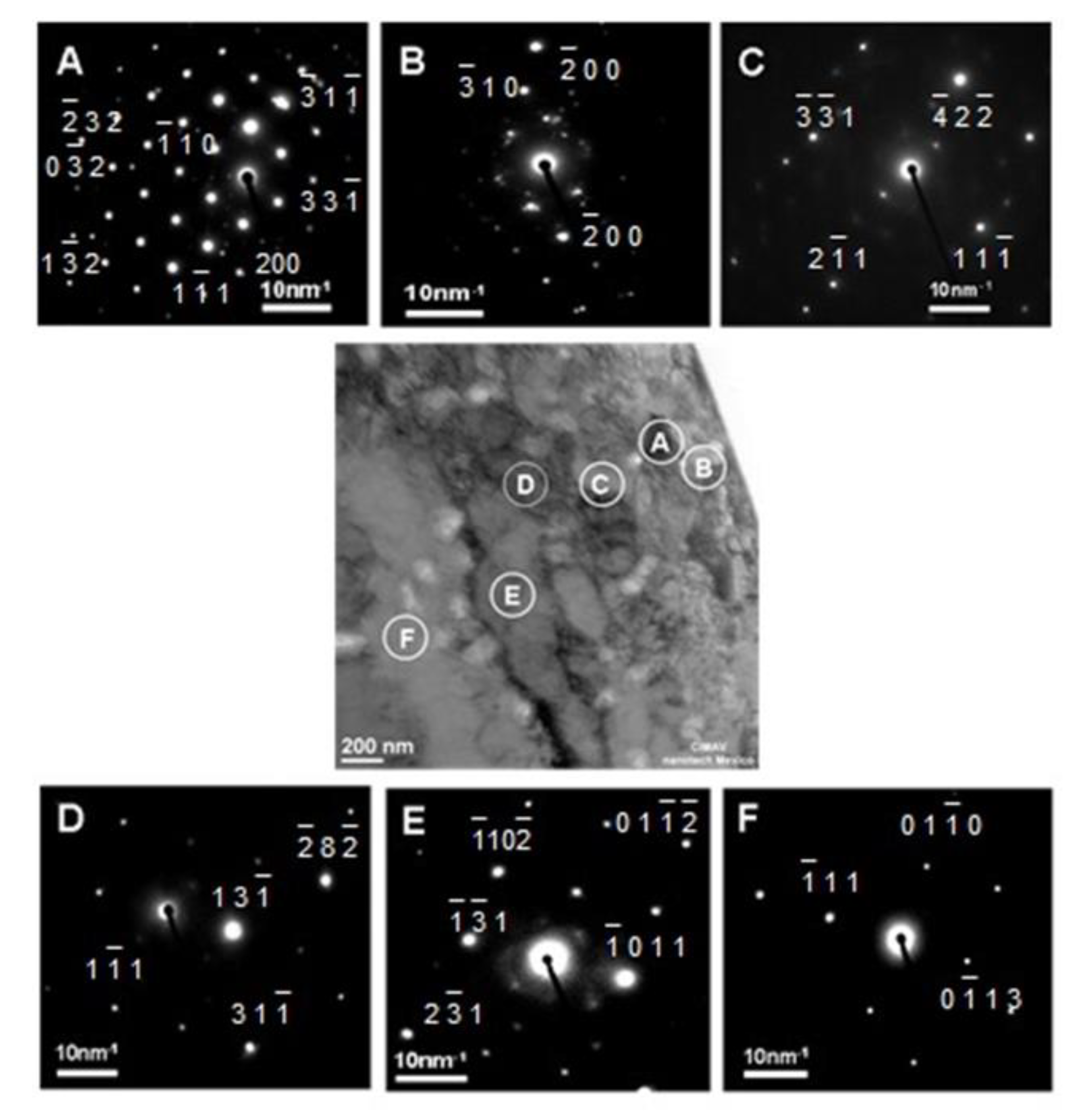
3.1.1. Morphology of Internal Oxides.
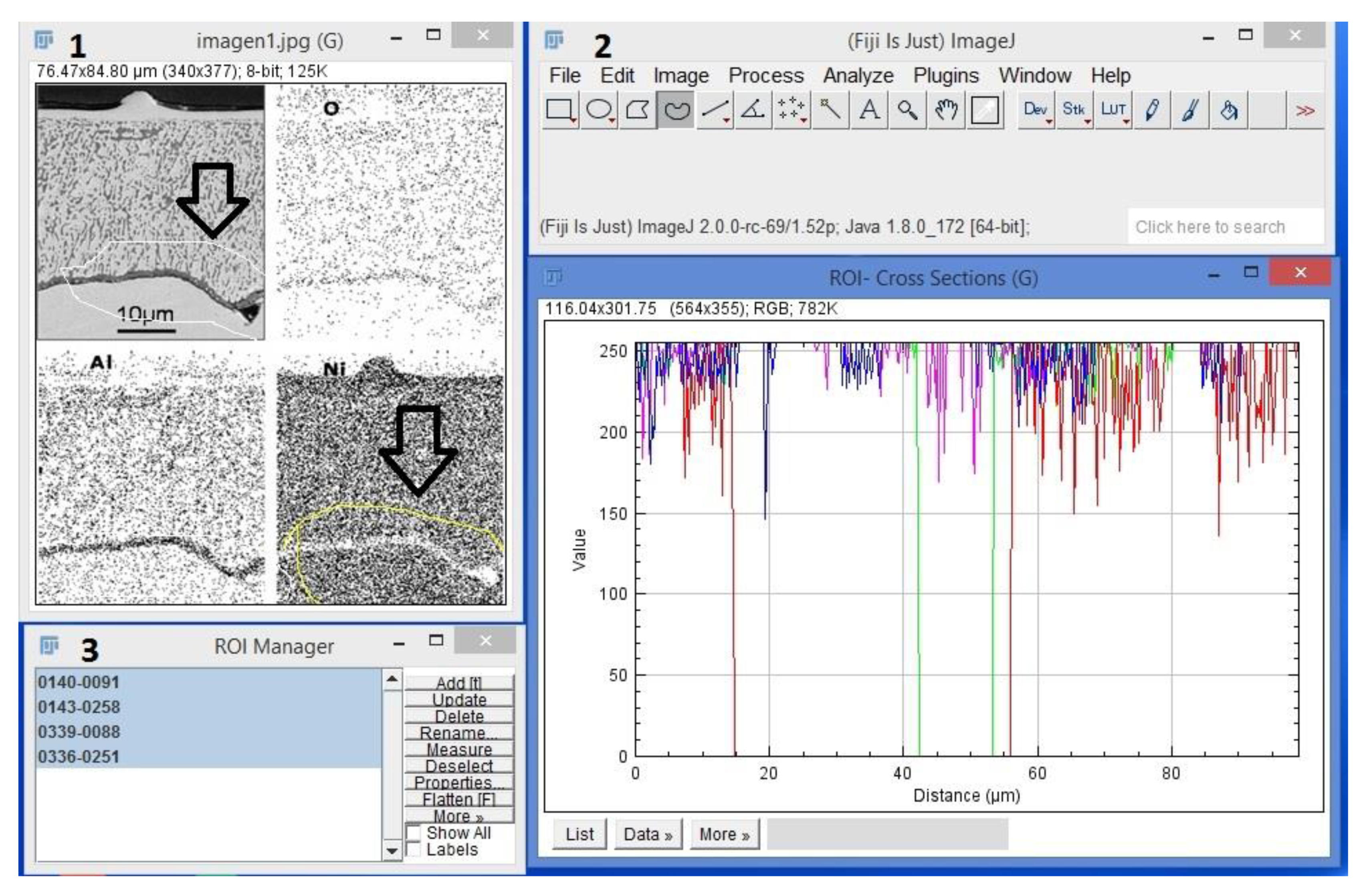
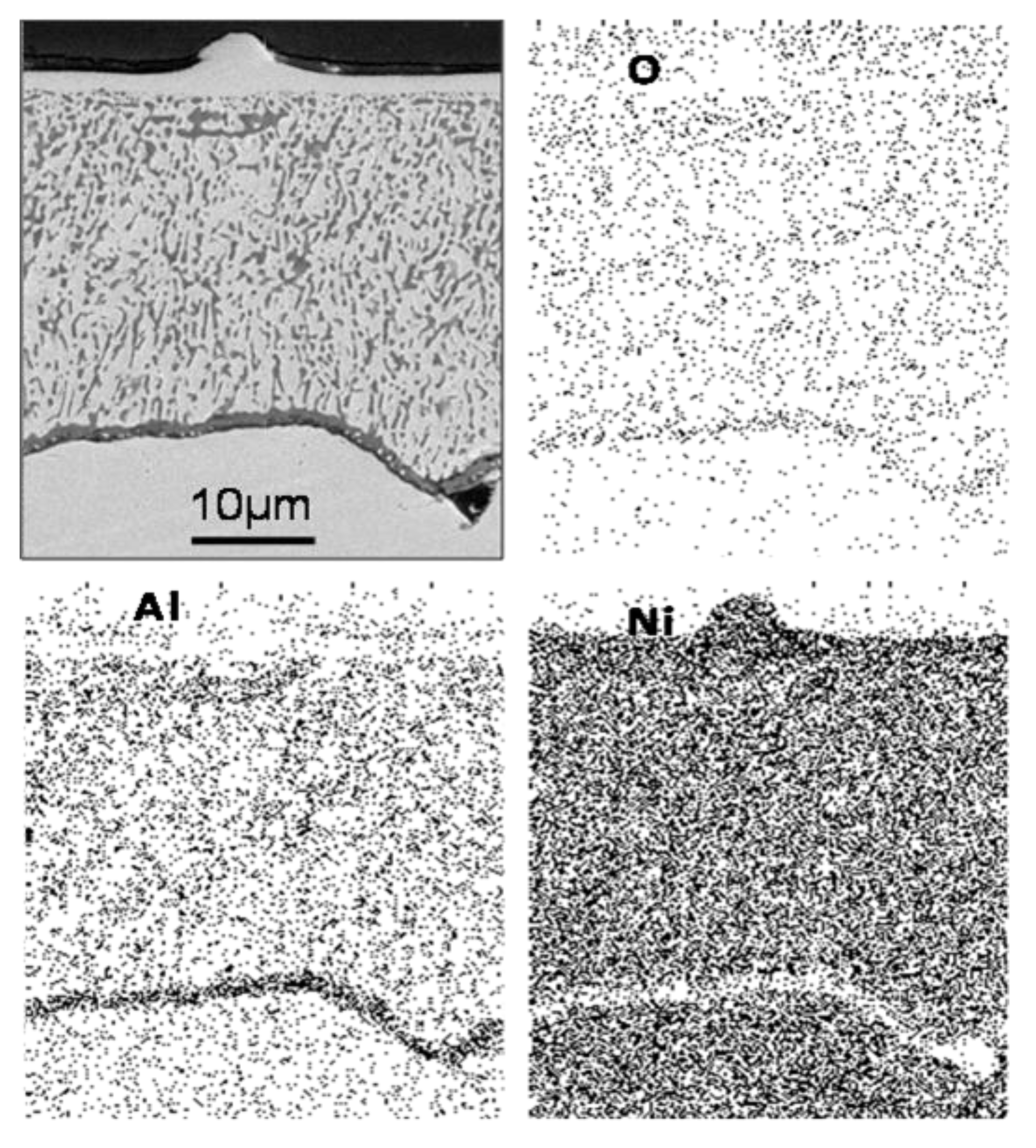
3.1.2. Morphology Analysis in Fiji Software
4. Discussion
5. Conclusions
- In the Ni-Al alloys, several internal oxide morphologies were observed, depending upon aluminum concentration after oxidation in Ni/NiO Rhines pack at a given temperature. At low concentrations (Ni-1.18% Al), the internal oxide precipitates were a mixture of spherical particles, fork-like triangles and acicular precipitates, extending across the internal oxidation zone; these morphologies were observed at temperatures from 800 °C to 1100 °C.
- In the Ni-2.47%Al alloy, the internal oxides at 1000 and 1100 °C were continuous rod-like precipitates extending across the internal zone. At 800 °C, a rod-like was observed. For the Ni-5.18% Al and Ni-8.67% Al alloys, only rod-like precipitates were observed for all temperature-time conditions studied. The cylindrical, rod-like precipitates were mainly closely-spaced and oriented approximately perpendicular to the specimen surface.
- The cylindrical rods had grown through the internal oxidation zone in a cone-shaped configuration, where the thin part of the cone was situated at the surface, and the broad base of the cone was situated at the end of the internal oxidation front.
- The particles were Al2O3 in the vicinity of the internal oxide front, and NiAl2O4 was detected in the vicinity of the surface by ROI analyzed. Morphologies observed had a mixture of figures like triangles, spheres and cones related to oxidation zone. Using ROI allows to understand a little bit about oxidation.
- In superalloys, observations are related to the microstructure with results such as a vacancy diffusion and creep dislocations. In these studies, pattern analysis in Figure 6 shows that reactions depend on crystal orientation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suárez, A.; Veiga, F.; Polvorosa, R.; Artaza, T.; Holmberg, J.; de Lacalle, L.N.L.; Wretland, A. Surface integrity and fatigue of non-conventional machined Alloy 718. J. Manuf. Process. 2019, 48, 44–50. [Google Scholar] [CrossRef]
- Li, M.F.; Kong, C.; Zhang, J.Q.; Zhou, C.G.; Young, D.J. Oxidation behavior of Ni-Al coating with and without a Ni-Re diffusion barrier in dry CO2 gas at 650 °C. Corros. Sci. 2019, 149, 236–243. [Google Scholar] [CrossRef]
- Hargather, C.Z.; Shang, S.L.; Liu, Z.K. A comprehensive first-principles study of solute elements in dilute Ni alloys: Diffusion coefficients and their implications to tailor creep rate. Acta Mater. 2018, 157, 126–141. [Google Scholar] [CrossRef]
- Eriş, R.; Akdeniz, M.V.; Mekhrabov, A.O. Atomic size effect of alloying elements on the formation, evolution and strengthening of γ′-Ni3Al precipitates in Ni-based superalloys. Intermetallics 2019, 109, 37–47. [Google Scholar] [CrossRef]
- Jullian, D.; Zhang, J.Q.; Hibbert, D.B.; Young, D.J. Oxygen solubility in austenitic Fe-Ni alloys at high temperatures. J. Alloys Compd. 2018, 732, 646–654. [Google Scholar] [CrossRef]
- Wood, G.C.; Stott, F.H.; Whittle, D.P.; Shida, Y.; Bastow, B.D. The high temperature internal oxidation and intergranular oxidation of nickel–chromium alloys. Corros. Sci. 1983, 23, 9–25. [Google Scholar] [CrossRef]
- Halem, Z.; Halem, N.; Abrudeanu, M.; Chekroude, S.; Petot, C.; Petot-Ervas, G. Transport properties of Al or Cr-doped nickel oxide relevant to the thermal oxidation of dilute Ni-Al and Ni-Cr alloys. Solid State Ion. 2016, 297, 13–19. [Google Scholar] [CrossRef]
- Kelesidis, G.A.; Pratsinis, S.E. Estimating the internal and surface oxidation of soot agglomerates. Combust. Flame 2019, 209, 493–499. [Google Scholar] [CrossRef]
- Goswami, K.N.; Mottura, A. A kinetic Monte Carlo study of vacancy diffusion in non-dilute Ni-Re alloys. Mater. Sci. Eng. A 2019, 743, 265–273. [Google Scholar] [CrossRef]
- Ren, R.; Hung, T.; Tan, K.C. Automatic Microstructure Defect Detection of Ti-6Al-4V Titanium Alloy by Regions-Based Graph. IEEE Trans. Emerg. Top. Comput. Intell. 2017, 1, 87–96. [Google Scholar] [CrossRef]
- Stott, F.H.; Martinez-Villafane, A.; Wood, G.C. The Influence of alloying element on the internal oxidation of Nickel-base alloys. In Proceedings of the 9th International Congress on Metallic Corrosion, Toronto, ON, Canada, 3–7 June 1984; National Research Council of Canada: Ottawa, ON, Canada, 1984; Volume 3, pp. 317–324. [Google Scholar]
- Stott, F.H.; Wood, G.C.; Whittle, D.P.; Bastow, B.D.; Shida, Y.; Martinez-Villafane, A. The transport of oxygen to the advancing internal oxide front during internal oxidation of Nickel-base alloys at high temperature. Solid State Ion. 1984, 12, 365–374. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C. Internal oxidation. Mater. Sci. Technol. 1988, 4, 1072–1078. [Google Scholar] [CrossRef]
- Guan, S.W.; Yi, H.C.; Smeltzer, W.W. Internal oxidation of ternary alloys. Part I: Kinetics in the absence of an external scale. Oxid. Met. 1994, 41, 377–387. [Google Scholar] [CrossRef]
- Martinez-Villafañe, A.; Stott, F.H.; Chacon-Nava, J.; Wood, G.C. Enhanced oxygen diffusion along internal oxide/metal matrix interfaces in Ni-Al Alloys during internal oxidation. Oxid. Met. 2002, 57, 267–279. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.J.; Wu, Y.; Gesmundo, F. The third-element effect in the oxidation of Ni–xCr–7Al (x = 0, 5, 10, 15 at.%) alloys in 1 atm O2 at 900–1000 °C. Corros. Sci. 2006, 48, 4020–4036. [Google Scholar] [CrossRef]
- Hayashi, S.; Narita, S.; Narita, T. Oxidation behavior of Ni-3,6, 10 wt.% Al alloys at 800 °C. Oxid. Met. 2006, 66, 191–207. [Google Scholar] [CrossRef]
- Simon, M.; Chalfoun, J.; Brady, M.; Bajcsy, P. Do We Trust Image Measurements? Variability, Accuracy and Traceability of Image Features. In Proceedings of the 2016 IEEE International Conference on Big Data (Big Data), Washington, DC, USA, 5–8 December 2016. [Google Scholar]
- Chang, S.Y.; Krupp, U.; Christ, H.J. Formation and compensation of internal stresses during internal nitridation of nickel-base alloys. Mater. Sci. Eng. A 2001, 301, 196–206. [Google Scholar] [CrossRef]
- Hernández-Negrete, O.; Martinez-Villafañe, A. Microstructural Study of Internal Oxidation of Dilute Ni-Al Alloys. Microsc. Microanal. 2018, 24 (Suppl. S1), 1106–1107. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.Y.; Wang, H.M.; Chena, S.H.; Wei, L.; Huang, J.H.; Yang, J.; Zhao, Z.Y. Interfacial reaction between solid Ni and liquid Al in tens of seconds: Dissolution kinetics of solid Ni and formation of intermetallic compounds. Mater. Charact. 2020, 159, 110043. [Google Scholar] [CrossRef]
- Hoier, P.; Klement, U.; Alagan, N.T.; Beno, T.; Wretland, A. Flank wear characteristics of WC-Co tools when turning Alloy 718 with high-pressure coolant supply. J. Manuf. Process. 2017, 30, 116–123. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Dino, C.G.; Martinez-Villafañe, A. Estimating the Internal Oxidation of Ni-Al Alloys. Materials 2020, 13, 48. https://doi.org/10.3390/ma13010048
Nava-Dino CG, Martinez-Villafañe A. Estimating the Internal Oxidation of Ni-Al Alloys. Materials. 2020; 13(1):48. https://doi.org/10.3390/ma13010048
Chicago/Turabian StyleNava-Dino, C.G., and A. Martinez-Villafañe. 2020. "Estimating the Internal Oxidation of Ni-Al Alloys" Materials 13, no. 1: 48. https://doi.org/10.3390/ma13010048



