Evaluation of a Zn–2Ag–1.8Au–0.2V Alloy for Absorbable Biocompatible Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructure Characterization and Mechanical Properties Test
2.3. Immersion Test
2.4. Cytotoxicity Test
2.5. Antibacterial Test
2.6. Statistical Analysis
3. Results and Discussion
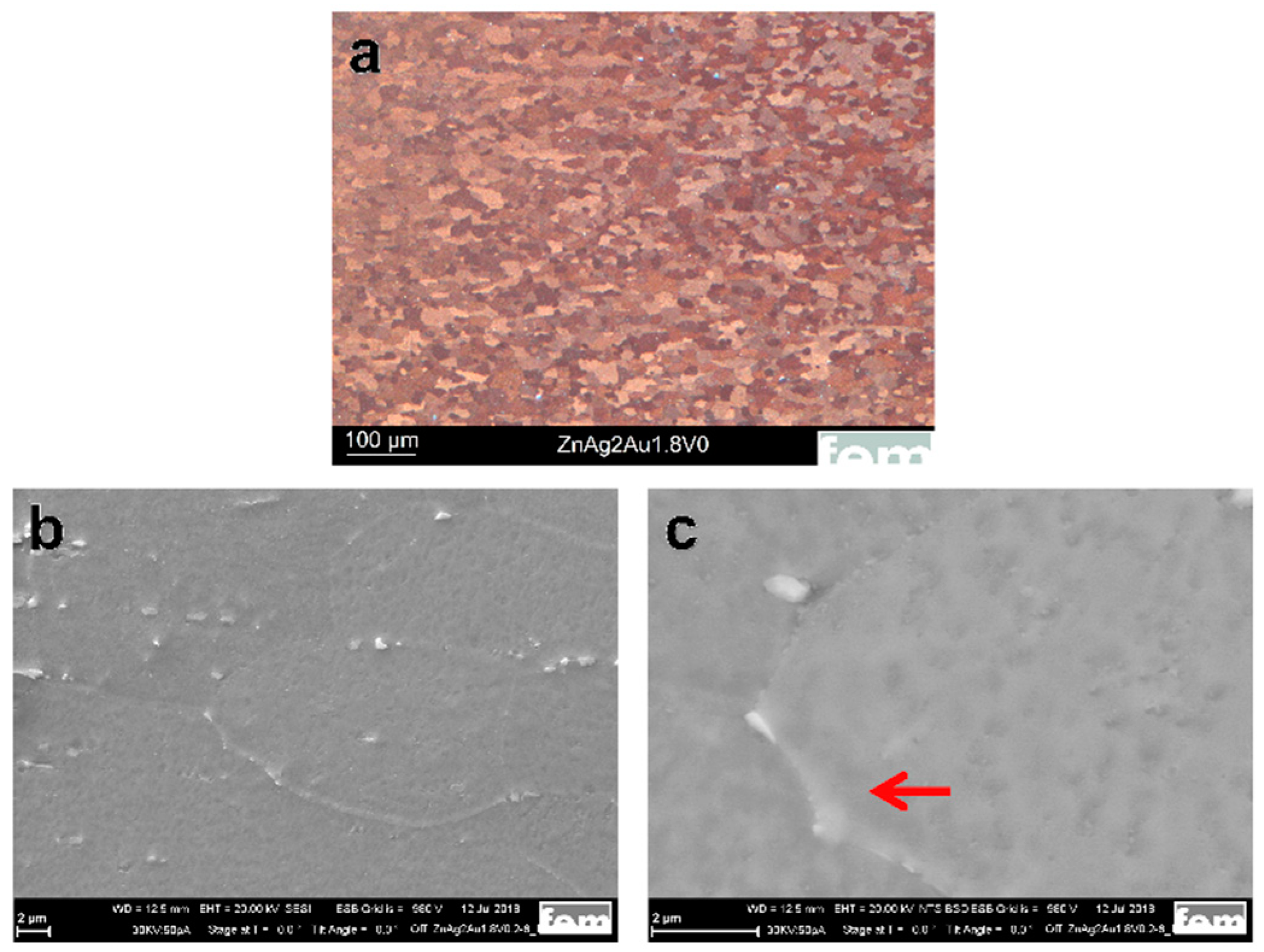
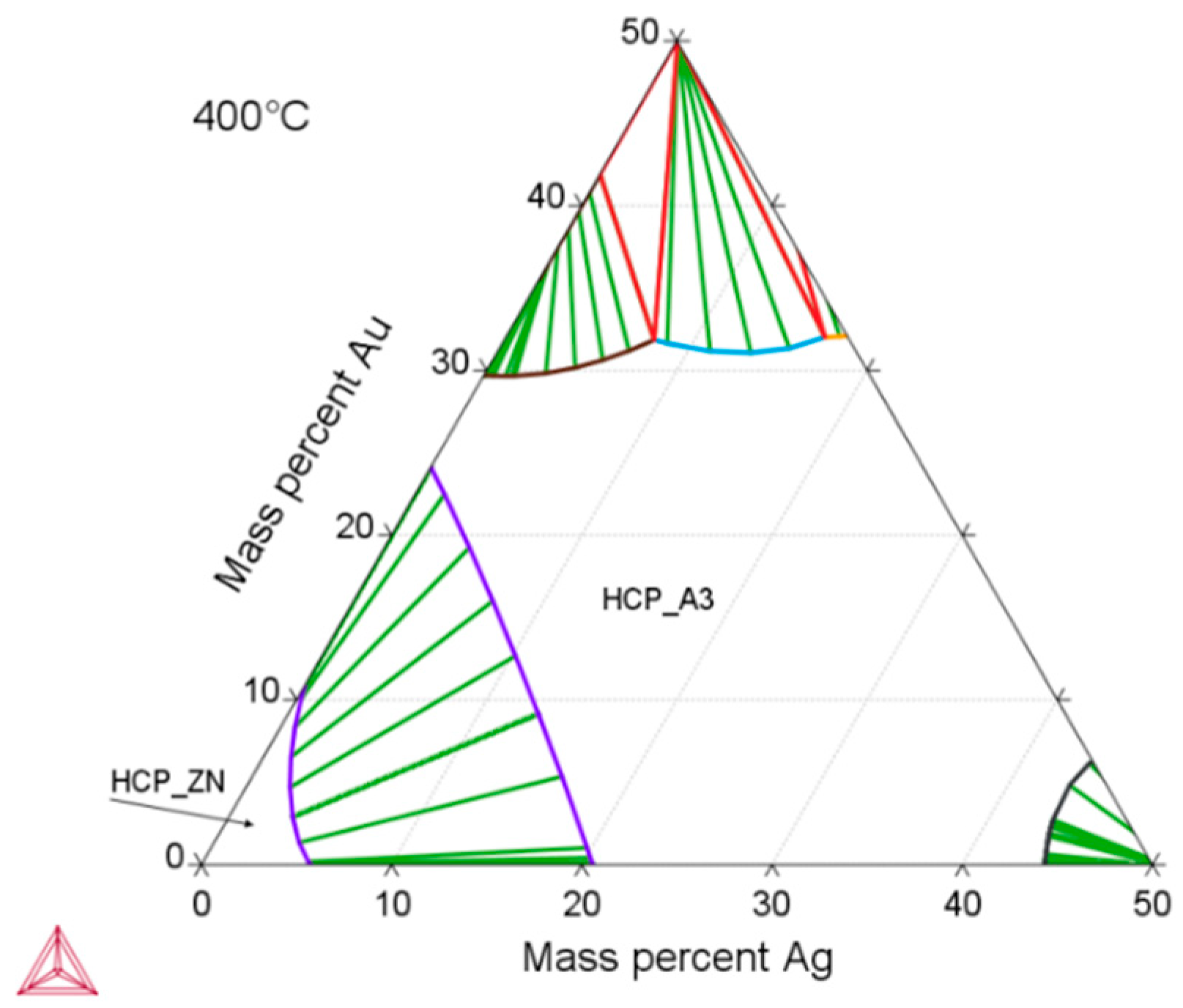
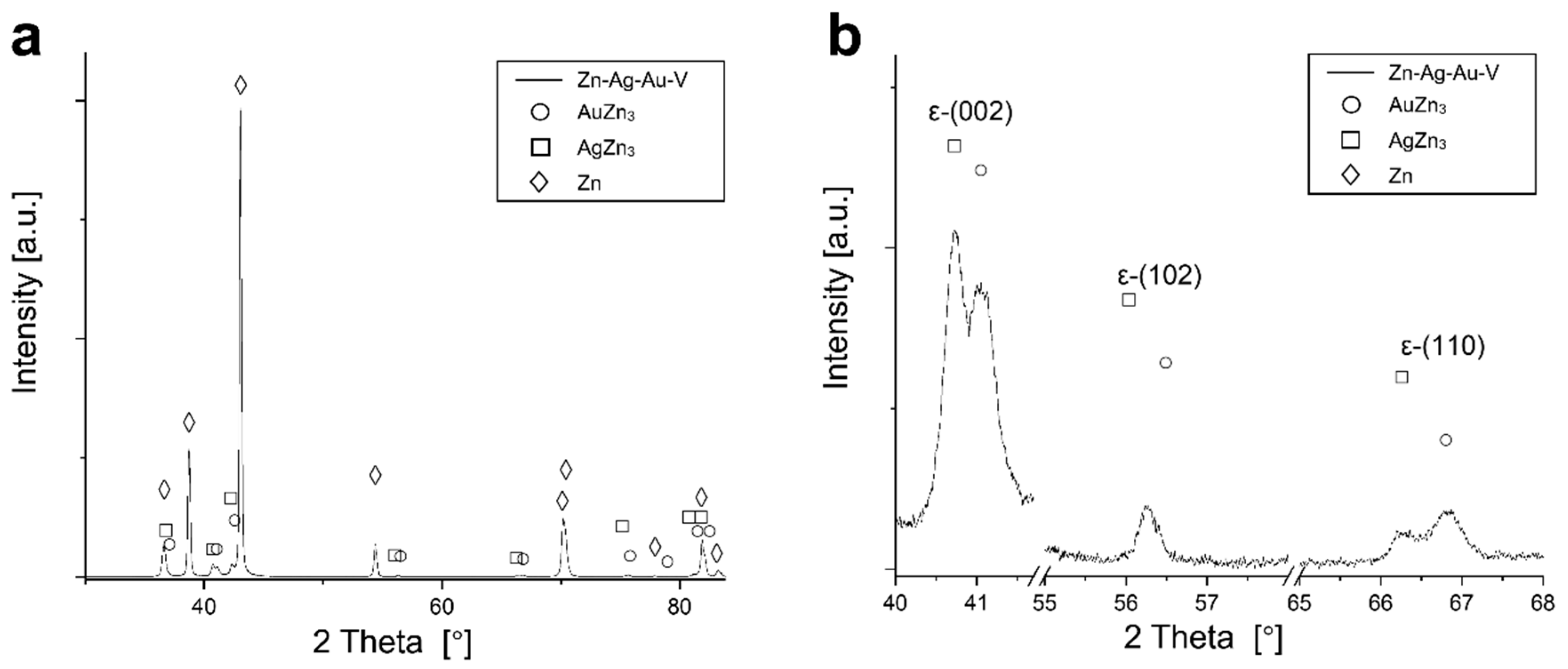
3.1. Microstructure and Mechanical Properties
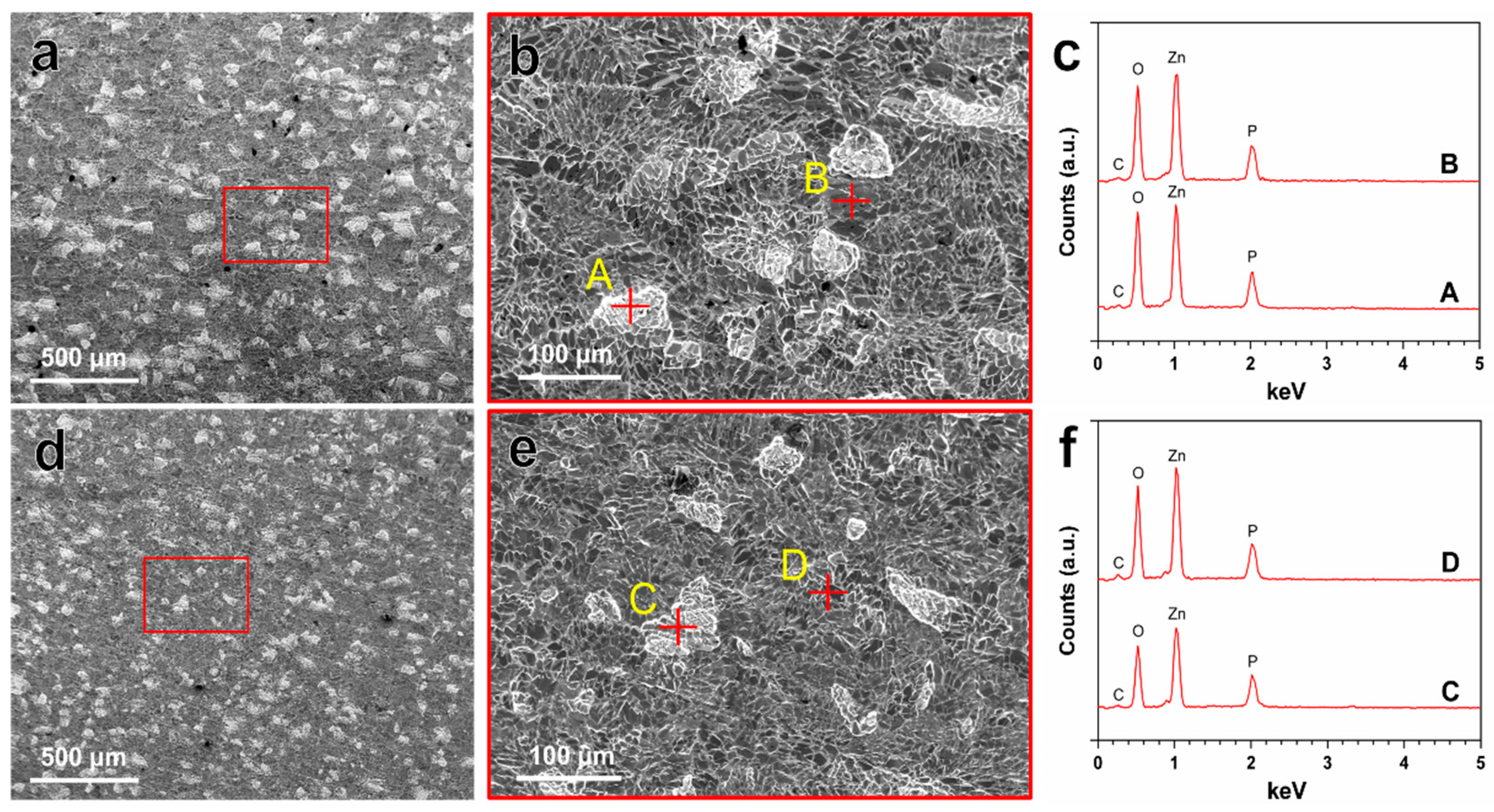
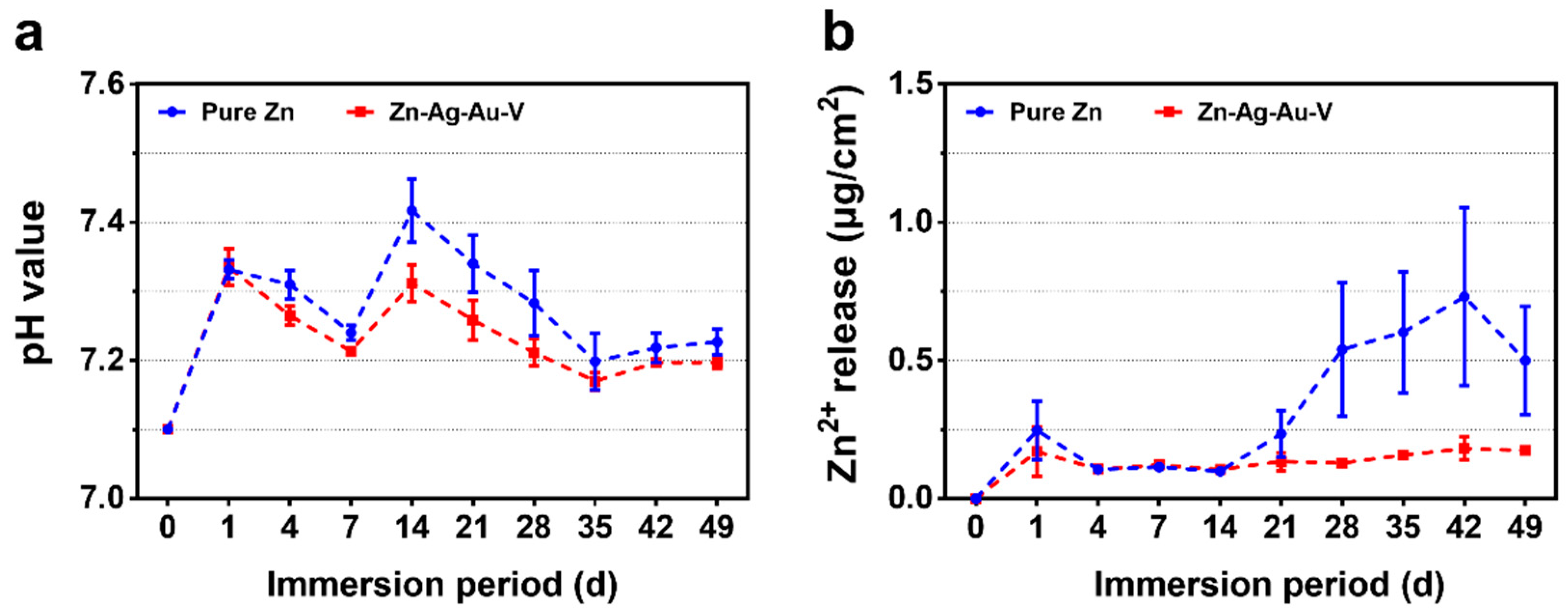
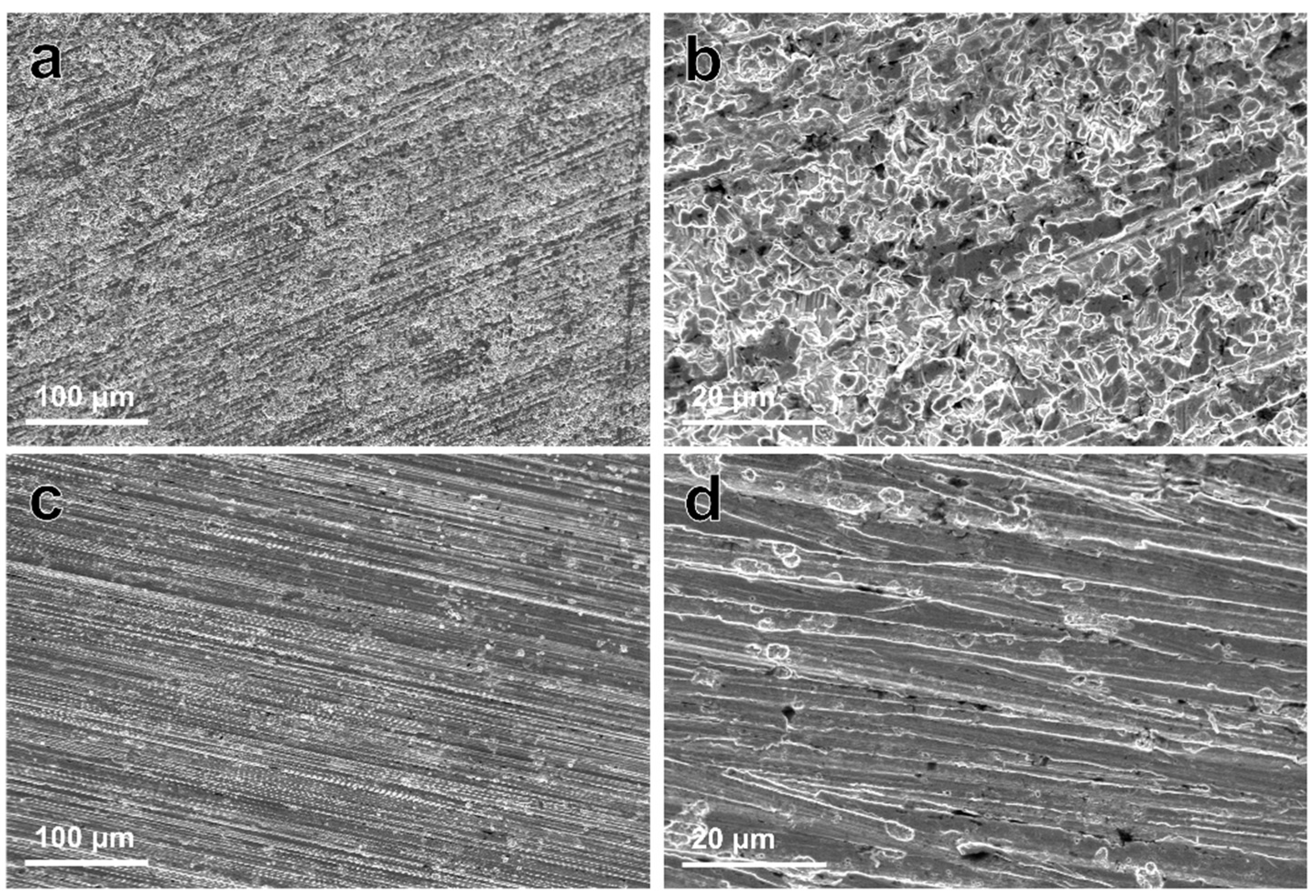
3.2. In Vitro Degradation Behavior
Zn(OH)2 → ZnO + H2O
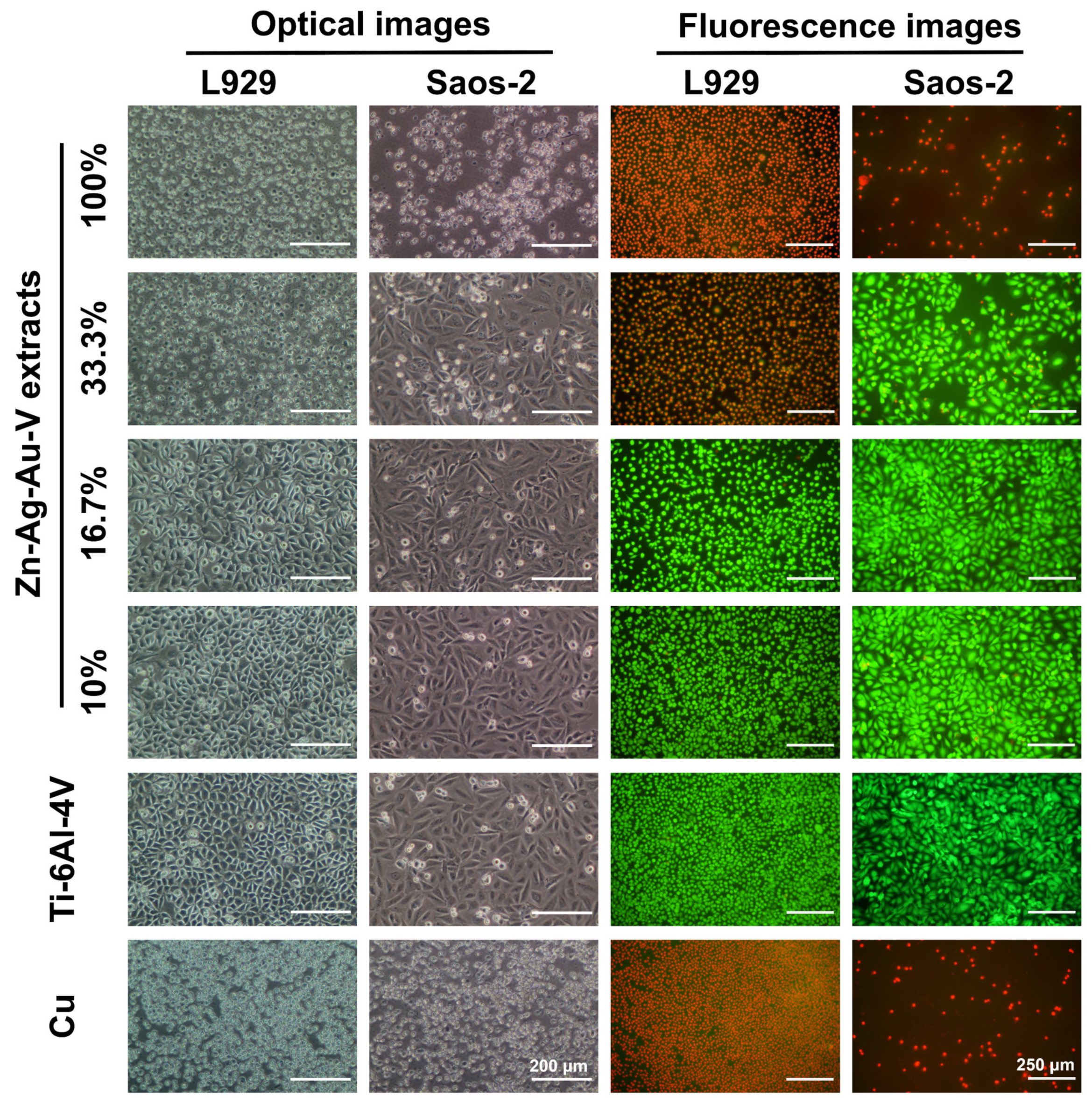
3.3. Cytotoxicity Evaluation
3.4. Antibacterial Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neacsu, P.N.; Ion, R.; Mitran, V.I.; Staras, A.; Cîmpean, A. State of the art and recent patents on Mg-based biodegradable bone implants. Recent Pat. Regen. Med. 2014, 4, 168–188. [Google Scholar] [CrossRef]
- Katarivas Levy, G.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants—A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, J.; Dargusch, M. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zhang, W.; Dai, J.; Xepapadeas, A.B.; Schweizer, E.; Alexander, D.; Scheideler, L.; Zhou, C.; Zhang, H.; Wan, G. Investigation of zinc-copper alloys as potential materials for craniomaxillofacial osteosynthesis implants. Mater. Sci. Eng. C 2019, 103, 109826. [Google Scholar] [CrossRef] [PubMed]
- Geis-Gerstorfer, J.; Schille, C.; Schweizer, E.; Rupp, F.; Scheideler, L.; Reichel, H.-P.; Hort, N.; Nolte, A.; Wendel, H.-P. Blood triggered corrosion of magnesium alloys. Mater. Sci. Eng. B 2011, 176, 1761–1766. [Google Scholar] [CrossRef]
- Neacsu, P.; Staras, A.; Voicu, S.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.; Pandele, A.; Croitoru, S.; Miculescu, F. Characterization and in vitro and in vivo assessment of a novel cellulose acetate-coated Mg-based alloy for orthopedic applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [Green Version]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. A 2012, 100, 58–67. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.; Leeflang, M.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, X.; Xu, Z.; Wang, J.-Q.; Cai, H. Metallic Biomaterials: New Directions and Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 161–188. [Google Scholar]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2018, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Drelich, A.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, X.; Zheng, Y.; Cong, Y.; Zhou, F.; Qiu, K.; Wang, X.; Chen, S.; Huang, L.; Tian, L. Development of biodegradable Zn-1x binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.; Mol, J.; Jahr, H. Additively manufactured biodegradable porous zinc. Acta Biomater. 2019, 101, 609–623. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Šedý, J.; Msallamová, Š.; Levorová, J.; Foltán, R.; Vojtěch, D. The fundamental comparison of Zn–2Mg and Mg–4Y–3Re alloys as a perspective biodegradable materials. Materials 2019, 12, 3745. [Google Scholar] [CrossRef] [Green Version]
- Dambatta, M.; Murni, N.; Izman, S.; Kurniawan, D.; Froemming, G.; Hermawan, H. In vitro degradation and cell viability assessment of Zn–3Mg alloy for biodegradable bone implants. Proc. Inst. Mech. Eng. J 2015, 229, 335–342. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Mechanical characteristics, in vitro degradation, cytotoxicity, and antibacterial evaluation of Zn-4.0 Ag alloy as a biodegradable material. Int. J. Mol. Sci. 2018, 19, 755. [Google Scholar] [CrossRef] [Green Version]
- Sikora-Jasinska, M.; Mostaed, E.; Mostaed, A.; Beanland, R.; Mantovani, D.; Vedani, M. Fabrication, mechanical properties and in vitro degradation behavior of newly developed Zn Ag alloys for degradable implant applications. Mater. Sci. Eng. C 2017, 77, 1170–1181. [Google Scholar] [CrossRef]
- Wątroba, M.; Bednarczyk, W.; Kawałko, J.; Mech, K.; Marciszko, M.; Boelter, G.; Banzhaf, M.; Bała, P. Design of novel Zn–Ag–Zr alloy with enhanced strength as a potential biodegradable implant material. Mater. Des. 2019, 183, 108154. [Google Scholar] [CrossRef]
- Hehrlein, C.; Schorch, B.; Kress, N.; Arab, A.; von zur Mühlen, C.; Bode, C.; Epting, T.; Haberstroh, J.; Mey, L.; Schwarzbach, H. Zn-alloy provides a novel platform for mechanically stable bioresorbable vascular stents. PLoS ONE 2019, 14, e0209111. [Google Scholar] [CrossRef]
- Shuai, C.; Xue, L.; Gao, C.; Yang, Y.; Peng, S.; Zhang, Y. Selective laser melting of Zn–Ag alloys for bone repair: Microstructure, mechanical properties and degradation behaviour. Virtual Phys. Prototyp. 2018, 13, 146–154. [Google Scholar] [CrossRef]
- Sotoudehbagha, P.; Sheibani, S.; Khakbiz, M.; Ebrahimi-Barough, S.; Hermawan, H. Novel antibacterial biodegradable Fe–Mn–Ag alloys produced by mechanical alloying. Mater. Sci. Eng. C 2018, 88, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, T.; Nishide, T.; Iwata, H.; Taki, W. Development of gold stents for the treatment of intracranial aneurysms: An experimental study in a canine model. AJNR Am. J. Neuroradiol. 2004, 25, 53–59. [Google Scholar] [PubMed]
- Buha, J. Grain refinement and improved age hardening of Mg–Zn alloy by a trace amount of V. Acta Mater. 2008, 56, 3533–3542. [Google Scholar] [CrossRef]
- International Organization for Standardization. Tensile Testing of Metallic Materials. Method of Test at Ambient Temperature; ISO 10002-1: 2001; International Organization for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- International Organization for Standardization. Dentistry-Corrosion Test Methods for Metallic Materials; ISO 10271: 2011; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Schille, C.; Braun, M.; Wendel, H.; Scheideler, L.; Hort, N.; Reichel, H.-P.; Schweizer, E.; Geis-Gerstorfer, J. Corrosion of experimental magnesium alloys in blood and PBS: A gravimetric and microscopic evaluation. Mater. Sci. Eng. B 2011, 176, 1797–1801. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Selection of extraction medium influences cytotoxicity of zinc and its alloys. Acta Biomater. 2019, 98, 235–245. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn–Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Jablonská, E.; Vojtěch, D.; Fousová, M.; Kubásek, J.; Lipov, J.; Fojt, J.; Ruml, T. Influence of surface pre-treatment on the cytocompatibility of a novel biodegradable ZnMg alloy. Mater. Sci. Eng. C 2016, 68, 198–204. [Google Scholar] [CrossRef]
- International Organization for Standardization. Corrosion of Metals and Alloys–Removal of Corrosion Products from Corrosion Test Specimens; ISO 8407: 2009; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- American Society for Testing and Materials. Standard Guide for Laboratory Immersion Corrosion Testing of Metals; ASTM G31-12a; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity; ISO 10993–5: 2009; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices–Part 12: Sample Preparation and Reference Materials; ISO 10993-12: 2012; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Wang, J.; Witte, F.; Xi, T.; Zheng, Y.; Yang, K.; Yang, Y.; Zhao, D.; Meng, J.; Li, Y.; Li, W. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015, 21, 237–249. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Yang, Y.; Pu, Z.; Zheng, Y. In vitro investigation of ultra-pure Zn and its mini-tube as potential bioabsorbable stent material. Mater. Lett. 2015, 161, 53–56. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Maitz, M.F.; Chen, M.; Zhang, H.; Mao, J.; Zhao, Y.; Huang, N.; Wan, G. Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corros. Sci. 2016, 111, 541–555. [Google Scholar] [CrossRef]
- Paramitha, D.; Chabaud, S.; Bolduc, S.; Hermawan, H. Biological assessment of Zn–based absorbable metals for ureteral stent applications. Materials 2019, 12, 3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yan, Y.; Xu, X.; Lu, Y.; Chen, L.; Li, D.; Dai, Y.; Kang, Y.; Yu, K. Investigation on the microstructure, mechanical properties, in vitro degradation behavior and biocompatibility of newly developed Zn–0.8% Li–(Mg, Ag) alloys for guided bone regeneration. Mater. Sci. Eng. C 2019, 99, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Qiu, K.; Yang, Y.; Pu, Z.; Li, L.; Zheng, Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5 Mg alloy. J. Alloy Comp. 2016, 664, 444–452. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L.-N. Initial formation of corrosion products on pure zinc in simulated body fluid. J. Mater. Sci. Technol. 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- Champagne, S.; Mostaed, E.; Safizadeh, F.; Ghali, E.; Vedani, M.; Hermawan, H. In vitro degradation of absorbable zinc alloys in artificial urine. Materials 2019, 12, 295. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Dai, J.; Schweizer, E.; Rupp, F.; Heiss, A.; Richter, A.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L.; Alexander, D. Response of human periosteal cells to degradation products of zinc and its alloy. Mater. Sci. Eng. C 2020, 108, 110208. [Google Scholar] [CrossRef]
- Murni, N.; Dambatta, M.; Yeap, S.; Froemming, G.R.A.; Hermawan, H. Cytotoxicity evaluation of biodegradable Zn–3Mg alloy toward normal human osteoblast cells. Mater. Sci. Eng. C 2015, 49, 560–566. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Bioabsorbable zinc ion induced biphasic cellular responses in vascular smooth muscle cells. Sci. Rep. 2016, 6, 26661. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, N.; Zhu, D. Endothelial cellular responses to biodegradable metal zinc. ACS Biomater. Sci. Eng. 2015, 1, 1174–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yang, H.; Li, X.; Zheng, Y. In vitro evaluation of the feasibility of commercial Zn alloys as biodegradable metals. J. Mater. Sci. Technol. 2016, 32, 909–918. [Google Scholar] [CrossRef]
- Wang, X.; Shao, X.; Dai, T.; Xu, F.; Zhou, J.G.; Qu, G.; Tian, L.; Liu, B.; Liu, Y. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system. Acta Biomater. 2019, 92, 351–361. [Google Scholar] [CrossRef]
- Spaey, Y.J.; Bettens, R.M.; Mommaerts, M.Y.; Adriaens, J.; Van Landuyt, H.W.; Abeloos, J.V.; De Clercq, C.A.; Lamoral, P.R.; Neyt, L.F. A prospective study on infectious complications in orthognathic surgery. J. Cranio Maxillofac. Surg. 2005, 33, 24–29. [Google Scholar] [CrossRef]
- Loo, C.; Corliss, D.; Ganeshkumar, N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. J. Bacteriol. 2000, 182, 1374–1382. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Low, H.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M. Fabrication of biodegradable Zn-Al-Mg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C 2017, 73, 215–219. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Phan, T.N.; Buckner, T.; Sheng, J.; Baldeck, J.; Marquis, R. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Mol. Oral Microbiol. 2004, 19, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.; Freisinger, B.; Held-Föhn, E. Enhanced antibacterial activity of silver-ruthenium coated hollow microparticles. Biointerphases 2017, 12, 05G608. [Google Scholar] [CrossRef] [PubMed]
- Königs, A.M.; Flemming, H.C.; Wingender, J. Nanosilver induces a non-culturable but metabolically active state in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 395. [Google Scholar] [CrossRef] [PubMed]
- Tie, D.; Feyerabend, F.; Mueller, W.-D.; Schade, R.; Liefeith, K.; Kainer, K.U.; Willumeit, R. Antibacterial biodegradable Mg-Ag alloys. Eur. Cell Mater. 2012, 25, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.K.; Ahn, R.; Tobias, M.E.; Hansen, L.A.; Luke-Marshall, N.R.; Wild, L.; Campagnari, A.A.; Ehrensberger, M.T. Magnesium alloy AZ91 exhibits antimicrobial properties in vitro but not in vivo. J. Biomed. Mater. Res. B 2018, 106, 221–227. [Google Scholar] [CrossRef]


| Composition | Human Extracellular Fluid | DPBS | DMEM | McCoy’s 5A | |
|---|---|---|---|---|---|
| Blood Plasma | Interstitial Fluid | ||||
| Inorganic Ions (mM) | |||||
| Na+ | 142.0 | 139.0 | 146.0 | 127.3 | 141.0 |
| K+ | 4.2 | 4.0 | 4.1 | 5.3 | 5.4 |
| Mg2+ | 0.8 | 0.7 | - | 0.8 | 0.8 |
| Ca2+ | 1.3 | 1.2 | - | 1.8 | 1.2 |
| Cl− | 106.0 | 108.0 | 140.6 | 90.8 | 117.2 |
| SO42− | 0.5 | 0.5 | - | 0.8 | 0.8 |
| HPO42− | 2.0 | 2.0 | 9.5 | 0.9 | 4.2 |
| HCO3− | 24.0 | 28.3 | - | 44.1 | 26.2 |
| Organic Components | |||||
| Protein | 1.2 (mM) | 0.2 (mM) | - | - | - |
| Glucose (mM) | 5.6 | 5.6 | - | 4.5 | 16.6 |
| Amino acids | 2.0 (mM) | 2.0 (mM) | - | 1.6 (g/L) | 0.4 (g/L) |
| Concentrations of Buffering Agents (mM) | |||||
| HCO3− | 24.0 | 28.3 | - | 44.1 | 26.2 |
| HPO42− | 2.0 | 2.0 | 9.5 | 0.9 | 4.2 |
| Tris-HCl | - | - | - | 25.0 | - |
| HPr | 16.0–18.0 | - | - | - | - |
| Total | 42.0–44.0 | 30.3 | 9.5 | 70.0 | 30.4 |
| Materials | Processing | Mechanical Properties | Reference | |||
|---|---|---|---|---|---|---|
| YS0.2 (MPa) | UTS (MPa) | Elongation (%) | Hardness (HV1) | |||
| Zn–Ag–Au–V | Thermomechanical treatment | 129 | 231 | 59 | 61 | In this study |
| Zn–Ag–Au–V | Thermomechanical treatment * | 168 | 233 | 17 | 96 | In this study |
| Pure Zn | As cast | 10 | 18 | 0.3 | 38 | [2] |
| Pure Zn | As extruded | 35 | 60 | 3.5 | - | [2] |
| Pure Zn | As hot rolled | 30–110 | 50–140 | 5.8–36 | 39 | [2] |
| PLA | - | - | 48–53 | 30–240 | - | [5] |
| PGA | - | - | 60–99 | 1.5–20 | - | [5] |
| WE43 | As extruded | 195.2 | 280.6 | 10.3 | - | [5] |
| Extracts | Zn ion Concentration (μM) | pH Value | ||
|---|---|---|---|---|
| DMEM + 10% FBS | McCoy’s 5A + 15% FBS | DMEM + 10% FBS | McCoy’s 5A + 15% FBS | |
| 100% | 738.9 | 313.7 | 8.0 | 8.2 |
| 33.3% | 249.3 | 111.9 | 7.9 | 8.2 |
| 16.7% | 126.5 | 61.3 | 7.8 | 8.1 |
| 10% | 77.2 | 41.0 | 7.8 | 8.1 |
| Original | 4.3 | 6.0 | 7.6 | 8.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Han, X.; Heiss, A.; Richter, A.; Legner, C.; Klotz, U.E.; et al. Evaluation of a Zn–2Ag–1.8Au–0.2V Alloy for Absorbable Biocompatible Materials. Materials 2020, 13, 56. https://doi.org/10.3390/ma13010056
Li P, Schille C, Schweizer E, Kimmerle-Müller E, Rupp F, Han X, Heiss A, Richter A, Legner C, Klotz UE, et al. Evaluation of a Zn–2Ag–1.8Au–0.2V Alloy for Absorbable Biocompatible Materials. Materials. 2020; 13(1):56. https://doi.org/10.3390/ma13010056
Chicago/Turabian StyleLi, Ping, Christine Schille, Ernst Schweizer, Evi Kimmerle-Müller, Frank Rupp, Xingting Han, Alexander Heiss, Andreas Richter, Claudia Legner, Ulrich E. Klotz, and et al. 2020. "Evaluation of a Zn–2Ag–1.8Au–0.2V Alloy for Absorbable Biocompatible Materials" Materials 13, no. 1: 56. https://doi.org/10.3390/ma13010056





