Releasing Behavior of Lipopolysaccharide from Gelatin Modulates Inflammation, Cellular Senescence, and Bone Formation in Critical-Sized Bone Defects in Rat Calvaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipopolysaccharide (LPS) Content Measurement
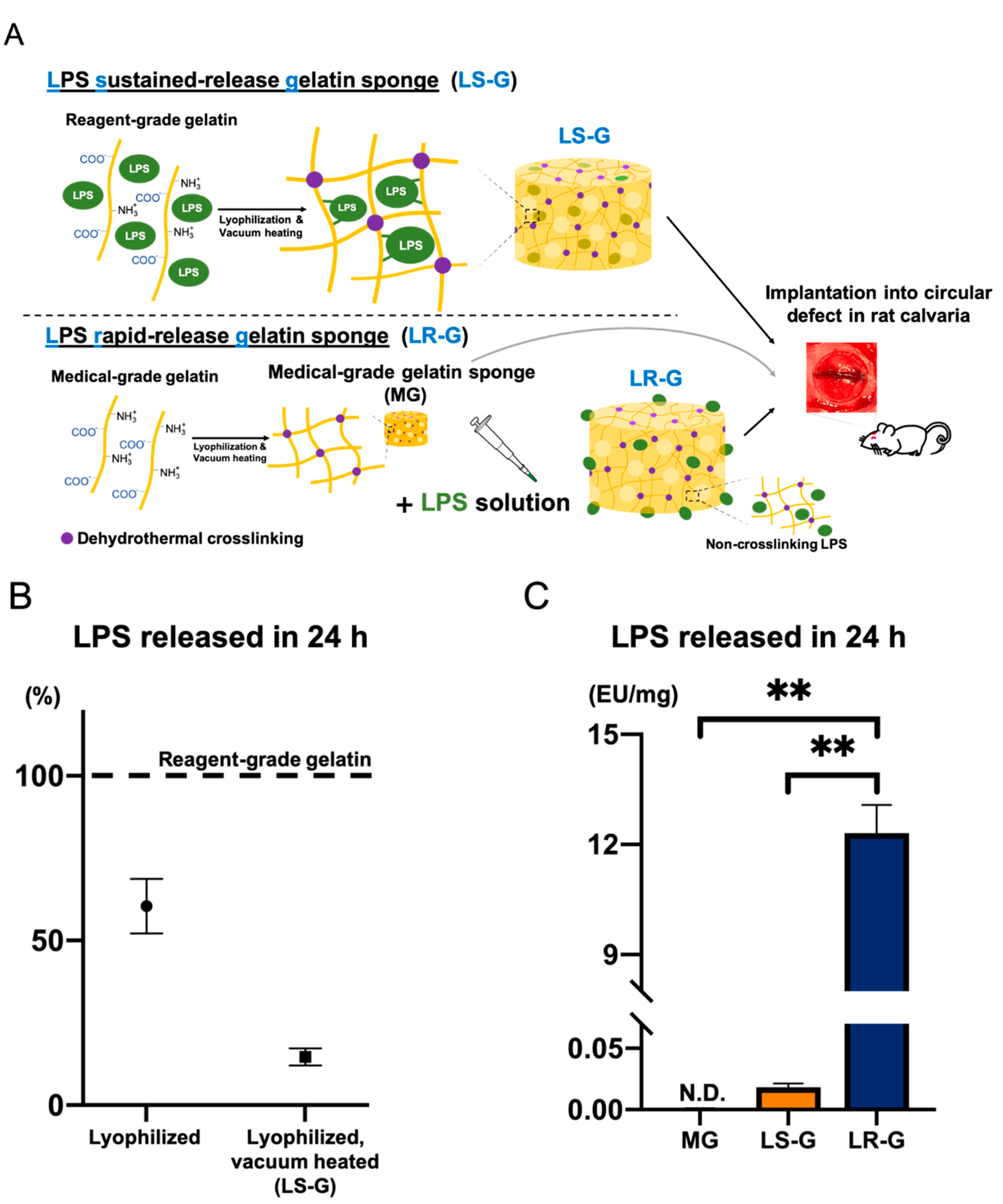
2.3. Preparation of Gelatin Sponges
2.3.1. Preparation of Medical-Grade Gelatin Sponge without LPS (MG)
2.3.2. Preparation of LPS Sustained-Release Gelatin Sponge (LS-G)
2.3.3. Preparation of LPS Rapid-Release Gelatin Sponge (LR-G)
2.4. Characterization of Sponges
2.5. LPS Release Experiments from Sponges
2.6. Implantation of Sponges
2.7. Hematoxylin-Eosin Staining
2.8. Lysate LPS-Level Test
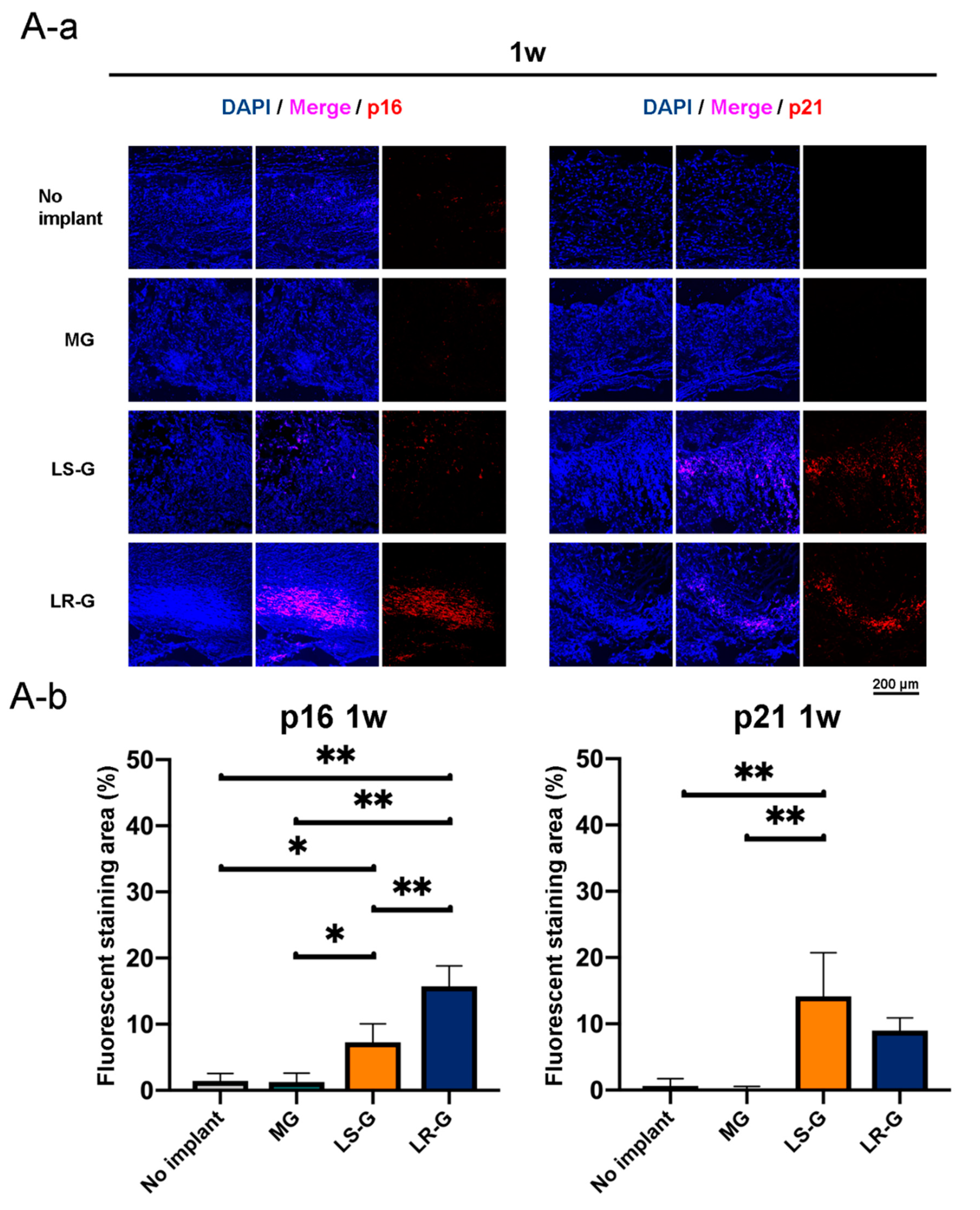
2.9. Immunohistochemistry Analysis
2.10. Bone Histomorphometric Analysis Utilizing Soft X-ray Imaging
2.11. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Sponges
3.2. Inflammatory Reactions of Defects
3.3. Senescent Cells in Defects
3.4. Bone Formation in Defects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arens, D.; Wilke, M.; Calabro, L.; Hackl, S.; Zeiter, S.; Zderic, I.; Richards, R.G.; Moriarty, T.F. A rabbit humerus model of plating and nailing osteosynthesis with and without staphylococcus aureus osteomyelitis. Eur. Cells Mater. 2015, 30, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Long-lived biomaterials. Nat. Biomed. Eng. 2017, 1, 0095. [CrossRef] [Green Version]
- Hosseini, B.; Byrd, W.C.; Preisser, J.S.; Khan, A.; Duggan, D.; Bencharit, S. Effects of antibiotics on bone and soft-tissue healing following immediate single-tooth implant placement into sites with apical pathology. J. Oral Implantol. 2015, 41, e202–e211. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef]
- Dixon, D.R.; Darveau, R.P. Lipopolysaccharide heterogeneity: Innate host responses to bacterial modification of lipid a structure. J. Dent. Res. 2005, 84, 584–595. [Google Scholar] [CrossRef]
- Reikerås, O.; Shegarfi, H.; Wang, J.E.; Utvåg, S.E. Lipopolysaccharide impairs fracture healing: An experimental study in rats. Acta Orthop. 2005, 76, 749–753. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Li, X.; Feng, J.; Du, J.; Guo, L.; Su, Y.; Zhou, J.; Ding, G.; Bai, Y.; et al. Aspirin inhibits LPS-induced macrophage activation via the NF-κB pathway. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Takami, M.; Kim, N.; Rho, J.; Choi, Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J. Immunol. 2002, 169, 1516–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.X.; Sun, X.X.; Li, W.; Xie, G.; Yang, Q.; Qu, Z.W.; Meng, Q.G. LPS at low concentration promotes the fracture healing through regulating the autophagy of osteoblasts via NF-κB signal pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1569–1579. [Google Scholar] [PubMed]
- Fridlyanskaya, I.; Alekseenko, L.; Nikolsky, N. Senescence as a general cellular response to stress: A mini-review. Exp. Gerontol. 2015, 72, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Sewerynek, E.; Ortiz, G.G.; Reiter, R.J.; Pablos, M.I.; Melchiorri, D.; Daniels, W.M.U. Lipopolysaccharide-induced DNA damage is greatly reduced in rats treated with the pineal hormone melatonin. Mol. Cell. Endocrinol. 1996, 117, 183–188. [Google Scholar] [CrossRef]
- Kim, C.O.; Huh, A.J.; Han, S.H.; Kim, J.M. Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch. Gerontol. Geriatr. 2012, 54, 35–41. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, K.; Cao, T.; Zhang, J.; Lian, M.; Huang, D.; Wei, C.; Gu, Z.; Feng, X. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology 2018, 70, 1023–1035. [Google Scholar] [CrossRef]
- Hoban, D.B.; Connaughton, E.; Connaughton, C.; Hogan, G.; Thornton, C.; Mulcahy, P.; Moloney, T.C.; Dowd, E. Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013, 27, 91–100. [Google Scholar] [CrossRef]
- Strålberg, F.; Kassem, A.; Kasprzykowski, F.; Abrahamson, M.; Grubb, A.; Lindholm, C.; Lerner, U.H. Inhibition of lipopolysaccharide-induced osteoclast formation and bone resorption in vitro and in vivo by cysteine proteinase inhibitors. J. Leukoc. Biol. 2017, 101, 1233–1243. [Google Scholar] [CrossRef]
- Unuma, K.; Aki, T.; Noritake, K.; Funakoshi, T.; Uemura, K. A CO-releasing molecule prevents annexin A2 down-regulation and associated disorders in LPS-administered rat lung. Biochem. Biophys. Res. Commun. 2017, 487, 748–754. [Google Scholar] [CrossRef]
- Abramova, A.Y.; Pertsov, S.S.; Kozlov, A.Y.; Nikenina, E.V.; Kalinichenko, L.S.; Dudnik, E.N.; Alekseeva, I.V. Cytokine levels in rat blood and brain structures after administration of lipopolysaccharide. Bull. Exp. Biol. Med. 2013, 155, 417–420. [Google Scholar] [CrossRef]
- Ayaz, G.; Halici, Z.; Albayrak, A.; Karakus, E.; Cadirci, E. Evaluation of 5-HT7 receptor trafficking on in vivo and in vitro model of lipopolysaccharide (LPS)-induced inflammatory cell injury in rats and LPS-treated A549 cells. Biochem. Genet. 2017, 55, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.; Braatz, R.D.; Hammond, P.T. Tunable staged release of therapeutics from layer-by-layer coatings with clay interlayer barrier. Biomaterials 2014, 35, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [Green Version]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomater. Appl. Nanomedicine, 1st ed.; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011; pp. 17–52. [Google Scholar]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [Green Version]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Kawamoto, T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch. Histol. Cytol. 2003, 66, 123–143. [Google Scholar] [CrossRef] [Green Version]
- Bingham, S.; Beswick, P.J.; Blum, D.E.; Gray, N.M.; Chessell, I.P. The role of the cylooxygenase pathway in nociception and pain. Semin. Cell Dev. Biol. 2006, 17, 544–554. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; VanSteeg, H.; Dollé, M.E.T.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell. 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Lu, Z.Y.; Zhang, X.; Liu, W.W.; Yao, G.D.; Liu, X.L.; Liu, W.; Wu, Q.J.; Hayashi, T.; Yamato, M.; et al. Gelatin promotes cell aggregation and pro-inflammatory cytokine production in PMA-stimulated U937 cells by augmenting endocytosis-autophagy pathway. Int. J. Biochem. Cell Biol. 2018, 95, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, Y.; Asaka, S.; Nakata, K. Endotoxin hepatotoxicity augmented by ethanol. Exp. Mol. Pathol. 1991, 55, 196–202. [Google Scholar] [CrossRef]
- Sidorczyk, Z.; Zahringer, U.; Rietschel, E.T. Chemical structure of the lipid A component of the lipopolysaccharide from a Proteus mirabilis Re-mutant. Eur. J. Biochem. 1983, 137, 15–22. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [Green Version]
- Bennett, R.R.; Pfeifer, C.R.; Irianto, J.; Xia, Y.; Discher, D.E.; Liu, A.J. Elastic-fluid model for DNA damage and mutation from nuclear fluid segregation due to cell migration. Biophys. J. 2017, 112, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, C.R.; Xia, Y.; Zhu, K.; Liu, D.; Irianto, J.; Morales García, V.M.; Santiago Millán, L.M.; Niese, B.; Harding, S.; Deviri, D.; et al. Constricted migration increases DNA damage and independently represses cell cycle. Mol. Biol. Cell 2018, 29, 1948–1962. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.C.; Meng, M.; Sun, Q.S.; Gao, S.M.; Sun, H. Linarin prevents LPSinduced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NFkappaB pathways. Int. J. Mol. Med. 2018, 42, 1460–1472. [Google Scholar]
- Miura, D.; Miura, Y.; Yagasaki, K. Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species-scavenging property. Clin. Exp. Metastasis 2004, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.A.; Crapo, J.D. Biology of disease: Free radicals and tissue injury. Lab. Investig. 1982, 47, 412–426. [Google Scholar] [PubMed]
- McCord, J.M. Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar] [PubMed]
- Tabibian, J.H.; O’Hara, S.P.; Splinter, P.L.; Trussoni, C.E.; LaRusso, N.F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014, 59, 2263–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croes, M.; Kruyt, M.C.; Loozen, L.; Kragten, A.H.M.; Yuan, H.; Dhert, W.J.; Öner, F.C.; Alblas, J. Local induction of inflammation affects bone formation. Eur. Cells Mater. 2017, 33, 211–226. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, L.; Wang, J.; Wang, F.; Yang, X.K.; Zhang, F.; Song, J.; Ma, X.; Cheng, Q.; Song, G. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in mc3t3-e1 cells. Inflammation 2014, 37, 621–631. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Honda, Y.; Tanaka, T.; Hashimoto, Y.; Matsumoto, N. Releasing Behavior of Lipopolysaccharide from Gelatin Modulates Inflammation, Cellular Senescence, and Bone Formation in Critical-Sized Bone Defects in Rat Calvaria. Materials 2020, 13, 95. https://doi.org/10.3390/ma13010095
Zhao J, Honda Y, Tanaka T, Hashimoto Y, Matsumoto N. Releasing Behavior of Lipopolysaccharide from Gelatin Modulates Inflammation, Cellular Senescence, and Bone Formation in Critical-Sized Bone Defects in Rat Calvaria. Materials. 2020; 13(1):95. https://doi.org/10.3390/ma13010095
Chicago/Turabian StyleZhao, Jianxin, Yoshitomo Honda, Tomonari Tanaka, Yoshiya Hashimoto, and Naoyuki Matsumoto. 2020. "Releasing Behavior of Lipopolysaccharide from Gelatin Modulates Inflammation, Cellular Senescence, and Bone Formation in Critical-Sized Bone Defects in Rat Calvaria" Materials 13, no. 1: 95. https://doi.org/10.3390/ma13010095
APA StyleZhao, J., Honda, Y., Tanaka, T., Hashimoto, Y., & Matsumoto, N. (2020). Releasing Behavior of Lipopolysaccharide from Gelatin Modulates Inflammation, Cellular Senescence, and Bone Formation in Critical-Sized Bone Defects in Rat Calvaria. Materials, 13(1), 95. https://doi.org/10.3390/ma13010095




