Efficacy of the Rotary Instrument XP-Endo Finisher in the Removal of Calcium Hydroxide Intracanal Medicament in Combination with Different Irrigation Techniques: A Microtomographic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Teeth Selection
2.2. Teeth Preparation
3. Standard Irrigation Protocol
4. Placement of Ca(OH)2
5. Removal of Ca(OH)2
5.1. Experimental Groups
5.1.1. Group A: Ca(OH)2 Removal Using the Master Apical File MAF
5.1.2. Group B: Ca(OH)2 Removal Using the XP File
5.2. Micro-CT Scanning Procedures and Evaluation Protocol
5.3. Outcome Assessment
6. Statistical Analysis
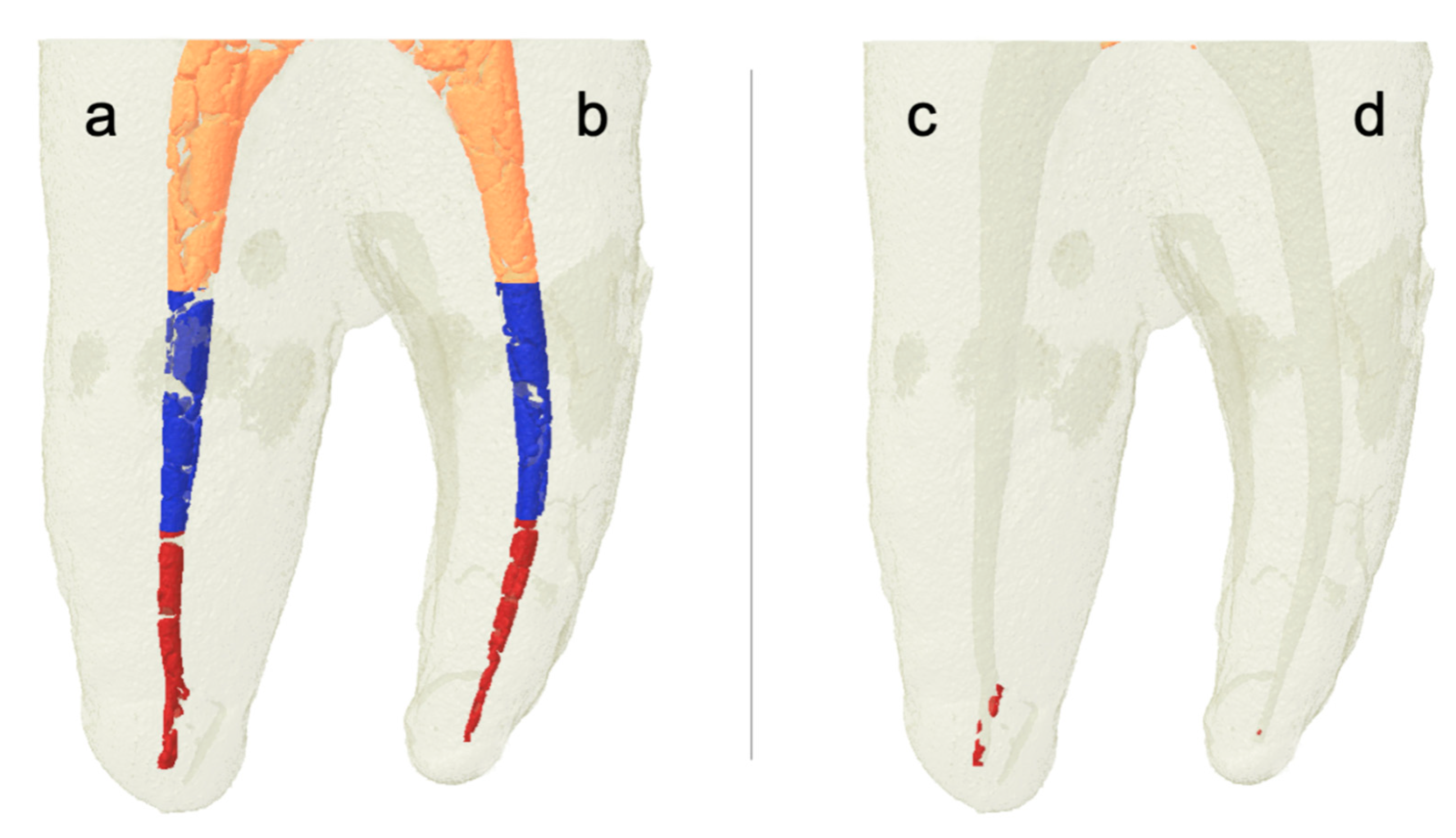
7. Results
7.1. Descriptive Analysis
7.2. Quantitative Analysis
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yucel, A.C.; Gurel, M.; Guler, E.; Karabucak, B. Comparison of final irrigation techniques in removal of calcium hydroxide. Aust. Endod. J. 2013, 39, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, A.; Sundqvist, G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Scand J Dent Res 1981, 89, 321–328. [Google Scholar] [CrossRef]
- Calt, S.; Serper, A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002, 28, 17–19. [Google Scholar] [CrossRef]
- Lambrianidis, T.; Margelos, J.; Beltes, P. Removal efficiency of calcium hydroxide dressing from the root canal. J. Endod. 1999, 25, 85–88. [Google Scholar] [CrossRef]
- Kuga, M.C.; Tanomaru-Filho, M.; Faria, G.; So, M.V.; Galletti, T.; Bavello, J.R. Calcium hydroxide intracanal dressing removal with different rotary instruments and irrigating solutions: A scanning electron microscopy study. Braz. Dent. J. 2010, 21, 310–314. [Google Scholar] [CrossRef]
- Hosoya, N.; Kurayama, H.; Iino, F.; Arai, T. Effects of calcium hydroxide on physical and sealing properties of canal sealers. Int. Endod. J. 2004, 37, 178–184. [Google Scholar] [CrossRef]
- Margelos, J.; Eliades, G.; Verdelis, C.; Palaghias, G. Interaction of calcium hydroxide with zinc oxide-eugenol type sealers: A potential clinical problem. J. Endod. 1997, 23, 43–48. [Google Scholar] [CrossRef]
- Schwartz, R.S. Adhesive dentistry and endodontics. Part 2: Bonding in the root canal system-the promise and the problems: A review. J. Endod. 2006, 32, 1125–1134. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.O. Influence of calcium hydroxide intracanal medication on apical seal. Int. Endod. J. 2002, 35, 623–628. [Google Scholar] [CrossRef]
- Kishen, A.; Peters, O.A.; Zehnder, M.; Diogenes, A.R.; Nair, M.K. Advances in endodontics: Potential applications in clinical practice. J. Conserv. Dent. JCD 2016, 19, 199. [Google Scholar] [CrossRef]
- Ricucci, D.; Langeland, K. Incomplete calcium hydroxide removal from the root canal: A case report. Int. Endod. J. 1997, 30, 418–421. [Google Scholar] [CrossRef]
- Schilder, H. Filling root canals in three dimensions. Dent Clin North Am 1967, 723–744. [Google Scholar] [CrossRef]
- Nandini, S.; Velmurugan, N.; Kandaswamy, D. Removal efficiency of calcium hydroxide intracanal medicament with two calcium chelators: Volumetric analysis using spiral CT, an in vitro study. J. Endod. 2006, 32, 1097–1101. [Google Scholar] [CrossRef]
- Kenee, D.M.; Allemang, J.D.; Johnson, J.D.; Hellstein, J.; Nichol, B.K. A quantitative assessment of efficacy of various calcium hydroxide removal techniques. J. Endod. 2006, 32, 563–565. [Google Scholar] [CrossRef]
- Aktemur Turker, S.; Uzunoglu, E.; Purali, N. Evaluation of dentinal tubule penetration depth and push-out bond strength of AH 26, BioRoot RCS, and MTA Plus root canal sealers in presence or absence of smear layer. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 294–298. [Google Scholar] [CrossRef]
- van der Sluis, L.W.; Wu, M.K.; Wesselink, P.R. The evaluation of removal of calcium hydroxide paste from an artificial standardized groove in the apical root canal using different irrigation methodologies. Int. Endod. J. 2007, 40, 52–57. [Google Scholar] [CrossRef]
- Salgado, R.J.; Moura-Netto, C.; Yamazaki, A.K.; Cardoso, L.N.; de Moura, A.A.; Prokopowitsch, I. Comparison of different irrigants on calcium hydroxide medication removal: Microscopic cleanliness evaluation. Oral Surg. Oral Medicine Oral Pathol. Oral Radiol. Endod. 2009, 107, 580–584. [Google Scholar] [CrossRef]
- Balvedi, R.P.; Versiani, M.A.; Manna, F.F.; Biffi, J.C. A comparison of two techniques for the removal of calcium hydroxide from root canals. Int. Endod. J. 2010, 43, 763–768. [Google Scholar] [CrossRef]
- Tasdemir, T.; Celik, D.; Er, K.; Yildirim, T.; Ceyhanli, K.T.; Yesilyurt, C. Efficacy of several techniques for the removal of calcium hydroxide medicament from root canals. Int. Endod. J. 2011, 44, 505–509. [Google Scholar] [CrossRef]
- Silva, L.J.; Pessoa, O.F.; Teixeira, M.B.; Gouveia, C.H.; Braga, R.R. Micro-CT evaluation of calcium hydroxide removal through passive ultrasonic irrigation associated with or without an additional instrument. Int. Endod. J. 2015, 48, 768–773. [Google Scholar] [CrossRef]
- Wiseman, A.; Cox, T.C.; Paranjpe, A.; Flake, N.M.; Cohenca, N.; Johnson, J.D. Efficacy of sonic and ultrasonic activation for removal of calcium hydroxide from mesial canals of mandibular molars: A microtomographic study. J. Endod. 2011, 37, 235–238. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.M.; Silveira, A.; Santos, E.; Prado, L.; Pessoa, O.F. Efficacy of sodium hypochlorite, ethylenediaminetetraacetic acid, citric acid and phosphoric acid in calcium hydroxide removal from the root canal: A microscopic cleanliness evaluation. Oral Surg. Oral Medicine Oral Pathol. Oral Radiol. Endod. 2011, 112, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Kvist, T.; Molander, A.; Dahlen, G.; Reit, C. Microbiological evaluation of one- and two-visit endodontic treatment of teeth with apical periodontitis: A randomized, clinical trial. J. Endod. 2004, 30, 572–576. [Google Scholar] [CrossRef]
- Kirar, D.S.; Jain, P.; Patni, P. Comparison of different irrigation and agitation methods for the removal of two types of calcium hydroxide medicaments from the root canal wall: An in-vitro study. Clujul Méd. 2017, 90, 327–332. [Google Scholar] [CrossRef]
- Kourti, E.; Pantelidou, O. Comparison of different agitation methods for the removal of calcium hydroxide from the root canal: Scanning electron microscopy study. J. Conserv. Dent. 2017, 20, 439–444. [Google Scholar] [CrossRef]
- Ahmad, M.; Pitt Ford, T.R.; Crum, L.A. Ultrasonic debridement of root canals: An insight into the mechanisms involved. J. Endod. 1987, 13, 93–101. [Google Scholar] [CrossRef]
- Camargo, C.H.; Leal, F.M.; Silva, G.O.; de Oliveira, T.R.; Madureira, P.G.; Camargo, S.E. Efficacy of different techniques for removal of calcium hydroxide-chlorhexidine paste from root canals. Gen. Dent. 2016, 64, e9–e12. [Google Scholar]
- Gu, Y.; Perinpanayagam, H.; Kum, D.J.; Yoo, Y.J.; Jeong, J.S.; Lim, S.M.; Chang, S.-W.; Baek, S.-H.; Zhu, Q.; Kum, K.-Y. Effect of Different Agitation Techniques on the Penetration of Irrigant and Sealer into Dentinal Tubules. Photomed. Laser Surg. 2017, 35, 71–77. [Google Scholar] [CrossRef]
- Adl, A.; Motamedifar, M.; Shams, M.S.; Mirzaie, A. Clinical investigation of the effect of calcium hydroxide intracanal dressing on bacterial lipopolysaccharide reduction from infected root canals. Aust. Endod. J. 2015, 41, 12–16. [Google Scholar] [CrossRef]
- Stamos, D.E.; Sadeghi, E.M.; Haasch, G.C.; Gerstein, H. An in vitro comparison study to quantitate the debridement ability of hand, sonic, and ultrasonic instrumentation. J. Endod. 1987, 13, 434–440. [Google Scholar] [CrossRef]
- Tamil, S.; Andamuthu, S.A.; Vaiyapuri, R.; Prasad, A.S.; Jambai, S.S.; Chittrarasu, M. A Comparative Evaluation of Intracanal Calcium Hydroxide Removal with Hand File, Rotary File, and Passive Ultrasonic Irrigation: An In Vitro Study. J. Pharm. Bioallied Sci. 2019, 11, S442–S445. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, B.; Alberton, C.S.; Tomazinho, F.S.F.; Gabardo, M.C.L.; Duarte, M.A.H.; Vivan, R.R.; Baratto-Filho, F. Effectiveness of five instruments when removing calcium hydroxide paste from simulated internal root resorption cavities in extracted maxillary central incisors. Int. Endod. J. 2020, 53, 366–375. [Google Scholar] [CrossRef]
- Wigler, R.; Dvir, R.; Weisman, A.; Matalon, S.; Kfir, A. Efficacy of XP-endo finisher files in the removal of calcium hydroxide paste from artificial standardized grooves in the apical third of oval root canals. Int. Endod. J. 2017, 50, 700–705. [Google Scholar] [CrossRef]
- Kfir, A.; Blau-Venezia, N.; Goldberger, T.; Abramovitz, I.; Wigler, R. Efficacy of self-adjusting file, XP-endo finisher and passive ultrasonic irrigation on the removal of calcium hydroxide paste from an artificial standardized groove. Aust. Endod. J. 2018, 44, 26–31. [Google Scholar] [CrossRef]




| Technique Used | Efficacy% |
|---|---|
| X3/SI | 92.3% ± 11.2% |
| X3/PUI | 95.7% ± 4.5% |
| XP/SI | 94.3% ± 5.4% |
| XP/PUI | 99.8% ± 0.002% |
| Technique Used | % of Remaining Ca(OH)2 | |||
|---|---|---|---|---|
| Whole Canal | Coronal Third | Middle Third | Apical Third | |
| X3/SI | 7.69% ± 11.25% | 0.02% ± 0.04% | 0% | 7.67% ± 11.26% |
| X3/PUI | 4.24% ± 4.51% | 0.008% ± 0.01% | 0% | 4.23% ± 4.51% |
| XP/SI | 5.65% ± 5.37% | 0% | 0.02 ± 0.04% | 5.63% ± 5.36% |
| XP/PUI | 0.18% ± 0.27% | 0% | 0.05 ± 0.11% | 0.12% ± 0.16% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denna, J.; Shafie, L.A.; Alsofi, L.; Al-Habib, M.; AlShwaimi, E. Efficacy of the Rotary Instrument XP-Endo Finisher in the Removal of Calcium Hydroxide Intracanal Medicament in Combination with Different Irrigation Techniques: A Microtomographic Study. Materials 2020, 13, 2222. https://doi.org/10.3390/ma13102222
Denna J, Shafie LA, Alsofi L, Al-Habib M, AlShwaimi E. Efficacy of the Rotary Instrument XP-Endo Finisher in the Removal of Calcium Hydroxide Intracanal Medicament in Combination with Different Irrigation Techniques: A Microtomographic Study. Materials. 2020; 13(10):2222. https://doi.org/10.3390/ma13102222
Chicago/Turabian StyleDenna, Jameela, Lubna A Shafie, Loai Alsofi, Mey Al-Habib, and Emad AlShwaimi. 2020. "Efficacy of the Rotary Instrument XP-Endo Finisher in the Removal of Calcium Hydroxide Intracanal Medicament in Combination with Different Irrigation Techniques: A Microtomographic Study" Materials 13, no. 10: 2222. https://doi.org/10.3390/ma13102222
APA StyleDenna, J., Shafie, L. A., Alsofi, L., Al-Habib, M., & AlShwaimi, E. (2020). Efficacy of the Rotary Instrument XP-Endo Finisher in the Removal of Calcium Hydroxide Intracanal Medicament in Combination with Different Irrigation Techniques: A Microtomographic Study. Materials, 13(10), 2222. https://doi.org/10.3390/ma13102222





