Highly Luminescent and Biocompatible P and N Co-Doped Passivated Carbon Nanodots for the Sensitive and Selective Determination of Rifampicin Using the Inner Filter Effect
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of CNDs
2.3. Instruments and Characterizations
2.4. Preparation of the Rifampicin Samples
2.5. Analytical Assay
2.6. Stability Experiments
2.7. Quantum Yield
2.8. Animals
2.9. Sub-Chronic Toxicity Assessment
2.10. Clinical Behaviour and Body Mass Analysis
2.11. Hematological and Blood Biochemistry Assay
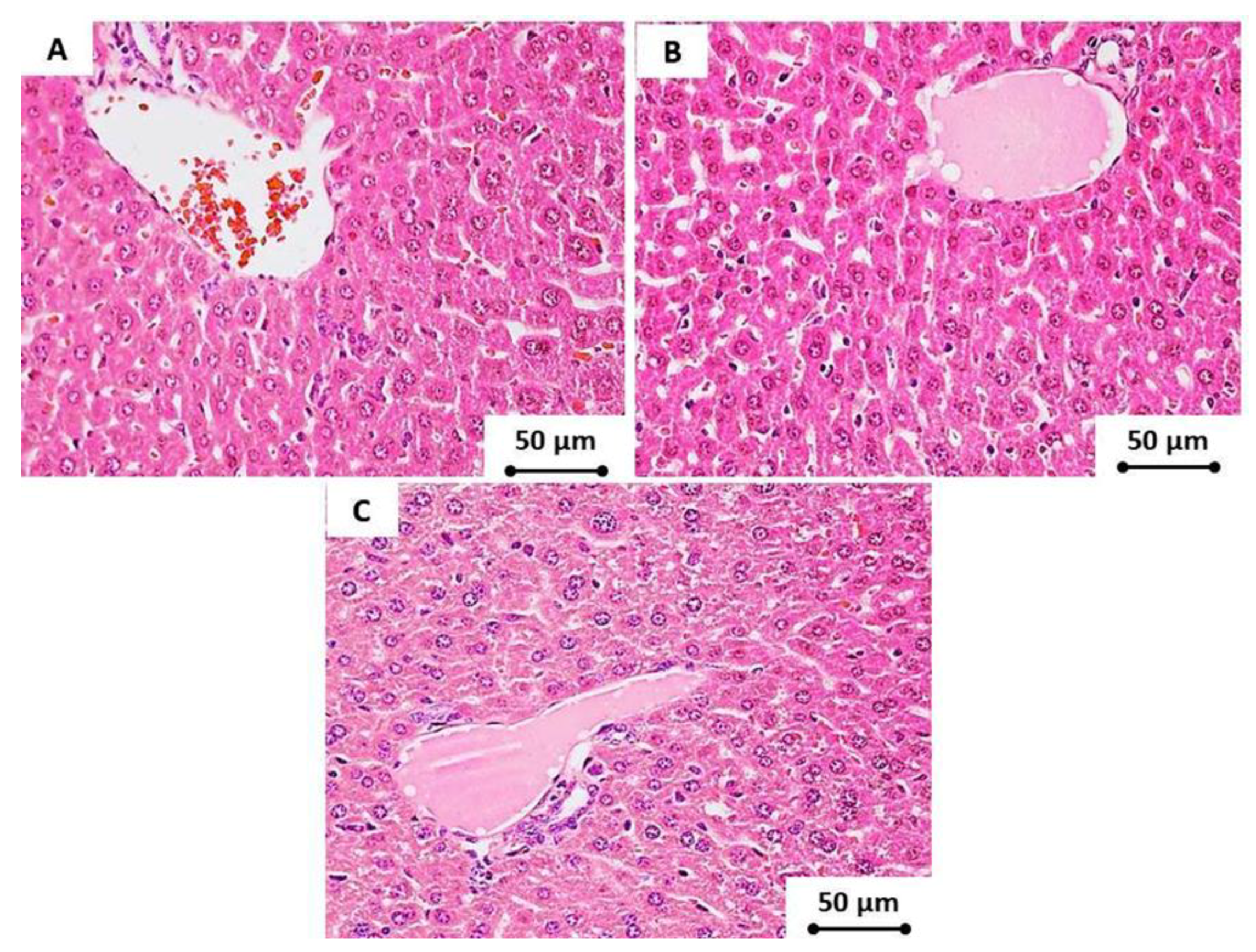
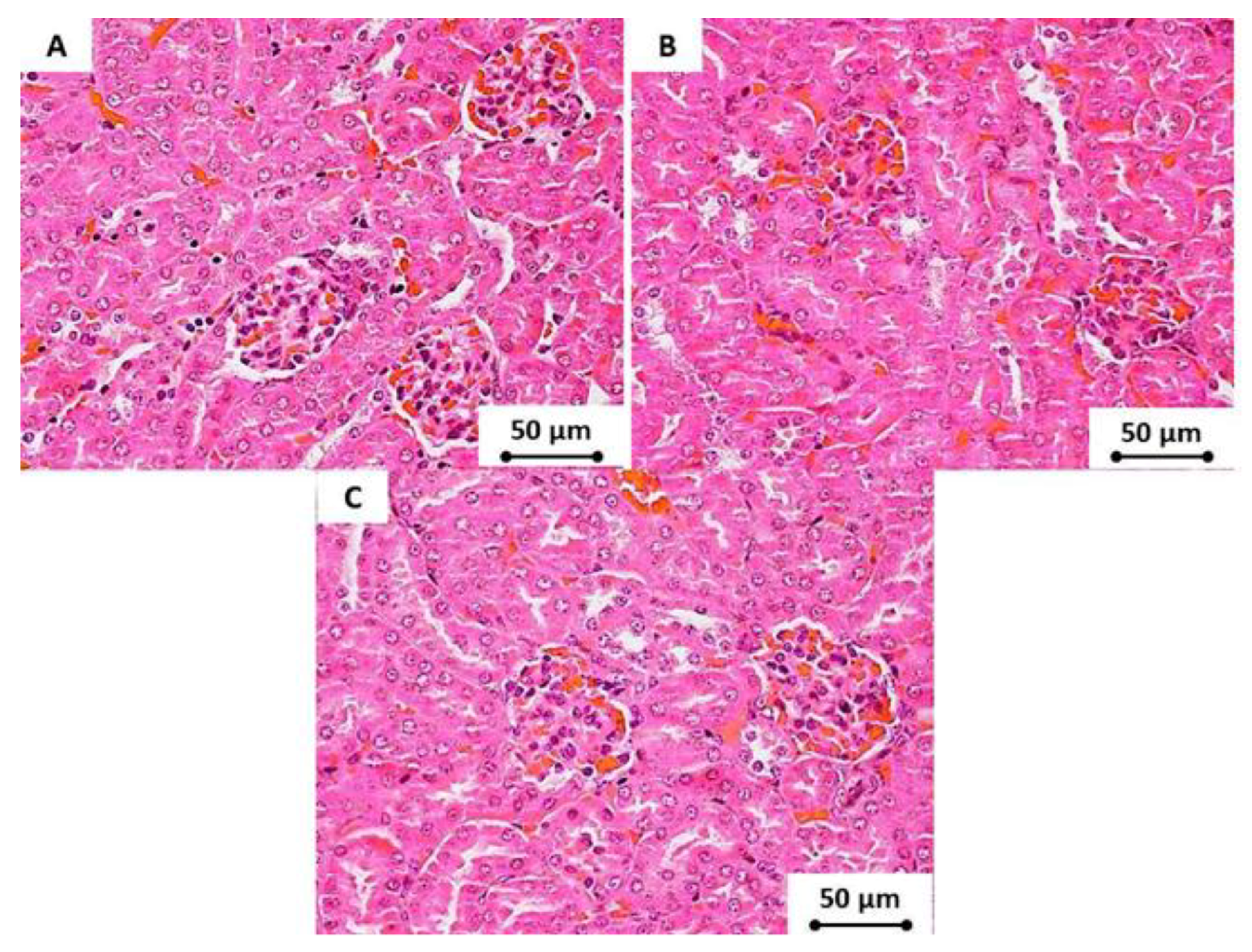
2.12. Histopathology
3. Results and Discussion
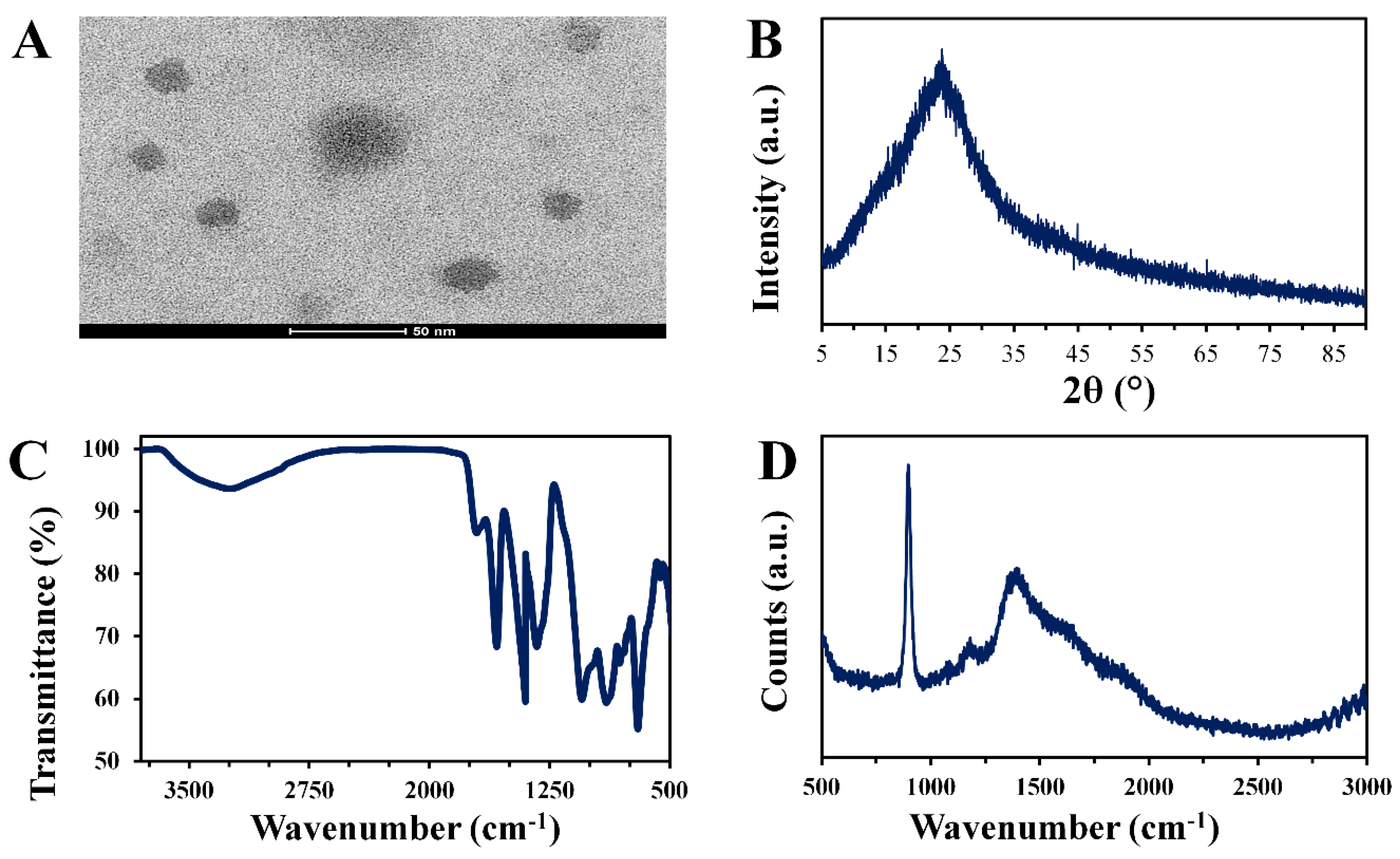
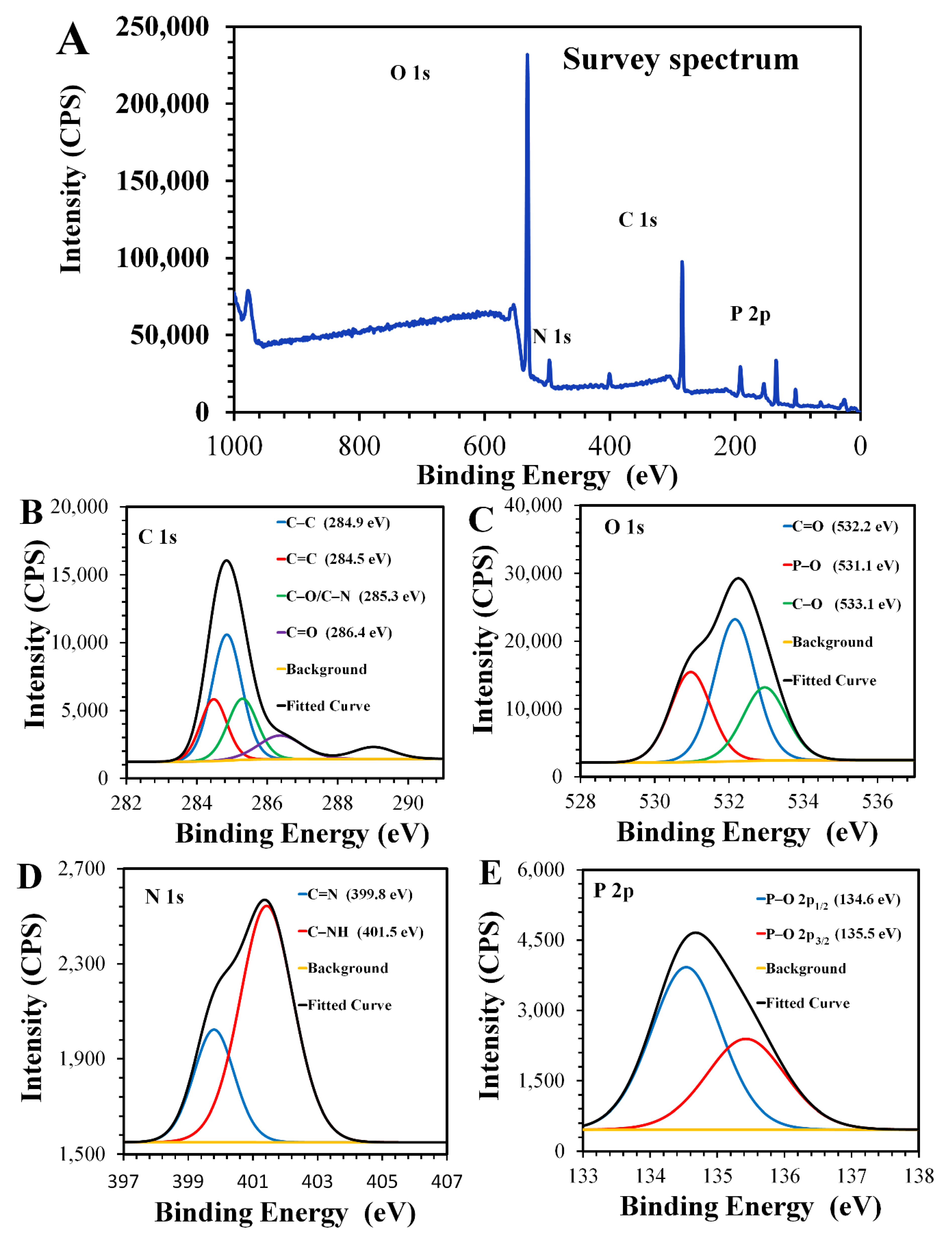
3.1. Morphology and Surface Composition
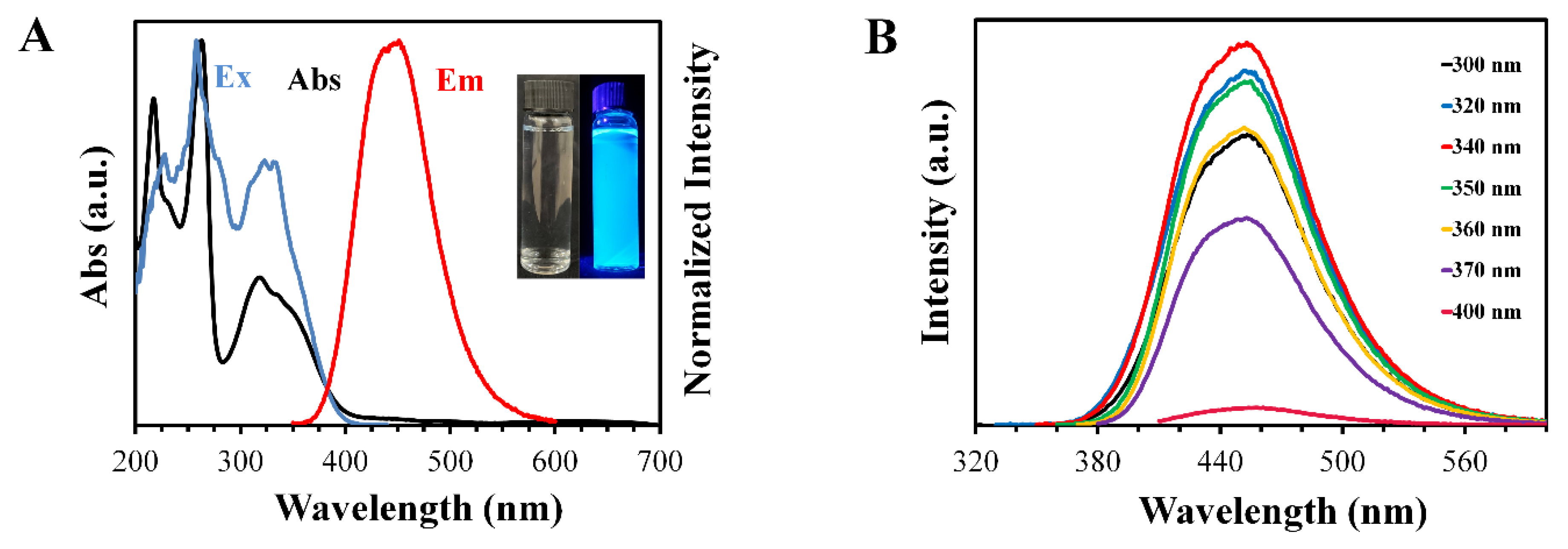
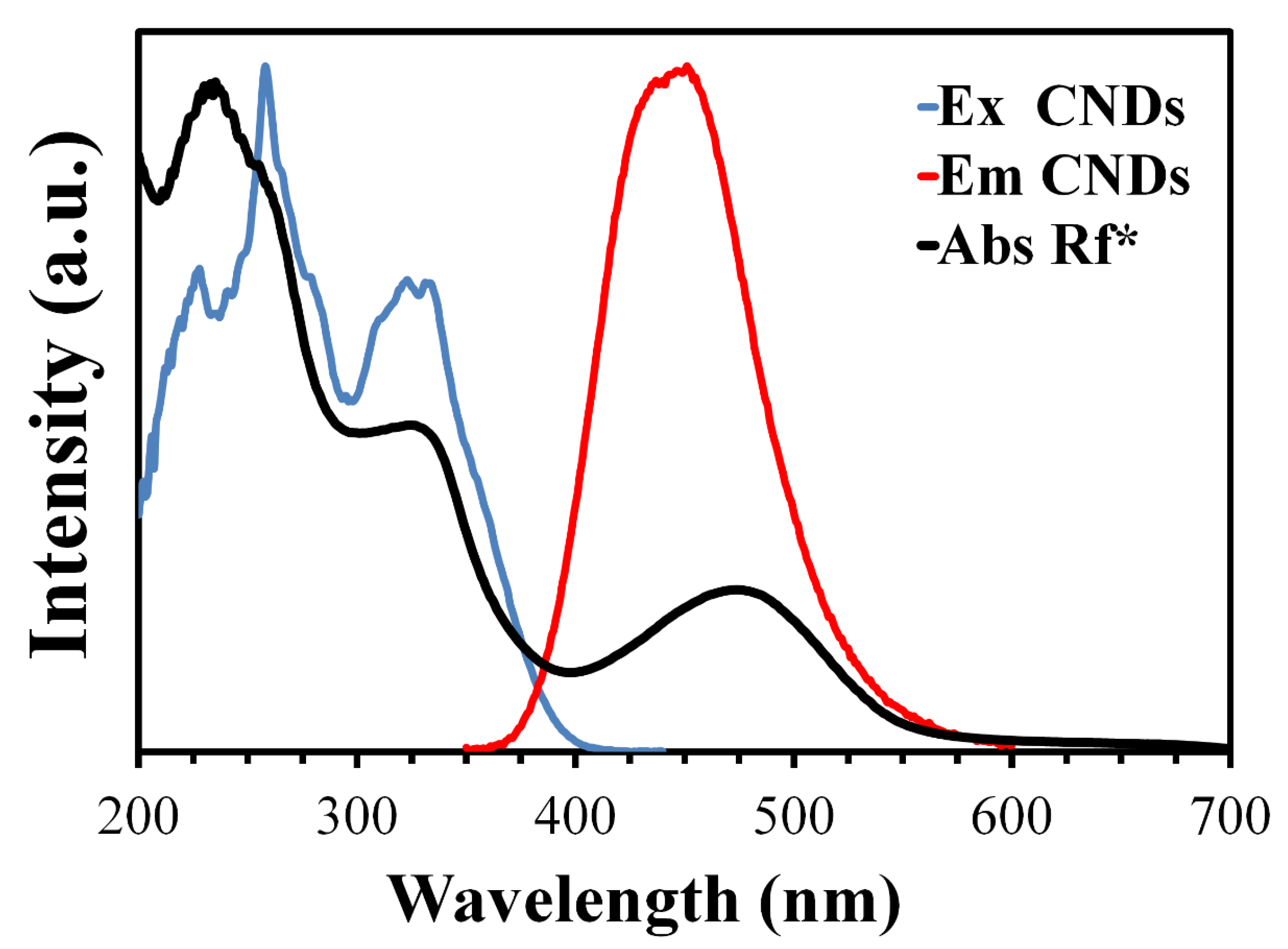
3.2. Optical Properties
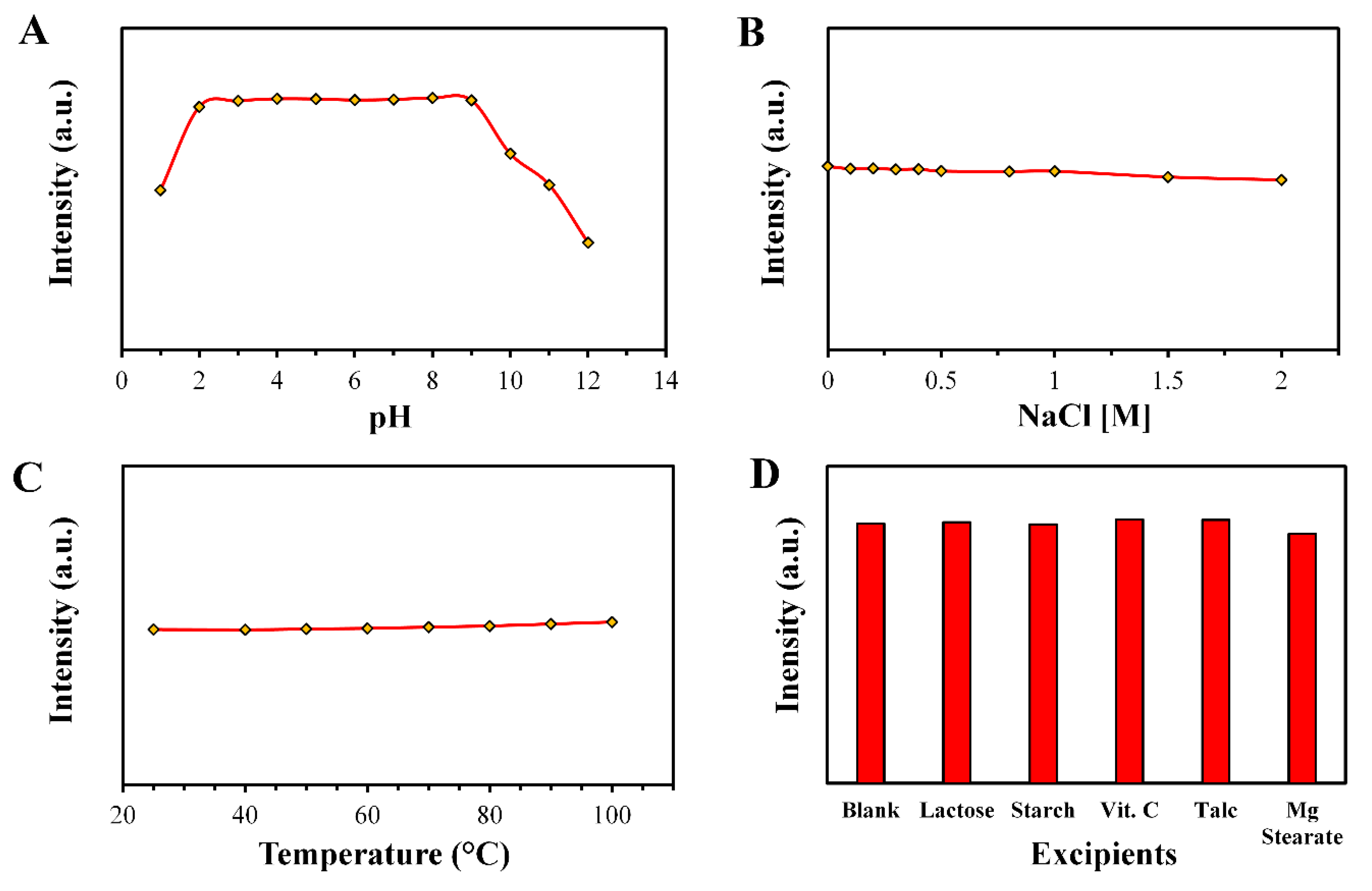
3.3. Stability of the CNDs
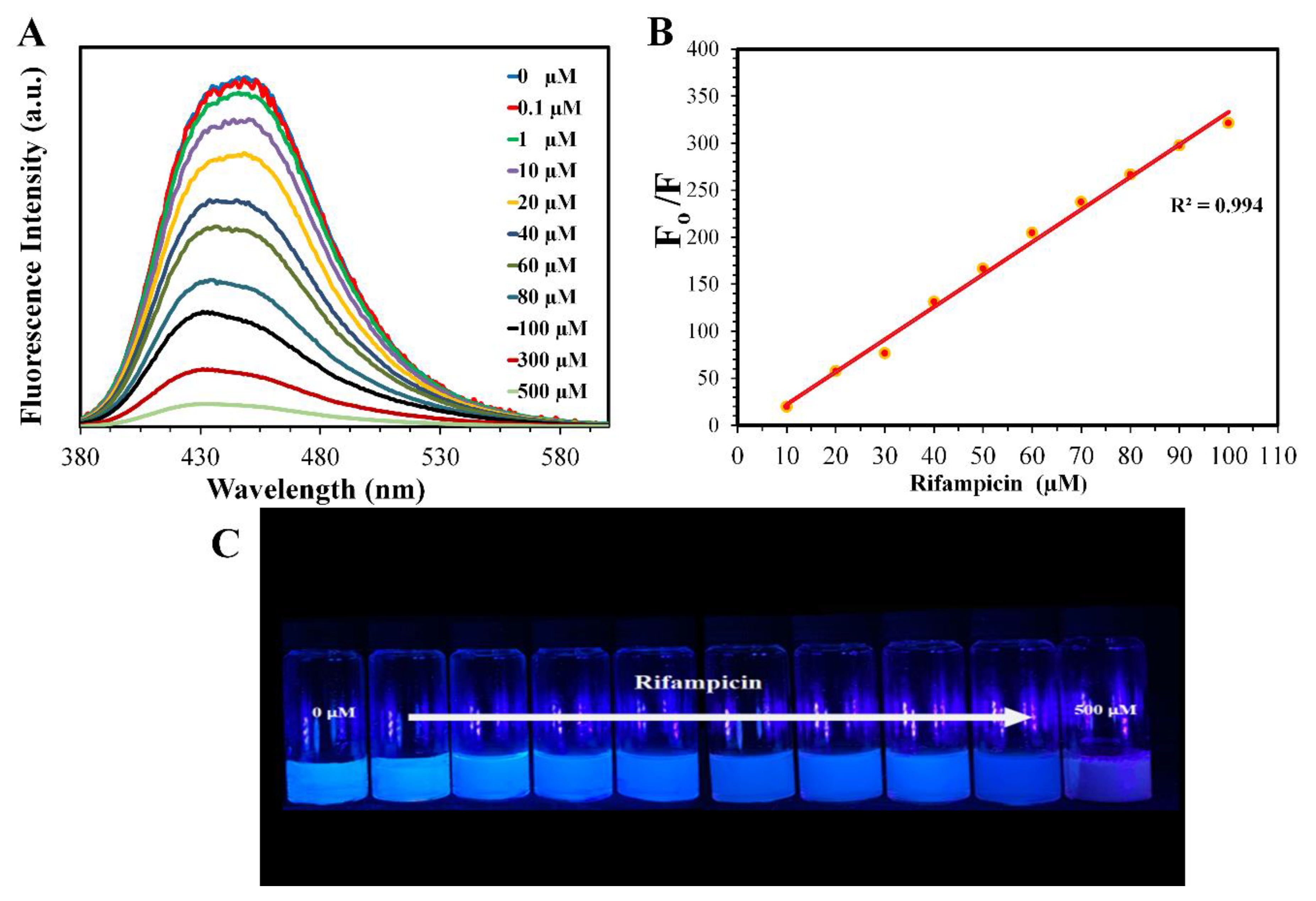
3.4. Selectivity of the CNDs
3.5. Mechanism of Quenching
3.6. Assay of Rifampicin Capsules
3.7. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akhgari, F.; Farhadi, K.; Samadi, N. Fluorescent Carbon Dot as Nanosensor for Sensitive and Selective Detection of Cefixime Based on Inner Filter Effect. J. Fluoresc. 2017, 27, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, K.; Guo, C. A fluorescent optode for sodium ion based on the inner filter effect. Anal. Chim. Acta 2000, 407, 45–52. [Google Scholar] [CrossRef]
- Yang, X.-F.; Liu, P.; Wang, L.; Zhao, M. A Chemosensing Ensemble for the Detection of Cysteine Based on the Inner Filter Effect Using a Rhodamine B Spirolactam. J. Fluoresc. 2007, 18, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Al-Lohedan, H.A. Spectroscopic and computational evaluation on the binding of safranal with human serum albumin: Role of inner filter effect in fluorescence spectral correction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 434–442. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, X.; Li, H.; Guan, F.; Guo, J.; Shen, F.; Luo, Y.; Sun, C.; Zhang, L. Sensitive fluorescent detection of melamine in raw milk based on the inner filter effect of Au nanoparticles on the fluorescence of CdTe quantum dots. Food Chem. 2012, 135, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, M.; Li, B. Homogeneous fluorescence-based immunoassay via inner filter effect of gold nanoparticles on fluorescence of CdTe quantum dots. Analyst 2012, 137, 3293. [Google Scholar] [CrossRef]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging in Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Smart Utilization of Carbon Dots in Semiconductor Photocatalysis. Adv. Mater. 2016, 28, 9454–9477. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Anilkumar, P.; Cao, L.; Yu, J.-J.; Tackett, K.N.; Wang, P.; Meziani, M.J.; Sun, Y.-P. Crosslinked carbon dots as ultra-bright fluorescence probes. Small 2013, 9, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, L.; Su, R.; Huang, R.; Qi, W.; He, Z.M. A carbon dot-based “off–on” fluorescent probe for highly selective and sensitive detection of phytic acid. Biosens. Bioelectron. 2015, 70, 232–238. [Google Scholar] [CrossRef]
- Omer, K.M.; Aziz, K.H.; Mohammed, S.J.; Mohammad, S. Improvement of selectivity via the surface modification of carbon nanodots towards the quantitative detection of mercury ions. New J. Chem. 2019, 43, 12979–12986. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury(II) ions. Sens. Actuators B Chem. 2013, 184, 156–162. [Google Scholar] [CrossRef]
- Grobbelaar, M.; Louw, G.E.; Sampson, S.L.; van Helden, P.D.; Donald, P.R.; Warren, R.M. Evolution of rifampicin treatment for tuberculosis. Infect. Genet. Evol. 2019, 74, 1–9. [Google Scholar]
- WHO. Global TB Report 2018, WHO Library Catalouging in Publication Data; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Campbell, E.; Korzheva, N.; Mustaev, A.; Murakami, K.S.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Goldstein, B.P. Resistance to rifampicin: A review. J. Antibiot. 2014, 67, 625–630. [Google Scholar] [CrossRef]
- Allanson, A.; Cotton, M.; Tettey, J.N.A.; Boyter, A. Determination of rifampicin in human plasma and blood spots by high performance liquid chromatography with UV detection: A potential method for therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2007, 44, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.C.; Josino, M.A.A.; Coelho, H.L.; Raffin, F.N. Development of a spectrophotometric analytical method for rifampicin, isoniazid and pyrazinamide assay and dissolution in combinated formulation. Indo Am. J. Pharm. Res. 2015, 5, 3716–3724. [Google Scholar]
- Jhamb, S.S.; Singh, R.P. Assay of rifampicin in pharmaceutical formulation: Comparison of spectrophotometric and microbiological methods. Int. J. Pharma. Bio Sci. 2010, 1, 294–302. [Google Scholar]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Cornell University. Price List|Institute of Biotechnology, Proteomics Services and Fees. Available online: http://www.biotech.cornell.edu/brc/proteomics/price-list (accessed on 17 April 2020).
- Fletcher, A.N. Quinine sulfate as a fluorescence quantum yield standard. Photochem. Photobiol. 1969, 9, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Zamberi, N.R.; Yeap, S.K.; Nordin, N.; Mohamad, N.E.; Romli, M.F.; Rasol, N.E.; Subramani, T.; Ismail, N.H.; Alitheen, N.B. Subchronic toxicity, immunoregulation and anti-breast tumor effect of Nordamnacantal, an anthraquinone extracted from the stems of Morinda citrifolia L. BMC Complement. Altern. Med. 2018, 18, 31. [Google Scholar] [CrossRef]
- Sciortino, A.; Cayuela, A.; Soriano, M.; Gelardi, F.; Cannas, M.; Valcárcel, M.; Messina, F. Different natures of surface electronic transitions of carbon nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 22670–22677. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.W.; Kim, M.-K.; Shin, D.Y.; Shin, D.H.; Kim, C.O.; Yang, S.B.; Park, J.H.; Hwang, E.; Choi, S.-H.; et al. Anomalous Behaviors of Visible Luminescence from Graphene Quantum Dots: Interplay between Size and Shape. ACS Nano 2012, 6, 8203–8208. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.-M.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Huang, H.; Liu, Y.; Li, H.; Ming, H.; Kang, Z. ZnO/carbon quantum dots nanocomposites: One-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature. New J. Chem. 2012, 36, 1031. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sahu, S.; Sinha, N.; Bhutia, S.K. Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 2015, 140, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Rong, M.; Lin, L.; Song, X.; Wang, Y.; Zhong, Y.; Yan, J.; Feng, Y.; Zeng, X.; Chen, X. Fluorescence sensing of chromium (VI) and ascorbic acid using graphitic carbon nitride nanosheets as a fluorescent “switch”. Biosens. Bioelectron. 2015, 68, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pastor, N.; Oehlerich, M.; Astilleros, J.M.; Kaliwoda, M.; Mayr, C.; Fernández-Díaz, L.; Schmahl, W.W. Crystallization of ikaite and its pseudomorphic transformation into calcite: Raman spectroscopy evidence. Geochim. Cosmochim. Acta 2016, 175, 271–281. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.-L.; Tian, Z.-Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D.-W. Electrochemical tuning of luminescent carbon nanodots: From preparation to luminescence mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef]
- Liu, D.; Qu, F.; Zhao, X.; You, J. Generalized One-Pot Strategy Enabling Different Surface Functionalizations of Carbon Nanodots to Produce Dual Emissions in Alcohol–Water Binary Systems. J. Phys. Chem. C 2015, 119, 17979–17987. [Google Scholar] [CrossRef]
- Ding, H.; Du, F.; Liu, P.; Chen, Z.; Shen, J. DNA–Carbon Dots Function as Fluorescent Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 6889–6897. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.-Z.; Yin, X.-B.; Hea, X.; Zhangad, Y. Nitrogen-Doped Carbon Dots: A Facile and General Preparation Method, Photoluminescence Investigation, and Imaging Applications. Chem. A Eur. J. 2013, 19, 2276–2283. [Google Scholar] [CrossRef]
- Hart, J.N.; May, P.W.; Allan, N.L.; Hallam, K.R.; Claeyssens, F.; Fuge, G.M.; Ruda, M.; Heard, P.J. Towards new binary compounds: Synthesis of amorphous phosphorus carbide by pulsed laser deposition. J. Solid State Chem. 2013, 198, 466–474. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, C.; Yan, X.; Wu, M. Observation of pH-, solvent-, spin-, and excitation-dependent blue photoluminescence from carbon nanoparticles. Chem. Commun. 2010, 46, 3681–3683. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Wang, T.; Wang, A.; Wang, R.; Liu, Z.; Sun, Y.; Shan, G.; Chen, Y.; Liu, Y. Carbon dots with molecular fluorescence and their application as a "turn-off" fluorescent probe for ferricyanide detection. Sci. Rep. 2019, 9, 10723–10729. [Google Scholar] [CrossRef] [PubMed]
- Omer, L.J.; Mohammed, K.M. Dual functional highly luminescence B, N Co-doped carbon nanodots as nanothermometer and Fe3+/Fe2+ sensor. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Aziz, K.H.; Omer, K.M.; Hamarawf, R.F. Lowering the detection limit towards nanomolar mercury ion detection via surface modification of N-doped carbon quantum dots. New J. Chem. 2019. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.; Wang, Y. Highly Green Emissive Nitrogen-Doped Carbon Dots with Excellent Thermal Stability for Bioimaging and Solid-State LED. Inorg. Chem. 2018, 57, 15229–15239. [Google Scholar] [CrossRef]
- Tang, D.-D.; Zhang, J.; Hou, X.; Wu, P. Phosphorescent Inner Filter Effect-based Sensing System for Determination of β -glucuronidase Using Manganese-doped Zinc Sulfide Quantum Dots. Chin. J. Anal. Chem. 2017, 45, 1909–1914. [Google Scholar] [CrossRef]
- Pawlak, K.; Skrzypczak, A.; Bialek-Bylka, E.G. Inner Filter Effect in the Fluorescence Emission Spectra of Room Temperature Ionic Liquids with-β-Carotene. In Applications of Ionic Liquids in Science and Technology; IntechOpen Limited: Londong, UK, 2011. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Schibelbain, A.F.; Neiva, E.; Zarbin, A.J.; Marcolino-Junior, L.H.; Bergamini, M.F. Nickel hexacyanoferrate supported at nickel nanoparticles for voltammetric determination of rifampicin. Sens. Actuators B Chem. 2018, 260, 816–823. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Zhang, W.; Gao, K.; He, Z. HPLC determination of rifampicin and related compounds in pharmaceuticals using monolithic column. J. Pharm. Biomed. Anal. 2008, 46, 405–409. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zhao, L.; Lin, J.-M. Determination of folic acid by chemiluminescence based on peroxomonosulfate-cobalt(II) system. Talanta 2008, 74, 1154–1159. [Google Scholar] [CrossRef]
- Han, Z.; Long, Y.; Pan, S.; Liu, H.; Yang, J.; Hu, X. Efficient one-pot synthesis of carbon dots as a fluorescent probe for the selective and sensitive detection of rifampicin based on the inner filter effect. Anal. Methods 2018, 10, 4085–4093. [Google Scholar] [CrossRef]
- Towers, M. British Pharmacopoeia 2009; The Stationery Office: London, UK, 2008. [Google Scholar] [CrossRef]

| Material | Method | LOD (μM) | Linearity Range (μM) | Reference |
|---|---|---|---|---|
| Nickel nanoparticles | Voltammetric | 2.6 | 5–500 | [51] |
| C18 monolithic column | HPLC | 0.2 | 5–200 | [52] |
| Peroxomonosulfate-Co (II) | Chemiluminescence | 0.008 | 0.06–1.21 | [53] |
| Carbon nanodots | Fluorometry | 0.15 | 0.52–59.5 | [54] |
| Carbon nanodots | Fluorometry | 0.06 | 1–100 | This work |
| Sample No. | Actual Amount (mg) | Measured Amount (mg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 298.39 | 302.4 | 100.80 | 1.03 |
| 2 | 297.98 | 302.2 | 100.73 | 0.98 |
| 3 | 297.88 | 298.8 | 99.60 | 0.18 |
| 4 | 298.26 | 304.4 | 101.47 | 1.50 |
| 5 | 298.38 | 300.2 | 100.07 | 0.51 |
| Animal Group | Day 0 | Day 14 | Day 28 |
|---|---|---|---|
| Control | 20.15 ± 1.3 | 25.0 ± 0.8 | 29.8 ± 1.0 |
| Low dose | 20.05 ± 1.2 | 24.15 ± 0.45 | 27.9 ± 1.5 |
| High dose | 20.3 ± 1.5 | 25.45 ± 0.75 | 28.66 ± 0.8 |
| Animal Group | ALP (U/L) | ALT (U/L) | AST (U/L) | Urea (mmol/L) | Creatinine (µmol/L) |
|---|---|---|---|---|---|
| Control | 110.5 ± 0.3 | 60.5 ± 1.0 | 145.5 ± 1.3 | 7.9.0 ± 0.5 | 30.5 ± 0.05 |
| Low dose | 111.75 ± 0.5 | 61.63 ± 1.5 | 144.2 ± 0.75 | 8.2 ± 1.2 | 30.1 ± 1.7 |
| High dose | 112.5 ± 0.65 | 61.07 ± 1.2 | 145.8 ± 0.7 | 8.0 ± 1.6 | 30.5 ± 1.9 |
| Animal Group | Total RBC (× 1012/L) | Hb (g/L) | PCV (L/L) | PLT (× 105/L) | Total WBC (× 103/µL) |
|---|---|---|---|---|---|
| Control | 8.3 ± 0.2 | 139 ± 1.3 | 0.39 ± 0.25 | 6.6 ± 0.85 | 2.6 ± 0.1 |
| Low dose | 8.4 ± 1.5 | 141 ± 1.7 | 0.41 ± 0.45 | 6.4 ± 0.33 | 2.55 ± 0.55 |
| High dose | 8.2 ± 0.75 | 142 ± 1.45 | 0.40 ± 0.3 | 6.3 ± 0.11 | 2.45 ± 0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hashimi, B.; Rahman, H.S.; Omer, K.M. Highly Luminescent and Biocompatible P and N Co-Doped Passivated Carbon Nanodots for the Sensitive and Selective Determination of Rifampicin Using the Inner Filter Effect. Materials 2020, 13, 2275. https://doi.org/10.3390/ma13102275
Al-Hashimi B, Rahman HS, Omer KM. Highly Luminescent and Biocompatible P and N Co-Doped Passivated Carbon Nanodots for the Sensitive and Selective Determination of Rifampicin Using the Inner Filter Effect. Materials. 2020; 13(10):2275. https://doi.org/10.3390/ma13102275
Chicago/Turabian StyleAl-Hashimi, Baraa, Heshu Sulaiman Rahman, and Khalid Mohammad Omer. 2020. "Highly Luminescent and Biocompatible P and N Co-Doped Passivated Carbon Nanodots for the Sensitive and Selective Determination of Rifampicin Using the Inner Filter Effect" Materials 13, no. 10: 2275. https://doi.org/10.3390/ma13102275
APA StyleAl-Hashimi, B., Rahman, H. S., & Omer, K. M. (2020). Highly Luminescent and Biocompatible P and N Co-Doped Passivated Carbon Nanodots for the Sensitive and Selective Determination of Rifampicin Using the Inner Filter Effect. Materials, 13(10), 2275. https://doi.org/10.3390/ma13102275






