A Novel Optimization Model and Application of Optimal Formula Design for CuxCo1−xFe2O4 Spinel-Based Coating Slurry in Relation to Near and Middle Infrared Radiation Strengthening
Abstract
:1. Introduction
2. Experimental
3. Results
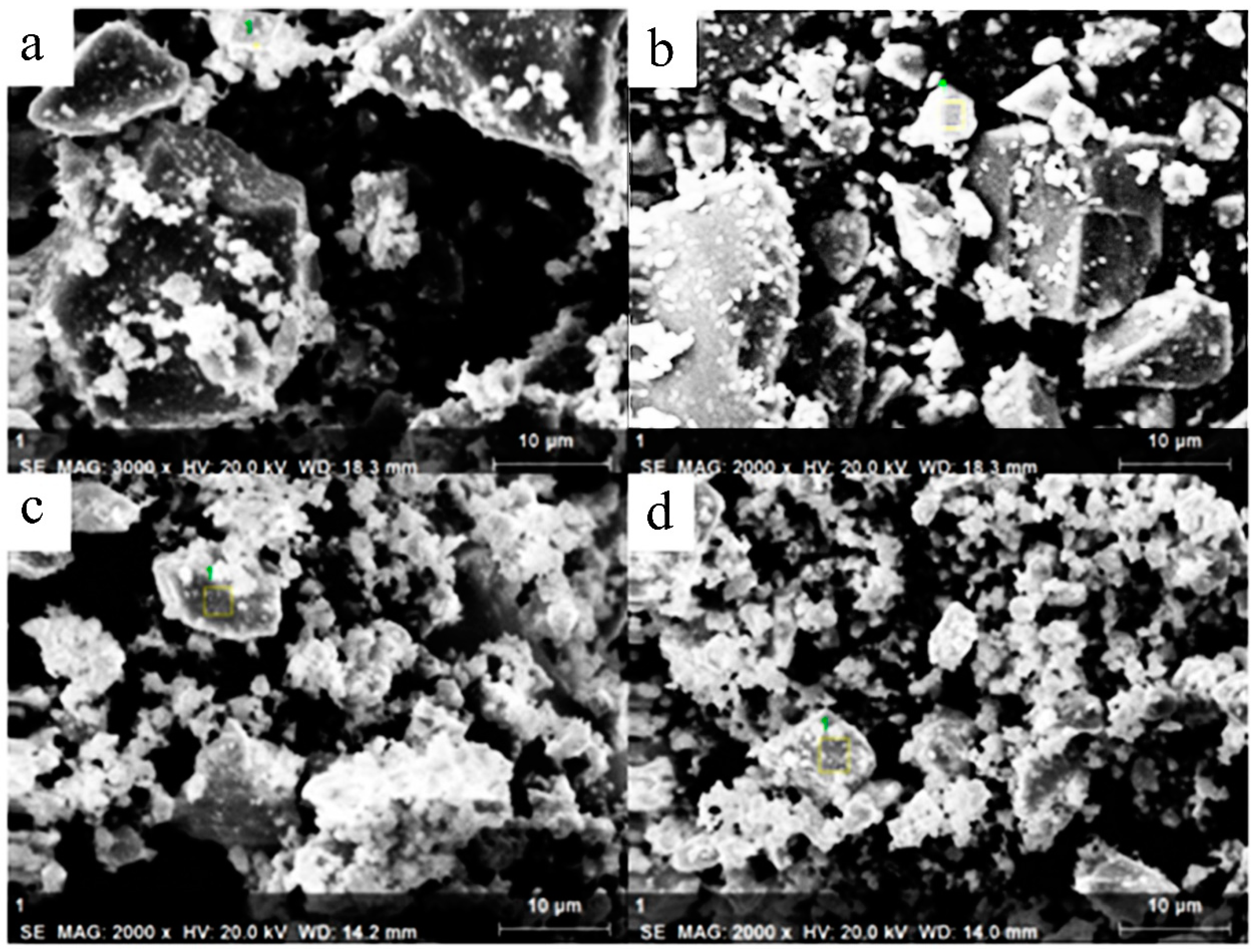
3.1. Morphology of Samples
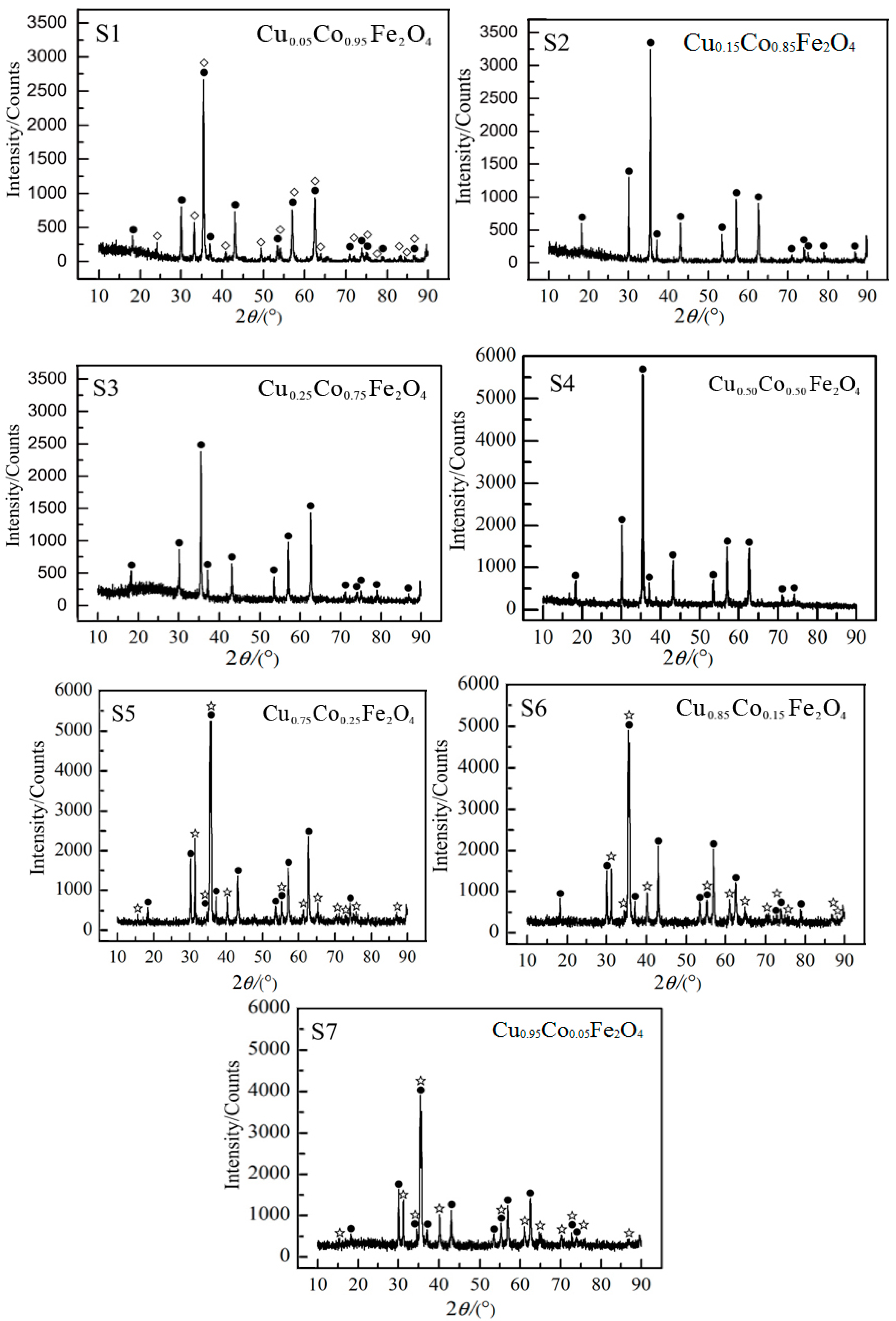
3.2. XRD Analysis
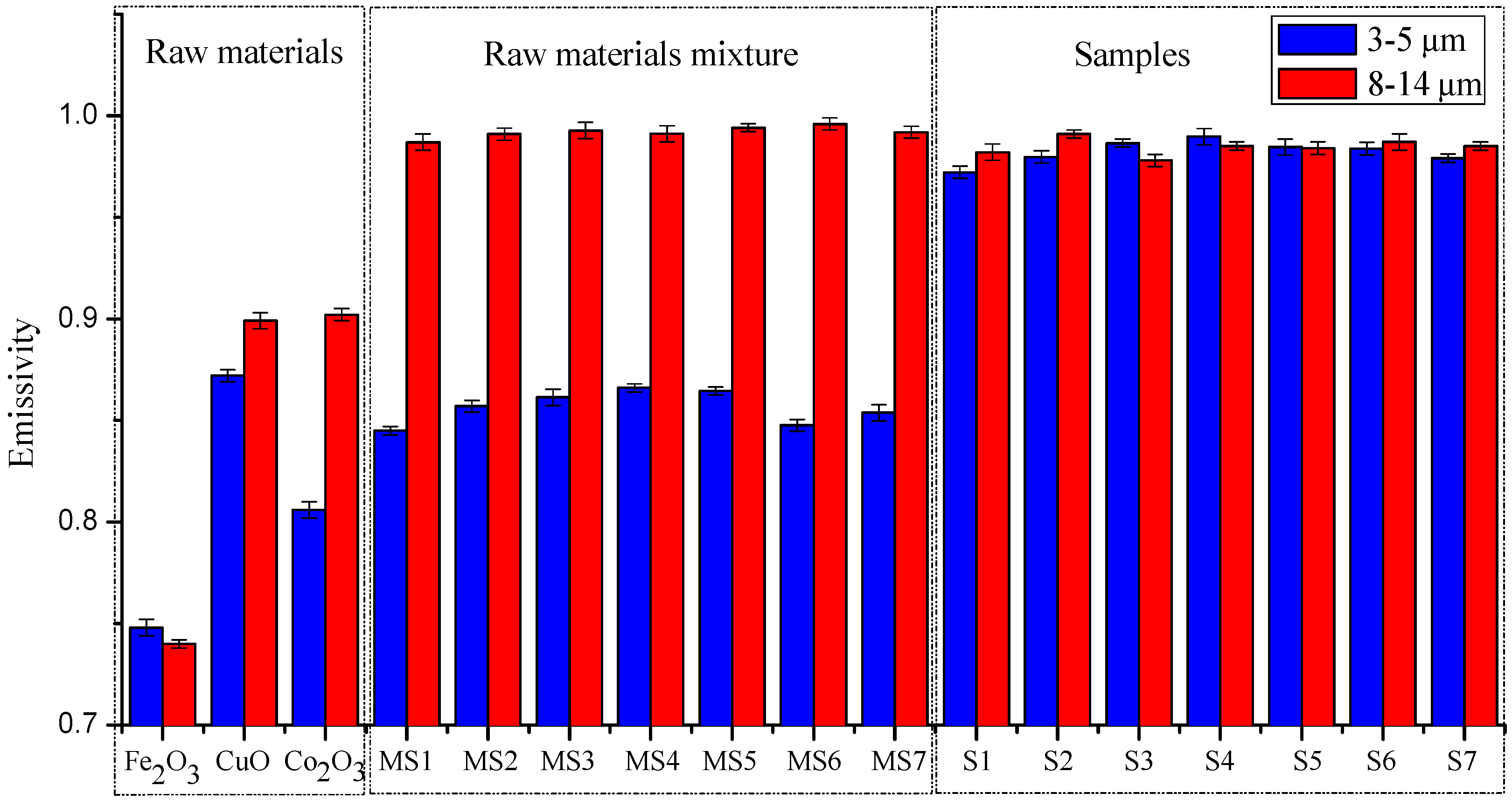
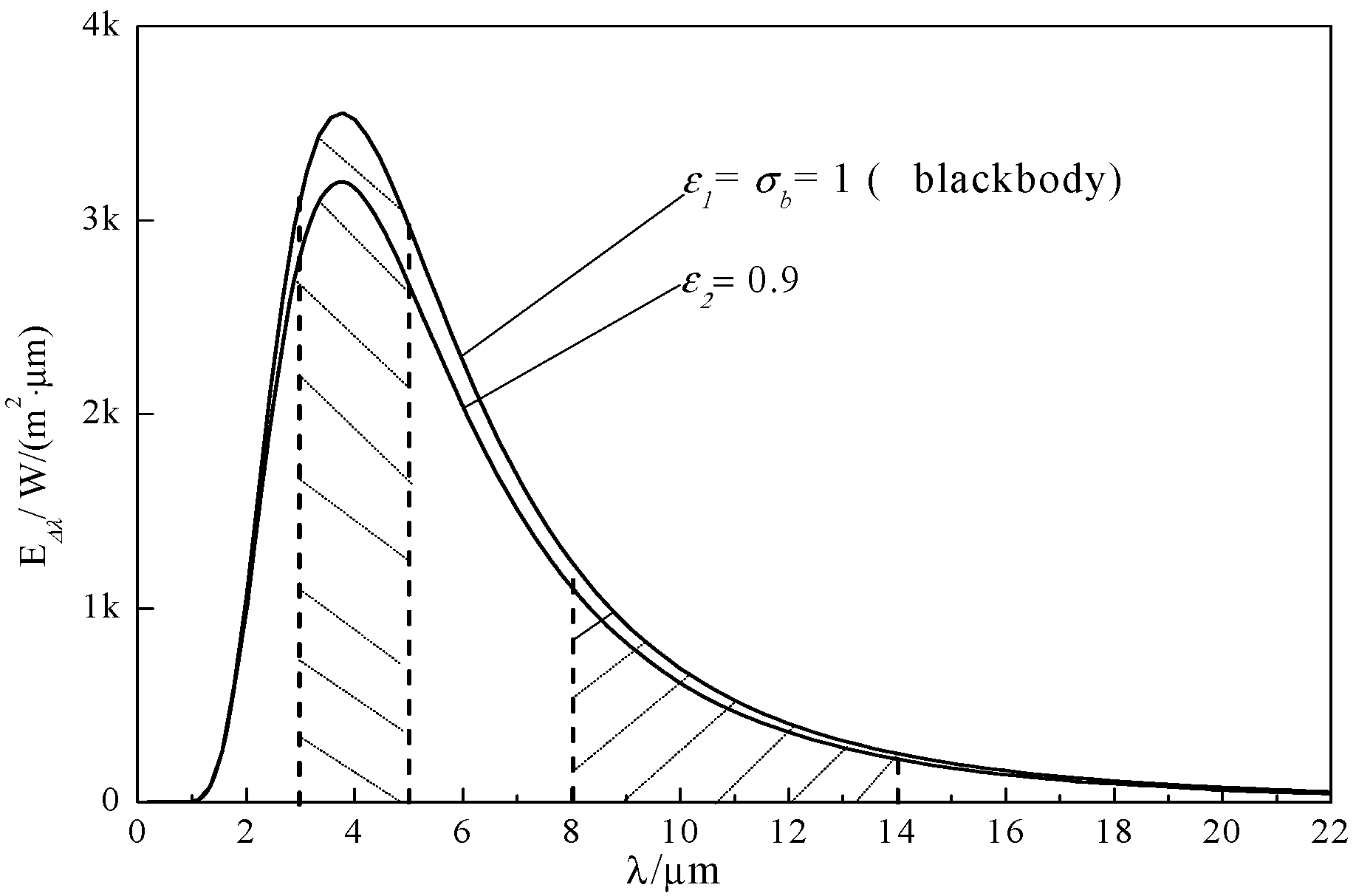
3.3. Strengthening Effect of Emissivity
4. Optimization Analysis for Formula Design of Coating Slurry
4.1. Optimization Model
4.2. Optimization of CuxCo1−xFe2O4 Material
4.3. Optimization of Coating Slurry
5. Conclusions
- (1)
- The optimal formula for the series infrared radiation material was CuO 16.98%, Co2O3 16.73% and Fe2O3 66.29%. At 773 K, the experimental emissivities of the formula were 0.986 and 0.977 in the 3–5 μm and 8–14 μm waveband, respectively.
- (2)
- Based on the series material, a spinel high emissivity coating was prepared and its performance analyzed. In addition, the optimized formula for high emissivity was obtained using the model with Matlab software. The optimal formula obtained was as follows: binder 30%; sodium hexametaphosphate 14.16%; bentonite 20% and water glass 35.84%. At 773 K, the emissivities of the formula were calculated as 0.931 and 0.905 in the 3–5 μm waveband and 8–14 μm waveband, respectively, which are close to the corresponding measured values.
- (3)
- In this study, the emissivity of the semiconductor with a spinel structure, such as the solid solution, in the near and middle infrared wavebands was a key consideration, and this was applied to the optimized process using the model, and as such is confirmed in the experiments.
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Q.; Li, X.; Li, F.; Zhou, D. The potential for energy saving and carbon emission reduction in China’s regional industrial sectors. Sci. Total Environ. 2020, 716. [Google Scholar] [CrossRef] [PubMed]
- Razdolski, I.; Chen, Y.; Giles, A.J.; Gewinner, S.; Schöllkopf, W.; Hong, M.; Wolf, M.; Giannini, V.; Caldwell, J.D.; Maier, S.A.; et al. Resonant Enhancement of Second-Harmonic Generation in the Mid-Infrared Using Localized Surface Phonon Polaritons in Subdiffractional Nanostructures. Nano Lett. 2016, 16, 6954–6959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Zhang, B.; Chong, R.; Li, R.; Yuan, B.; Lu, S.-M.; Li, C. Selective oxidation of sulfides on Pt/BiVO 4 photocatalyst under visible light irradiation using water as the oxygen source and dioxygen as the electron acceptor. J. Catal. 2015, 332, 95–100. [Google Scholar] [CrossRef]
- Zhu, X.; Ruiz, R.; Li, S.; Li, X. An effective heuristic for project scheduling with resource availability cost. Eur. J. Oper. Res. 2017, 257, 746–762. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, D. Relationship between infrared radiation and crystal structure in Fe-Mn-Co-Cu-O spinels. Acta Met. Sin. English Lett. 2008, 21, 15–20. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Mao, J.; Li, Q.; Zeng, X. Effects of rare-earth oxide doping on the thermal radiation performance of HfO2 coating. Ceram. Int. 2019, 45, 13004–13010. [Google Scholar] [CrossRef]
- Mao, J.; Ding, S.; Li, Y.; Li, S.; Liu, F.; Zeng, X.; Cheng, X. Preparation and investigation of MoSi2/SiC coating with high infrared emissivity at high temperature. Surf. Coat. Tech. 2019, 358, 873–878. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liu, P.A.; Zeng, F.C.; Liao, Y.F. Preparation and Properties of High Thermal Shock-Resistant Infrared Radiant Energy-Saving Coatings. Jscut 2014, 42, 46–50. [Google Scholar]
- Debnath, S.; Das, R. Study of the optical properties of Zn doped Mn spinel ferrite nanocrystals shows multiple emission peaks in the visible range–a promising soft ferrite nanomaterial for deep blue LED. J. Mol. Struct. 2020, 1199. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhang, L.; Pang, G.; Yuan, C.; Zhang, X. Self-Sacrifice Template Fabrication of Hierarchical Mesoporous Bi-Component-Active ZnO/ZnFe2O4 Sub-Microcubes as Superior Anode Towards High-Performance Lithium-Ion Battery. Adv. Funct. Mater. 2014, 25, 238–246. [Google Scholar] [CrossRef]
- Al-Meer, S.; Ghouri, Z.K.; Elsaid, K.; Easa, A.; Al-Qahtani, M.T.; Akhtar, M.S. Engineering of magnetically separable ZnFe2O4@ TiO2 nanofibers for dye-sensitized solar cells and removal of pollutant from water. J. Alloy Compd. 2017, 723, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, S.; Shao, M.; Huang, J.; Deng, X.; Hou, P.; Xu, X. Fabrication of ZnO/ZnFe2O4 hollow nanocages through metal organic frameworks route with enhanced gas sensing properties. Sens. Actuators B Chem. 2017, 251, 27–33. [Google Scholar] [CrossRef]
- Yuan, C.; Cao, H.; Zhu, S.; Hua, H.; Hou, L. Core–shell ZnO/ZnFe2O4@C mesoporous nanospheres with enhanced lithium storage properties towards high-performance Li-ion batteries. J. Mater. Chem. A 2015, 3, 20389–20398. [Google Scholar] [CrossRef]
- Lian, L.; Hou, L.; Zhou, L.; Wang, L.; Yuan, C. Rapid low-temperature synthesis of mesoporous nanophase ZnFe2O4 with enhanced lithium storage properties for Li-ion batteries. RSC Adv. 2014, 4, 49212–49218. [Google Scholar] [CrossRef]
- Vadiyar, M.M.; Kolekar, S.S.; Chang, J.Y.; Ye, Z.B.; Ghue, A.V. Anchoring ultrafine ZnFe2O4/C nanoparticles on 3D ZnFe2O4 nanoflakes for boosting cycle stability and energy density of flexible asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 26016–26028. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, H.; Han, Y.; Wang, F.; He, S.; Liu, P.; Zhang, Z. The effect of CuFe2O4 ferrite phase evolution on 3–5 μm waveband emissivity. Ceram. Int. 2020, 46, 7694–7702. [Google Scholar] [CrossRef]
- Wang, S. Effects of Fe on Crystallization and Properties of a New High Infrared Radiance Glass-Ceramics. Environ. Sci. Technol. 2010, 44, 4816–4820. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, S.Q.; Yang, C.; Bao, R.Q.; Tong, L.N.; Hou, L.R.; Zhang, X.G.; Yuan, C.Z. Metal-organic-framework-derived two-dimensional ultrathin mesoporous hetero-ZnFe2O4/ZnO nanosheets with enhanced lithium storage properties for Li-ion batteries. Nanotechnology 2016, 27, 46. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, Z.-G.; Ouyang, J.-H.; Wang, Y.; Wang, Y. Dependence of the infrared emissivity on SiC content and microstructure of microarc oxidation ceramic coatings formed in Na2SiO3 electrolyte. Appl. Surf. Sci. 2018, 431, 17–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, D. Infrared emission properties of RE (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, and Dy) and Mn co-doped Co0.6Zn0.4Fe2O4 ferrites. Mater. Chem. Phys. 2012, 131, 575–580. [Google Scholar] [CrossRef]
- Wu, X.; Yu, H.; Dong, H.; Geng, L. Enhanced infrared radiation properties of CoFe2O4 by single Ce3+-doping with energy-efficient preparation. Ceram. Int. 2014, 40, 5905–5911. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, H.; Wei, W.; Ding, Y.; Zhang, X.; Yuan, H.; Zhang, Z. The effect of microstructure on the middle and short waveband emissivity of CuO-doped CuxCo1−xFe2O4 spinel. J. Alloy Compd. 2019, 787, 638–648. [Google Scholar] [CrossRef]
- Lu, L.; Fan, X.; Zhang, J.; Hu, X.; Li, G.; Zhang, Z. Evolution of structure and infrared radiation properties for ferrite-based amorphous coating. Appl. Surf. Sci. 2014, 316, 82–87. [Google Scholar] [CrossRef]
- Novikov, V.V.; Mitroshenkov, N.V.; Matovnikov, A.V.; Kuznetsov, S. Specific features of the lattice dynamics of CaxSr1−x F2 solid solutions. Mater. Chem. Phys. 2020, 240. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Ma, L.; Huang, X. Microemulsion-assisted hydrothermal preparation and infrared radiation property of TiO2 nanomaterials with tunable morphologies and crystal form. Mater. Sci. Semicond. Process. 2015, 31, 295–301. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Mao, J.; Li, S.; Shao, H.; Liu, T.; Yamaguchi, T.; Zeng, X. Fabrication and characterization of Pr6O11-HfO2 ultra-high temperature infrared radiation coating. J. Eur. Ceram. Soc. 2019, 39, 4208–4215. [Google Scholar] [CrossRef]
- Song, K.L.; Xie, M.; Ai, Q.; Yang, L.; Tan, H.P. Effects of size, volume fraction, and orientation of metallic flake particles on infrared radiation characteristics of Al/acrylic resin composite coatings Progress in Organic Coatings. Prog. Org. Coat. 2020, 145. [Google Scholar] [CrossRef]
- Tao, X.; Xu, X.; Guo, L.; Hong, W.; Gou, A.; Hou, F.; Liu, J. MoSi2-borosilicate glass coating on fibrous ceramics prepared by in-situ reaction method for infrared radiation Materials & Design. Mater. Des. 2016, 1035, 144–151. [Google Scholar]
- Shao, G.; Wu, X.; Kong, Y.; Cui, S.; Shen, X.; Jiao, C.; Jiao, J. Thermal shock behavior and infrared radiation property of integrative insulations consisting of MoSi2/borosilicate glass coating and fibrous ZrO2 ceramic substrate.Surface and Coatings Technology. Surf. Coat. Technol. 2015, 270, 154–163. [Google Scholar] [CrossRef]
- Inculet, I.I.; Bergougnou, M. Temperature distribution in an electrostatically applied powder coating layer to be cured with infrared radiation. J. Electrost. 2008, 66, 564–566. [Google Scholar] [CrossRef]
- Ding, S.; Mao, J.; Zeng, X.; Cheng, X. Enhanced infrared emission property of NiCr spinel coating doped with MnO2 and rare-earth oxides. Surf. Coatings Technol. 2018, 344, 418–422. [Google Scholar] [CrossRef]
- Chuanzhi, S.; Yao, M.; Xv, L.; Xiaoming, W.; Liu, Y.; Jiubin, T. Ring and circular aperture hexagonal array resonance micromesh coating with infrared dual-bandpass extraordinary transmission and strong electromagnetic shielding. Mater. Chem. Phys. 2019, 234, 323–328. [Google Scholar] [CrossRef]
- Gan, X.; Xu, D.; Lv, Y. Fabrication of TiO2-coated ZrO2 fibers for heat radiative applications. Mater. Chem. Phys. 2020, 251. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Uvida, M.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. A comparative study on PMMA-TiO2 and PMMA-ZrO2 protective coatings. Prog. Org. Coat. 2020, 140. [Google Scholar] [CrossRef]
- Stojadinovic, S.; Tadić, N.; Vasilić, R. Down- and up-conversion photoluminescence of ZrO2:Ho3+ and ZrO2:Ho3+/Yb3+ coatings formed by plasma electrolytic oxidation. J. Alloy Compd. 2019, 785, 1222–1232. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Wang, L.; Zeng, M.-Q.; Zeng, R.-C.; Kannan, M.B.; Lin, C.-G.; Zheng, Y.-F. Biodegradation behavior of micro-arc oxidation coating on magnesium alloy-from a protein perspective. Bioact. Mater. 2020, 5, 398–409. [Google Scholar] [CrossRef]
- Wei, G.; Yang, D.; Zhang, T.; Yue, X.; Qiu, F. Fabrication of multifunctional coating with high luminous transmittance, self-cleaning and radiative cooling performances for energy-efficient windows. Sol. Energy Mater. Sol. Cells 2019, 202. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jiang, Z.-T.; Yin, C.-Y.; Chuah, L.S.; Lee, H.L.; Amri, A.; Goh, B.-M.; Wood, B.J.; Creagh, C.; Mondinos, N.; et al. Structural Thermal Stability of Graphene Oxide-Doped Copper–Cobalt Oxide Coatings as a Solar Selective Surface. J. Mater. Sci. Technol. 2016, 32, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Li, K.-Z.; Gu, S.; Liu, Q. Comparing ablation properties of NbC and NbC-25 mol.% ZrC coating on SiC-coated C/C composites. Ceram. Int. 2020, 46, 7055–7064. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, L.-F.; Xiang, L.; Zhang, Q.; Zhang, L. Effect of SiC coating and heat treatment on the thermal radiation properties of C/SiC composites. J. Eur. Ceram. Soc. 2014, 34, 1667–1672. [Google Scholar] [CrossRef]
- Zheng, J.-Q.; Chen, J.; Zhang, B.-H.; Liu, X.-J.; Chen, Z.-M.; Wu, H.-B.; Huang, Z.-R. Electrical percolation and infrared emissivity of pressureless sintered SiC-MoSi2 composites tailored by sintering temperature. J. Eur. Ceram. Soc. 2019, 39, 3981–3987. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, X.; Lu, L.; Hu, X.; Li, G. Ferrites based infrared radiation coatings with high emissivity and high thermal shock resistance and their application on energy-saving kettle. Appl. Surf. Sci. 2015, 344, 223–229. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Wu, C.; Zhang, J.; Wang, F. Solvothermal synthesis of spinel ZnFe2O4 nanoparticles with enhanced infrared radiation property. Chem. Phys. Lett. 2019, 732. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Xue, M.-M.; Lv, Y.-L.; Li, R.-H.; Tong, J.-M.; Shi, G.-H.; Yang, Y.; Dong, Y.-C. Study on spectral selective absorbing coatings with spinel structures fabricated via plasma spraying. Vacuum 2020, 174. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Murali, N.; Margarette, S.; Samatha, K.; Veeraiah, V. Comparative study of synthesis, structural and magnetic properties of Cu2+ substituted Co-Ni, Co-Zn and Co-Mg nano ferrites. Phys. B Condens. Matter 2018, 530, 251–257. [Google Scholar] [CrossRef]
- Li, N.-H.; Lo, S.-L.; Hu, C.-Y.; Hsieh, C.-H.; Chen, C.-L. Stabilization and phase transformation of CuFe2O4 sintered from simulated copper-laden sludge. J. Hazard. Mater. 2011, 190, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Mason, P.; Williams, A. A compilation of data on the radiant emissivity of some materials at high temperatures. J. Energy Inst. 2019, 92, 523–534. [Google Scholar] [CrossRef]
- Quandt, N.; Roth, R.; Syrowatka, F.; Steimecke, M.; Ebbinghaus, S.G. Spin-Coating and Characterization of Multiferroic MFe2O4 (M = Co, Ni)/BaTiO3 Bilayers. J. Solid State Chem. 2016, 233, 82–89. [Google Scholar] [CrossRef]
- Kalousek, R.; Spousta, J.; Zlámal, J.; Dub, P.; Šikola, T.; Shen, Z.; Salamon, D.; Maca, K. Rapid heating of zirconia nanoparticle-powder compacts by infrared radiation heat transfer. J. Eur. Ceram. Soc. 2017, 37, 1067–1072. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, X.; Lu, L.; Hu, X. Plasma sprayed ferrite-based infrared radiation coating directly from transition metal oxides without high-temperature roasting. Mater. Lett. 2015, 161, 348–351. [Google Scholar] [CrossRef]
- Del Campo, L.; Pérez-Sáez, R.B.; Tello, M.J. Iron oxidation kinetics study by using infrared spectral emissivity measurements below 570 °C. Corros. Sci. 2008, 50, 194–199. [Google Scholar] [CrossRef]
- Castro, C.A.D.O.; Nunes, A.C.P.; Roque, J.V.; Teófilo, R.F.; Santos, O.P.; Santos, G.A.; Gallo, R.; Pantuza, I.B.; Resende, M.D.V. Optimization of Eucalyptus benthamii progeny test based on Near-Infrared Spectroscopy approach and volumetric production. Ind. Crop. Prod. 2019, 141. [Google Scholar] [CrossRef]
- Shen, S.; Yuan, Y.; Ruan, Z.; Tan, H. Optimizing the design of an embedded grating polarizer for infrared polarization light field imaging. Results Phys. 2019, 12, 21–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, S.; Yu, K.; Cong, M. A new k-interval optimization technique for atmospheric upwelling radiance calculation in infrared absorption bands. J. Quant. Spectrosc. Radiat. Transf. 2015, 160, 75–84. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, F.; Gong, H.; Hu, P.; Song, S.; Zhen, Q. Study on ZrSiO4 -aluminosilicate glass coating with high infrared emissivity and anti-oxidation properties. Compos. Commun. 2017, 4, 16–19. [Google Scholar] [CrossRef]


| Test Number | Formula/g | Cu Doped Ratio/% | ||
|---|---|---|---|---|
| CuO | Co2O3 | Fe2O3 | ||
| S1 | 1.6925 | 33.5258 | 67.9524 | 5 |
| S2 | 5.0774 | 29.9968 | 67.9524 | 15 |
| S3 | 8.4623 | 26.4678 | 67.9524 | 25 |
| S4 | 16.9246 | 17.6452 | 67.9524 | 50 |
| S5 | 25.3868 | 8.8226 | 67.9524 | 75 |
| S6 | 28.7717 | 5.2936 | 67.9524 | 85 |
| S7 | 32.1567 | 1.7645 | 67.9524 | 95 |
| Samples | The Mole Fraction of Each Element/% | Cu/(Cu + Co)/% | |||
|---|---|---|---|---|---|
| O | Fe | Cu | Co | ||
| S2 | 58.12 | 27.28 | 2.04 | 12.57 | 13.9 |
| S3 | 55.30 | 30.02 | 3.48 | 11.20 | 23.7 |
| S5 | 56.24 | 31.19 | 8.16 | 4.40 | 64.97 |
| S7 | 45.81 | 37.02 | 15.96 | 1.22 | 92.8 |
| Waveband | 1–3 μm | 3–5 μm | 5–8 μm | 8–14 μm | 14–22 μm |
|---|---|---|---|---|---|
| Proportion | 0.13 | 0.34 | 0.30 | 0.18 | 0.05 |
| Composition | CuO | Co2O3 | Fe2O3 |
|---|---|---|---|
| Mass Content/% | 16.98 | 16.73 | 66.29 |
| Waveband | 3–5 μm | 8–14 μm |
|---|---|---|
| Fitted values | 0.99 | 0.986 |
| Experimental values | 0.986 | 0.977 |
| Deviation | 0.41% | 0.92% |
| Number | x1 | x2 | x3 | x4 | |
|---|---|---|---|---|---|
| Extreme Vertices Design | 1 | 0.50 | 0.05 | 0.15 | 0.30 |
| 2 | 0.30 | 0.05 | 0.15 | 0.50 | |
| 3 | 0.45 | 0.05 | 0.20 | 0.30 | |
| 4 | 0.40 | 0.15 | 0.15 | 0.30 | |
| 5 | 0.35 | 0.15 | 0.20 | 0.30 | |
| 6 | 0.30 | 0.05 | 0.20 | 0.45 | |
| 7 | 0.30 | 0.15 | 0.15 | 0.40 | |
| 8 | 0.30 | 0.15 | 0.20 | 0.35 | |
| Boundary Surface Centroid Design | 9 | 0.36 | 0.10 | 0.18 | 0.36 |
| 10 | 0.30 | 0.10 | 0.18 | 0.42 | |
| 11 | 0.39 | 0.05 | 0.18 | 0.38 | |
| 12 | 0.34 | 0.15 | 0.18 | 0.33 | |
| 13 | 0.38 | 0.10 | 0.15 | 0.37 | |
| 14 | 0.35 | 0.10 | 0.20 | 0.35 | |
| Overall Centroid Design | 15 | 0.42 | 0.10 | 0.18 | 0.30 |
| Number | x1 | x2 | x3 | x4 | y1 | y2 |
|---|---|---|---|---|---|---|
| 1 | 0.50 | 0.05 | 0.15 | 0.30 | 0.928 | 0.914 |
| 2 | 0.30 | 0.05 | 0.15 | 0.50 | 0.830 | 0.970 |
| 3 | 0.45 | 0.05 | 0.20 | 0.30 | 0.849 | 0.887 |
| 4 | 0.40 | 0.15 | 0.15 | 0.30 | 0.912 | 0.900 |
| 5 | 0.35 | 0.15 | 0.20 | 0.30 | 0.900 | 0.900 |
| 6 | 0.30 | 0.05 | 0.20 | 0.45 | 0.845 | 0.947 |
| 7 | 0.30 | 0.15 | 0.15 | 0.40 | 0.950 | 0.897 |
| 8 | 0.30 | 0.15 | 0.15 | 0.40 | 0.943 | 0.905 |
| 9 | 0.36 | 0.10 | 0.18 | 0.36 | 0.926 | 0.903 |
| 10 | 0.30 | 0.10 | 0.18 | 0.42 | 0.927 | 0.899 |
| 11 | 0.39 | 0.05 | 0.18 | 0.38 | 0.867 | 0.939 |
| 12 | 0.34 | 0.15 | 0.18 | 0.33 | 0.923 | 0.894 |
| 13 | 0.38 | 0.10 | 0.15 | 0.37 | 0.935 | 0.913 |
| 14 | 0.35 | 0.10 | 0.20 | 0.35 | 0.910 | 0.894 |
| 15 | 0.42 | 0.10 | 0.18 | 0.30 | 0.905 | 0.876 |
| Waveband | 3–5 μm | 8–14 μm |
|---|---|---|
| Fitting values | 0.944 | 0.901 |
| Experimental values | 0.931 | 0.905 |
| Deviation | 1.4% | 0.44% |
| Waveband | 3–5 μm | 8–14 μm |
|---|---|---|
| Optimal materials | 0.986 | 0.977 |
| Optimal coating | 0.931 | 0.905 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; An, H.; Zhang, J.; Ding, Y.; Lian, C.; Bai, H. A Novel Optimization Model and Application of Optimal Formula Design for CuxCo1−xFe2O4 Spinel-Based Coating Slurry in Relation to Near and Middle Infrared Radiation Strengthening. Materials 2020, 13, 2332. https://doi.org/10.3390/ma13102332
Du H, An H, Zhang J, Ding Y, Lian C, Bai H. A Novel Optimization Model and Application of Optimal Formula Design for CuxCo1−xFe2O4 Spinel-Based Coating Slurry in Relation to Near and Middle Infrared Radiation Strengthening. Materials. 2020; 13(10):2332. https://doi.org/10.3390/ma13102332
Chicago/Turabian StyleDu, Haiqing, Haifei An, Jian Zhang, Yuhao Ding, Chao Lian, and Hao Bai. 2020. "A Novel Optimization Model and Application of Optimal Formula Design for CuxCo1−xFe2O4 Spinel-Based Coating Slurry in Relation to Near and Middle Infrared Radiation Strengthening" Materials 13, no. 10: 2332. https://doi.org/10.3390/ma13102332




