High Oxygen and Water-Vapor Transmission Rate and In Vitro Cytotoxicity Assessment with Illite-Polyethylene Packaging Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Preparation of Illite-PE Masterbatch
2.3. Preparation of Illite-PE Composite Film
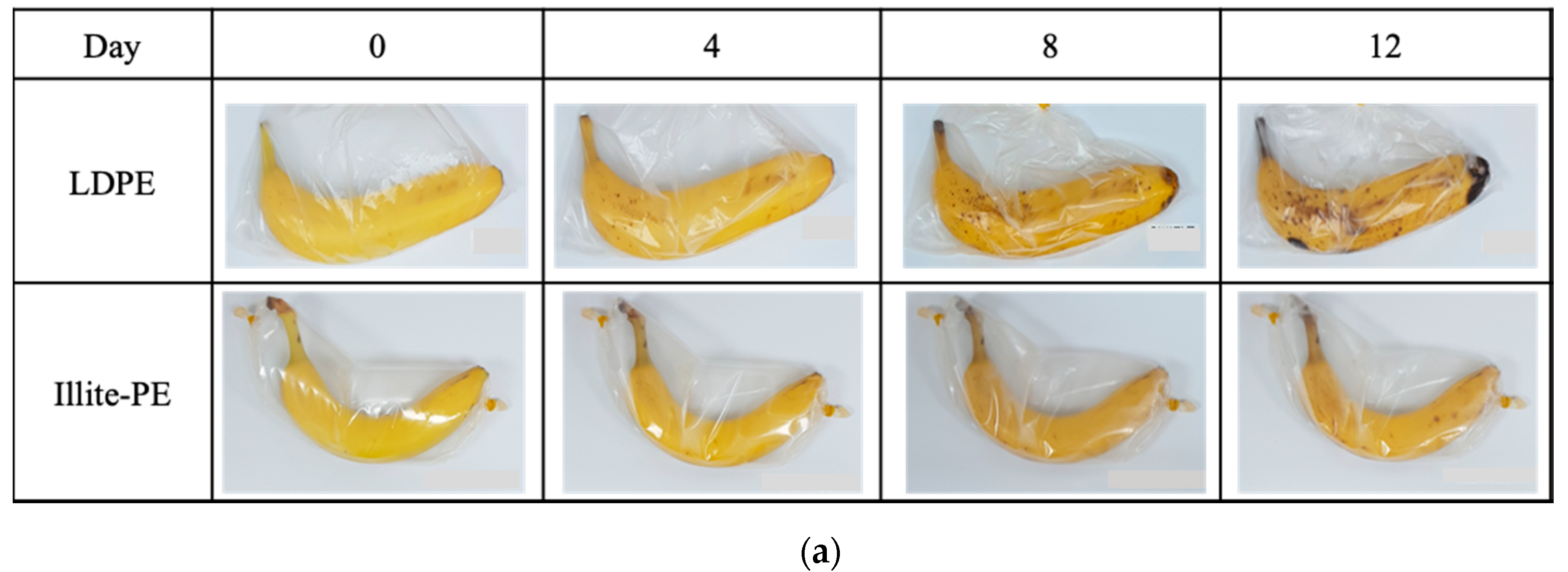
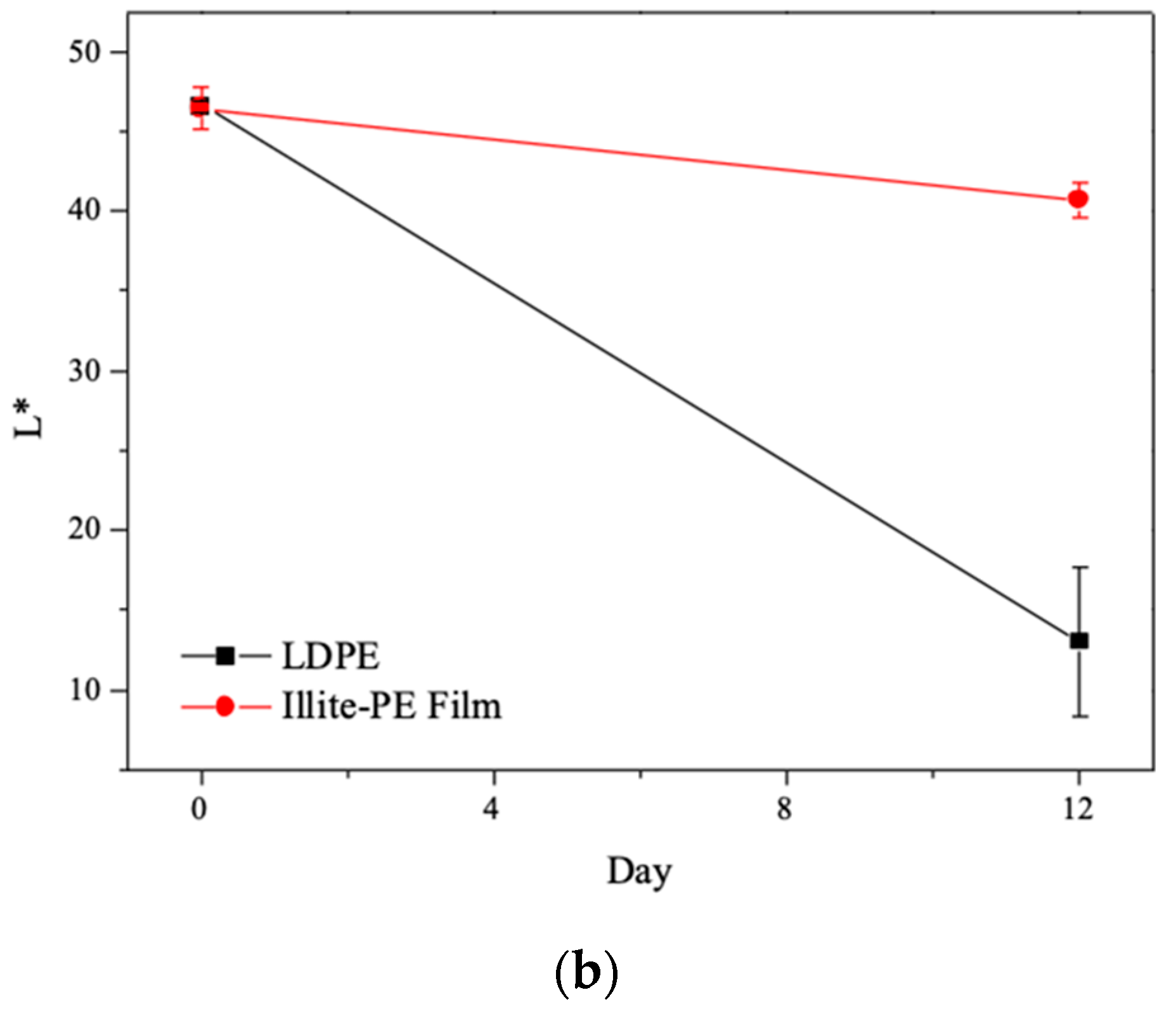
2.4. Assessments of Freshness-Maintaining Performance of Illite-PE Composite Film
2.5. In Vitro Cytotoxicity of Illite-PE Composite Film
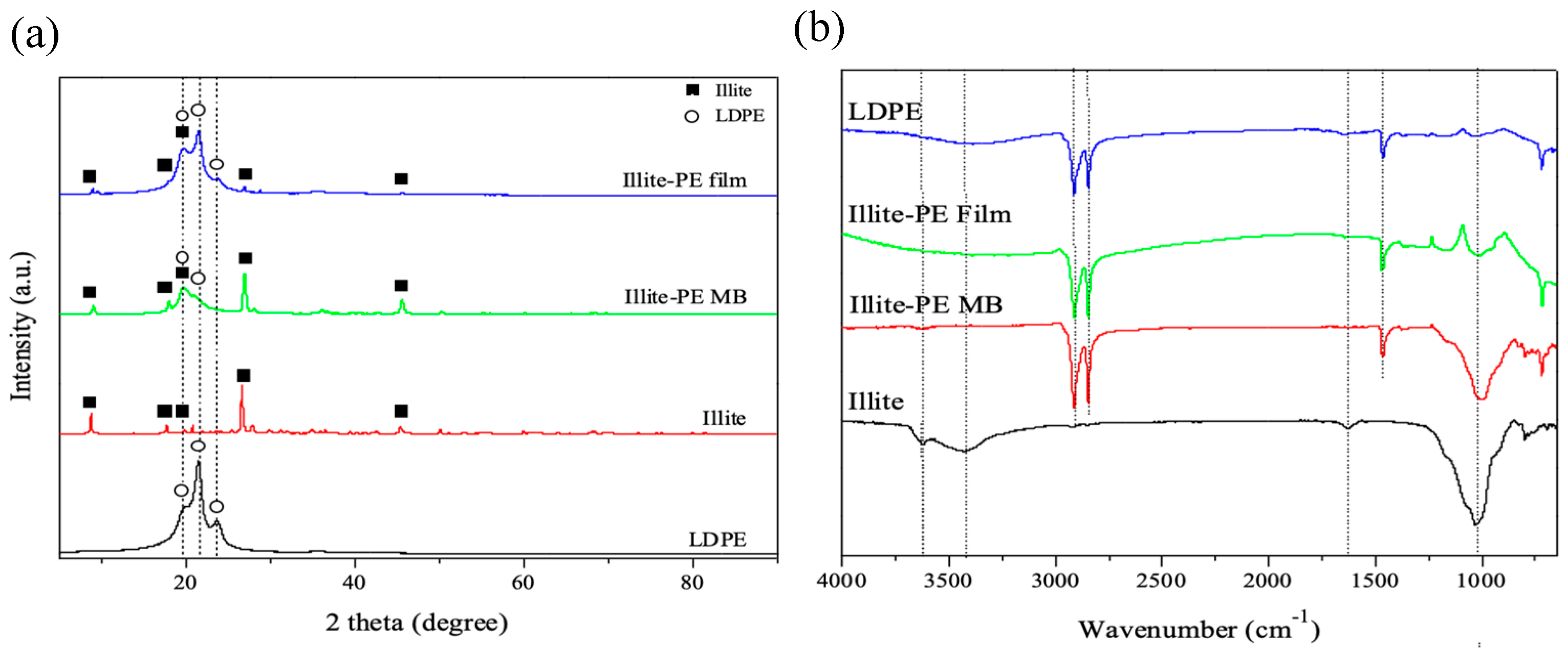
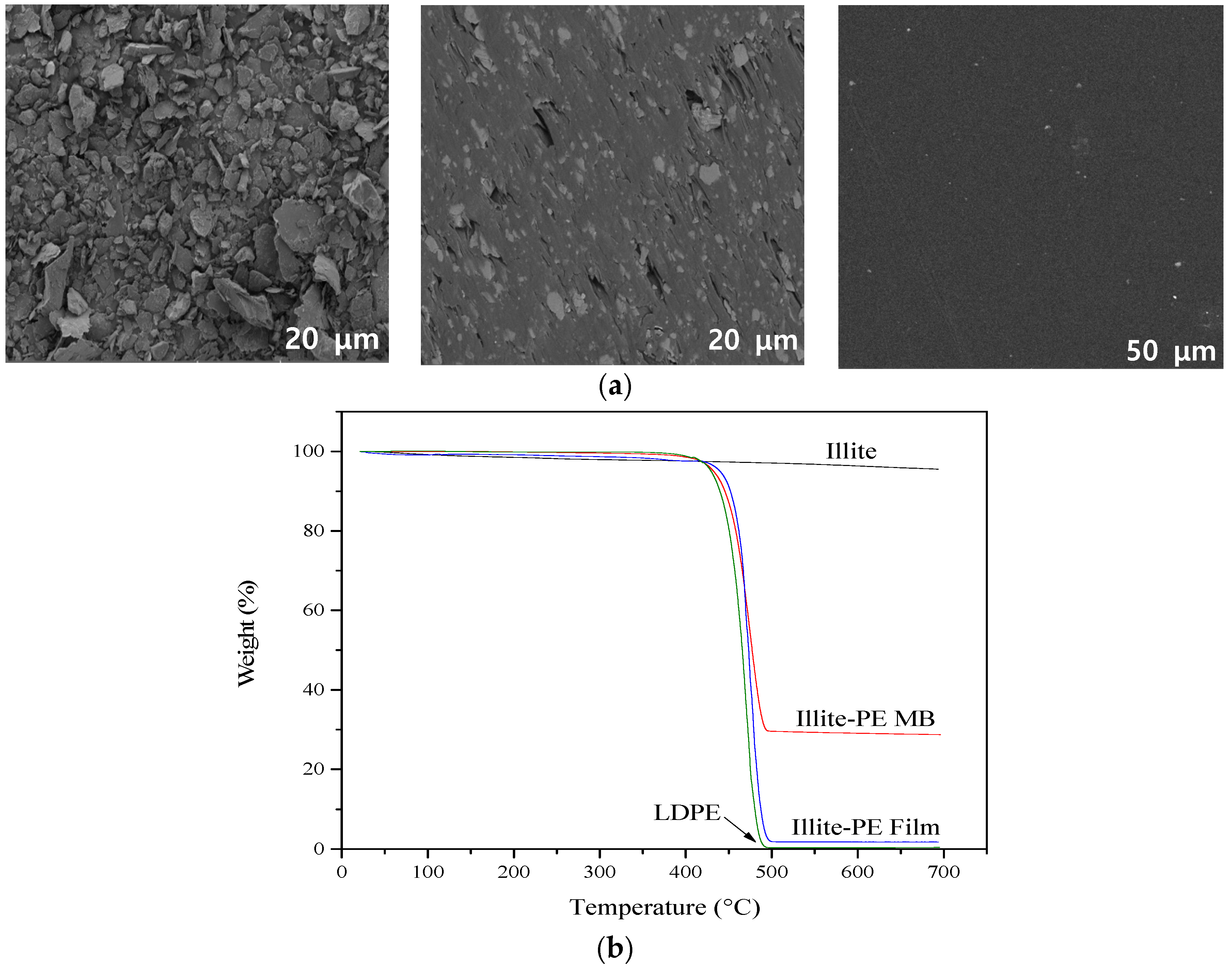
2.6. Instrumental Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Xing, L.Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C. Modified atmosphere packaging of pomegranate fruit and arils: A review. Food Bioprocess Technol. 2011, 5, 15–30. [Google Scholar] [CrossRef]
- Han, J.H. A Review of food packaging technologies and innovations. Innov. Food Packag. 2014, 3–12. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.-L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.K.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Compr. Rev. Food Sci. Food Saf. 2015, 15, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Pech, J.; Bouzayen, M.; Latché, A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.; Ferrer, A.; Oria, R.; Salvador, M.L. Determination of O2 and CO2 transmission rates through microperforated films for modified atmosphere packaging of fresh fruits and vegetables. J. Food Eng. 2008, 86, 194–201. [Google Scholar] [CrossRef]
- Kartal, S.; Aday, M.S.; Caner, C. Use of microperforated films and oxygen scavengers to maintain storage stability of fresh strawberries. Postharvest Boil. Technol. 2012, 71, 32–40. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Du, Z.; Chan, J.K.Y.; Teoh, S.H.; Thian, E.S.; Hong, M. Direct laser microperforation of bioresponsive surface-patterned films with through-hole arrays for vascular tissue-engineering application. ACS Biomater. Sci. Eng. 2015, 1, 1239–1249. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Fasihi, M.; Abolghasemi, M.R. Oxygen barrier and mechanical properties of masterbatch-based PA6/nanoclay composite films. J. Appl. Polym. Sci. 2012, 125, E2–E8. [Google Scholar] [CrossRef]
- Pokharel, P.; Lee, D.S. High performance polyurethane nanocomposite films prepared from a masterbatch of graphene oxide in polyether polyol. Chem. Eng. J. 2014, 253, 356–365. [Google Scholar] [CrossRef]
- Adak, B.; Joshi, M.; Butola, B.S. Polyurethane/clay nanocomposites with improved helium gas barrier and mechanical properties: Direct versus master-batch melt mixing route. J. Appl. Polym. Sci. 2018, 135, 46422–46426. [Google Scholar] [CrossRef]
- Hong, S.-I.; Rhim, J.-W. Preparation and properties of melt-intercalated linear low density polyethylene/clay nanocomposite films prepared by blow extrusion. LWT-Food Sci. Technol. 2012, 48, 43–51. [Google Scholar] [CrossRef]
- Rosa, D.S.; Neto, I.C.; Calil, M.R.; Pedroso, A.G.; Fonseca, C.P.; Neves, S. Evaluation of the thermal and mechanical properties of poly(ε-caprolactone), low-density polyethylene, and their blends. J. Appl. Polym. Sci. 2004, 91, 3909–3914. [Google Scholar] [CrossRef]
- Hasegawa, N.; Okamoto, H.; Kato, M.; Usuki, A. Preparation and mechanical properties of polypropylene-clay hybrids based on modified polypropylene and organophilic clay. J. Appl. Polym. Sci. 2000, 78, 1918–1922. [Google Scholar] [CrossRef]
- Mangaraj, S.; Goswami, T.K.; Panda, D.K. Modeling of gas transmission properties of polymeric films used for MA packaging of fruits. J. Food Sci. Technol. 2014, 52, 5456–5469. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Easteal, A.J.; Chen, X.D. Ethylene and oxygen permeability through polyethylene packaging films. Packag. Technol. Sci. 1998, 11, 169–178. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.-H. HDPE/expanded graphite nanocomposites prepared via masterbatch process. Polym. Eng. Sci. 2007, 47, 882–888. [Google Scholar] [CrossRef]
- Gomes, E.V.D.; Visconte, L.L.Y.; Pacheco, E.B.A.V. Morphological, thermal and mechanical properties of polypropylene and vermiculite blends. Int. J. Polym. Mater. 2008, 57, 957–968. [Google Scholar] [CrossRef]
- Elleithy, R.; Ali, I.; Ali, M.A.; Al-Zahrani, S.M.; Al-Zahrani, S.M. High density polyethylene/micro calcium carbonate composites: A study of the morphological, thermal, and viscoelastic properties. J. Appl. Polym. Sci. 2010, 117, 2413–2421. [Google Scholar] [CrossRef]
- Raji, M.; Essabir, H.; Essassi, E.M.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Morphological, thermal, mechanical, and rheological properties of high density polyethylene reinforced with illite clay. Polym. Compos. 2016, 39, 1522–1533. [Google Scholar] [CrossRef]
- Gomes, J.F.S.; Vieira, R.R.; De Oliveira, I.A.A.; Leta, F.R. Influence of illumination on the characterization of banana ripening. J. Food Eng. 2014, 120, 215–222. [Google Scholar] [CrossRef]
- Villanueva, M.; Cabedo, L.; Gimenez, E.; Lagaron, J.; Coates, P.; Kelly, A. Study of the dispersion of nanoclays in a LDPE matrix using microscopy and in-process ultrasonic monitoring. Polym. Test. 2009, 28, 277–287. [Google Scholar] [CrossRef]
- Xia, X.; Cai, S.; Xie, C. Preparation, structure and thermal stability of Cu/LDPE nanocomposites. Mater. Chem. Phys. 2006, 95, 122–129. [Google Scholar] [CrossRef]
- Gulmine, J.; Janissek, P.; Heise, H.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Prashantha, K. Multi-walled carbon nanotube filled polypropylene nanocomposites based on masterbatch route: Improvement of dispersion and mechanical properties through PP-g-MA addition. Express Polym. Lett. 2008, 2, 735–745. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Sorbitol- vs glycerol-plasticized whey protein edible films: Integrated oxygen permeability and tensile property evaluation. J. Agric. Food Chem. 1994, 42, 841–845. [Google Scholar] [CrossRef]
- Shi, Q.P.; Li, D.L.; Xu, W.C. LDPE/EVA Modified film prepared by melt blend method and study of its mechanical performances and barrier property. Mater. Sci. Forum 2011, 689, 321–327. [Google Scholar] [CrossRef]
- Liang, J.-Z. Estimation of tensile strength of inorganic plate-like particulate reinforced polymer composites. Polym. Eng. Sci. 2012, 53, 1823–1827. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Saha, P. HaCaT Keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, D.M.; Lee, H.; Kim, J.; Chang, J.H. High Oxygen and Water-Vapor Transmission Rate and In Vitro Cytotoxicity Assessment with Illite-Polyethylene Packaging Films. Materials 2020, 13, 2382. https://doi.org/10.3390/ma13102382
Seong DM, Lee H, Kim J, Chang JH. High Oxygen and Water-Vapor Transmission Rate and In Vitro Cytotoxicity Assessment with Illite-Polyethylene Packaging Films. Materials. 2020; 13(10):2382. https://doi.org/10.3390/ma13102382
Chicago/Turabian StyleSeong, Dong Min, Heysun Lee, Jungbae Kim, and Jeong Ho Chang. 2020. "High Oxygen and Water-Vapor Transmission Rate and In Vitro Cytotoxicity Assessment with Illite-Polyethylene Packaging Films" Materials 13, no. 10: 2382. https://doi.org/10.3390/ma13102382





